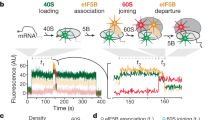
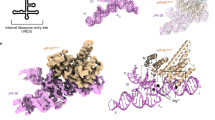
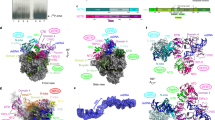
Abstract
Many viruses potently inhibit host protein synthesis, termed host shutoff, while employing strategies to sustain their own translation. How and why certain host mRNAs continue to be translated at later infection stages remains unclear. Here, using RNAseq and polysome profiling, we show that during shutoff by vaccinia virus (VacV), several host mRNAs increase in polysome occupancy but only a few, primarily JUN that encodes the Jun transcription factor, result in increased protein abundance across multiple cell lines. While dispensable for Jun production, translation of viral mRNAs depended on the small ribosomal protein, Receptor for Activated C Kinase 1 (RACK1) and the eukaryotic Initiation Factor, eIF3. These differential eIF3 dependencies are associated with structurally distinct 5′ untranslated regions in viral versus JUN mRNAs. Cryo-electron microscopy structures of 40S ribosomes from mock-infected or VacV-infected cells showed that when bound to eIF3, the rotational range of the RACK1-containing 40S head domain broadens during infection. Our data reveal how eIF3-bound 40S ribosomes are remodelled late in infection and the distinct strategies of translation initiation that arise during shutoff to produce host and viral proteins required for poxvirus spread.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its supplementary files. Cryo-EM maps are deposited in Electron Microscopy Data Bank (EMDB) as follows: eIF3-bound 40S, state 1, from mock-infected cells (EMD-48646); eIF3-bound 40S, state 2, from mock-infected cells (EMD-48647); free 40S, state 1, from mock-infected cells (EMD-48644), free 40S, state 2, from mock-infected cells (EMD-48645); eIF3-bound 40S consensus structure, from mock-infected cells (EMD-48642); free 40S consensus structure, from mock-infected cells (EMD-48643); eIF3-bound 40S, state 1, from VacV-infected cells (EMD-46432); eIF3-bound 40S, state 2, from VacV-infected cells (EMD-46447); free 40S, state 1, from VacV-infected cells (EMD-46449), free 40S, state 2, from VacV-infected cells (EMD-46450); eIF3-bound 40S consensus structure, from VacV-infected cells (EMD-48640); free 40S consensus structure, from VacV-infected cells (EMD-48641). Raw and processed sequencing data are deposited in NCBI Gene Expression Omnibus (GEO) under accession number GSE278320. Source data are provided with this paper.
Code availability
All code is available on CodeOcean at https://doi.org/10.24433/CO.5984508.v2 (ref. 75).
References
Burgess, H. M., Vink, E. I. & Mohr, I. Minding the message: tactics controlling RNA decay, modification, and translation in virus-infected cells. Genes Dev. 36, 108–132 (2022).
Rozman, B., Fisher, T. & Stern-Ginossar, N. Translation-A tug of war during viral infection. Mol. Cell 83, 481–495 (2023).
Jan, E., Mohr, I. & Walsh, D. A cap-to-tail guide to mRNA translation strategies in virus-infected cells. Annu. Rev. Virol. 3, 283–307 (2016).
Pelletier, J. & Sonenberg, N. The organizing principles of eukaryotic ribosome recruitment. Annu. Rev. Biochem. 88, 307–335 (2019).
Brito Querido, J., Diaz-Lopez, I. & Ramakrishnan, V. The molecular basis of translation initiation and its regulation in eukaryotes. Nat. Rev. Mol. Cell Biol. 25, 168–186 (2024).
Brito Querido, J. et al. The structure of a human translation initiation complex reveals two independent roles for the helicase eIF4A. Nat. Struct. Mol. Biol. 31, 455–464 (2024).
Park, C. & Walsh, D. Ribosomes in poxvirus infection. Curr. Opin. Virol. 56, 101256 (2022).
Moss, B., Ahn, B. Y., Amegadzie, B., Gershon, P. D. & Keck, J. G. Cytoplasmic transcription system encoded by vaccinia virus. J. Biol. Chem. 266, 1355–1358 (1991).
Parrish, S. & Moss, B. Characterization of a second vaccinia virus mRNA-decapping enzyme conserved in poxviruses. J. Virol. 81, 12973–12978 (2007).
Ly, M., Burgess, H. M., Shah, S. B., Mohr, I. & Glaunsinger, B. A. Vaccinia virus D10 has broad decapping activity that is regulated by mRNA splicing. PLoS Pathog. 18, e1010099 (2022).
Dhungel, P., Cao, S. & Yang, Z. The 5′-poly(A) leader of poxvirus mRNA confers a translational advantage that can be achieved in cells with impaired cap-dependent translation. PLoS Pathog. 13, e1006602 (2017).
Rollins, M. G. et al. Negative charge in the RACK1 loop broadens the translational capacity of the human ribosome. Cell Rep. 36, 109663 (2021).
Park, C. & Walsh, D. RACK1 regulates poxvirus protein synthesis independently of its role in ribosome-based stress signaling. J. Virol. 96, e0109322 (2022).
Jha, S. et al. Trans-kingdom mimicry underlies ribosome customization by a poxvirus kinase. Nature 546, 651–655 (2017).
DiGiuseppe, S. et al. Proteomic and mechanistic dissection of the poxvirus-customized ribosome. J. Cell Sci. https://doi.org/10.1242/jcs.246603 (2020).
Yang, Z., Martens, C. A., Bruno, D. P., Porcella, S. F. & Moss, B. Pervasive initiation and 3′-end formation of poxvirus postreplicative RNAs. J. Biol. Chem. 287, 31050–31060 (2012).
Shirokikh, N. E. & Spirin, A. S. Poly(A) leader of eukaryotic mRNA bypasses the dependence of translation on initiation factors. Proc. Natl Acad. Sci. USA 105, 10738–10743 (2008).
Yamamoto, H. et al. Molecular architecture of the ribosome-bound Hepatitis C Virus internal ribosomal entry site RNA. EMBO J. 34, 3042–3058 (2015).
Spahn, C. M. et al. Hepatitis C virus IRES RNA-induced changes in the conformation of the 40s ribosomal subunit. Science 291, 1959–1962 (2001).
Quade, N., Boehringer, D., Leibundgut, M., van den Heuvel, J. & Ban, N. Cryo-EM structure of Hepatitis C virus IRES bound to the human ribosome at 3.9-Å resolution. Nat. Commun. 6, 7646 (2015).
Murray, J. et al. Structural characterization of ribosome recruitment and translocation by type IV IRES. Elife https://doi.org/10.7554/eLife.13567 (2016).
Acosta-Reyes, F., Neupane, R., Frank, J. & Fernandez, I. S. The Israeli acute paralysis virus IRES captures host ribosomes by mimicking a ribosomal state with hybrid tRNAs. EMBO J. 38, e102226 (2019).
Johannes, G., Carter, M. S., Eisen, M. B., Brown, P. O. & Sarnow, P. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc. Natl Acad. Sci. USA 96, 13118–13123 (1999).
Glaunsinger, B. & Ganem, D. Highly selective escape from KSHV-mediated host mRNA shutoff and its implications for viral pathogenesis. J. Exp. Med. 200, 391–398 (2004).
Hutin, S., Lee, Y. & Glaunsinger, B. A. An RNA element in human interleukin 6 confers escape from degradation by the gammaherpesvirus SOX protein. J. Virol. 87, 4672–4682 (2013).
Bercovich-Kinori, A. et al. A systematic view on influenza induced host shutoff. Elife https://doi.org/10.7554/eLife.18311 (2016).
Finkel, Y. et al. SARS-CoV-2 uses a multipronged strategy to impede host protein synthesis. Nature 594, 240–245 (2021).
Puray-Chavez, M. et al. The translational landscape of SARS-CoV-2-infected cells reveals suppression of innate immune genes. mBio 13, e0081522 (2022).
Dai, A. et al. Ribosome profiling reveals translational upregulation of cellular oxidative phosphorylation mRNAs during vaccinia virus-induced host shutoff. J. Virol. https://doi.org/10.1128/JVI.01858-16 (2017).
Soday, L. et al. Quantitative temporal proteomic analysis of vaccinia virus infection reveals regulation of histone deacetylases by an interferon antagonist. Cell Rep. 27, 1920–1933.e7 (2019).
Guerra, S. et al. Cellular gene expression survey of vaccinia virus infection of human HeLa cells. J. Virol. 77, 6493–6506 (2003).
Molina, J. A. & Yang, Z. Rapid and quantitative evaluation of vaccinia virus-induced host shutoff using newly generated cell lines stably expressing secreted Gaussia luciferase. J. Med. Virol. 94, 3811–3819 (2022).
Erez, N., Wyatt, L. S., Americo, J. L., Xiao, W. & Moss, B. Spontaneous and targeted mutations in the decapping enzyme enhance replication of modified vaccinia virus Ankara (MVA) in monkey cells. J. Virol. 95, e0110421 (2021).
Liu, S. W., Katsafanas, G. C., Liu, R., Wyatt, L. S. & Moss, B. Poxvirus decapping enzymes enhance virulence by preventing the accumulation of dsRNA and the induction of innate antiviral responses. Cell Host Microbe 17, 320–331 (2015).
Hesser, C. R. & Walsh, D. YTHDF2 is downregulated in response to host shutoff induced by DNA virus infection and regulates interferon-stimulated gene expression. J. Virol. 97, e0175822 (2023).
Meade, N. et al. Poxviruses evade cytosolic sensing through disruption of an mTORC1-mTORC2 regulatory circuit. Cell 174, 1143–1157.e17 (2018).
Meade, N., King, M., Munger, J. & Walsh, D. mTOR dysregulation by vaccinia virus F17 controls multiple processes with varying roles in infection. J. Virol. https://doi.org/10.1128/JVI.00784-19 (2019).
Walsh, D. et al. Eukaryotic translation initiation factor 4F architectural alterations accompany translation initiation factor redistribution in poxvirus-infected cells. Mol. Cell. Biol. 28, 2648–2658 (2008).
Hochstoeger, T. & Chao, J. A. Towards a molecular understanding of the 5′TOP motif in regulating translation of ribosomal mRNAs. Semin. Cell Dev. Biol. 154, 99–104 (2024).
Filone, C. M. et al. The master regulator of the cellular stress response (HSF1) is critical for orthopoxvirus infection. PLoS Pathog. 10, e1003904 (2014).
Brum, L. M., Lopez, M. C., Varela, J. C., Baker, H. V. & Moyer, R. W. Microarray analysis of A549 cells infected with rabbitpox virus (RPV): a comparison of wild-type RPV and RPV deleted for the host range gene, SPI-1. Virology 315, 322–334 (2003).
Marzluff, W. F., Wagner, E. J. & Duronio, R. J. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat. Rev. Genet. 9, 843–854 (2008).
Gershon, P. D., Ahn, B. Y., Garfield, M. & Moss, B. Poly(A) polymerase and a dissociable polyadenylation stimulatory factor encoded by vaccinia virus. Cell 66, 1269–1278 (1991).
Gonzalez-Sanchez, A. M., Castellanos-Silva, E. A., Diaz-Figueroa, G. & Cate, J. H. D. JUN mRNA translation regulation is mediated by multiple 5′ UTR and start codon features. PLoS ONE 19, e0299779 (2024).
de la Parra, C. et al. A widespread alternate form of cap-dependent mRNA translation initiation. Nat. Commun. 9, 3068 (2018).
Lee, A. S., Kranzusch, P. J. & Cate, J. H. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 522, 111–114 (2015).
Mukhopadhyay, S., Amodeo, M. E. & Lee, A. S. Y. eIF3d controls the persistent integrated stress response. Mol. Cell 83, 3303–3313.e6 (2023).
Lee, A. S., Kranzusch, P. J., Doudna, J. A. & Cate, J. H. eIF3d is an mRNA cap-binding protein that is required for specialized translation initiation. Nature 536, 96–99 (2016).
Lamper, A. M., Fleming, R. H., Ladd, K. M. & Lee, A. S. Y. A phosphorylation-regulated eIF3d translation switch mediates cellular adaptation to metabolic stress. Science 370, 853–856 (2020).
Thompson, L., Depledge, D. P., Burgess, H. M. & Mohr, I. An eIF3d-dependent switch regulates HCMV replication by remodeling the infected cell translation landscape to mimic chronic ER stress. Cell Rep. 39, 110767 (2022).
Walsh, D. & Mohr, I. Coupling 40S ribosome recruitment to modification of a cap-binding initiation factor by eIF3 subunit e. Genes Dev. 28, 835–840 (2014).
Kratzat, H. et al. A structural inventory of native ribosomal ABCE1-43S pre-initiation complexes. EMBO J. 40, e105179 (2021).
Iwasaki, S., Floor, S. N. & Ingolia, N. T. Rocaglates convert DEAD-box protein eIF4A into a sequence-selective translational repressor. Nature 534, 558–561 (2016).
Sawai, M., Ishikawa, Y., Ota, A. & Sakurai, H. The proto-oncogene JUN is a target of the heat shock transcription factor HSF1. FEBS J. 280, 6672–6680 (2013).
Pereira, A. C. et al. A vaccinia virus-driven interplay between the MKK4/7-JNK1/2 pathway and cytoskeleton reorganization. J. Virol. 86, 172–184 (2012).
Saba, J. A. et al. LARP1 binds ribosomes and TOP mRNAs in repressed complexes. EMBO J. 43, 6555–6572 (2024).
Schneider, C. et al. An unusual mode of baseline translation adjusts cellular protein synthesis capacity to metabolic needs. Cell Rep. 41, 111467 (2022).
Zaborowska, I. & Walsh, D. PI3K signaling regulates rapamycin-insensitive translation initiation complex formation in vaccinia virus-infected cells. J. Virol. 83, 3988–3992 (2009).
Meade, N. et al. The poxvirus F17 protein counteracts mitochondrially orchestrated antiviral responses. Nat. Commun. 14, 7889 (2023).
Jindal, S. & Young, R. A. Vaccinia virus infection induces a stress response that leads to association of Hsp70 with viral proteins. J. Virol. 66, 5357–5362 (1992).
Kowalczyk, A., Guzik, K., Slezak, K., Dziedzic, J. & Rokita, H. Heat shock protein and heat shock factor 1 expression and localization in vaccinia virus infected human monocyte derived macrophages. J. Inflamm. 2, 12 (2005).
Sedger, L. et al. Vaccinia virus replication is independent of cellular HSP72 expression which is induced during virus infection. Virology 225, 423–427 (1996).
Sedger, L. & Ruby, J. Heat shock response to vaccinia virus infection. J. Virol. 68, 4685–4689 (1994).
Oudart, M. et al. The ribosome-associated protein RACK1 represses Kir4.1 translation in astrocytes and influences neuronal activity. Cell Rep. 42, 112456 (2023).
Rollins, M. G., Jha, S., Bartom, E. T. & Walsh, D. RACK1 evolved species-specific multifunctionality in translational control through sequence plasticity within a loop domain. J. Cell Sci. https://doi.org/10.1242/jcs.228908 (2019).
Yi, S. H. et al. Conformational rearrangements upon start codon recognition in human 48S translation initiation complex. Nucleic Acids Res. 50, 5282–5298 (2022).
Llacer, J. L. et al. Conformational differences between open and closed states of the eukaryotic translation initiation complex. Mol. Cell 59, 399–412 (2015).
Hussain, T. et al. Structural changes enable start codon recognition by the eukaryotic translation initiation complex. Cell 159, 597–607 (2014).
Dower, K., Rubins, K. H., Hensley, L. E. & Connor, J. H. Development of Vaccinia reporter viruses for rapid, high content analysis of viral function at all stages of gene expression. Antiviral Res. 91, 72–80 (2011).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pellegrino, S., Dent, K. C., Spikes, T. & Warren, A. J. Cryo-EM reconstruction of the human 40S ribosomal subunit at 2.15 Å resolution. Nucleic Acids Res. 51, 4043–4054 (2023).
Park, C., Meade, N., Ferrell, A. & Shen. P. Distinct modes of non-canonical initiation regulate selective host versus viral mRNA translation during poxvirus-induced shutoff [Source code]. CodeOcean https://doi.org/10.24433/CO.5984508.v2 (2025).
Acknowledgements
We thank E. Bartom and C. Rosencrance (Northwestern University) for guidance on sequencing and bioinformatics; S. Rothenburg (UC Davis), M. Mendillo (Northwestern University), D. Evans (University of Alberta), P. Traktman (The Medical University of South Carolina), J. Connor (Boston University), J. Cao (The Public Health Agency of Canada) and Y. Xiang (The University of Texas Health San Antonio) for plasmids and antibodies; and C. Norbury (Penn State College of Medicine) for ECTV-infected lysates. We thank the University of Utah Beckman Center for Cryo-EM and Center for High Performance Computing for cryo-EM imaging and computational support. This work was supported by funding from the National Institutes of Health under grant numbers R35GM133772 to P.S.S., and R01AI127456 and R01AI179744 to P.S.S. and D.W. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
C.P. designed the research, performed experiments and analysed data. A.J.F. performed cryo-EM imaging, processing and data analysis. N.M. assisted with experiments. P.S.S. and D.W. secured funding and oversaw the project, experimental design and data interpretation. All authors contributed to writing and approving the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 GO Term analysis of transcriptomic changes during VacV infection.
GO Term analysis of differentially expressed genes that were upregulated (left) or downregulated (right) in total, 80S or polysome samples detailed in Fig. 1. DESeq2 uses Wald tests to evaluate the significance of differential expression and applies Benjamini-Hochberg (BH) correction to control the false discovery rate (FDR). Adjusted p-values (padj) identify significantly differentially expressed genes after multiple testing correction.
Extended Data Fig. 2 Translational downregulation of 5’TOP mRNAs during infection.
a. In uninfected cells, specific genes highly enriched in association with the 80 s ribosome were identified. A heatmap of differential gene expression between total and 80 s or total and polysome samples is annotated above with 5’TOP genes in red. Beneath: 20 nucleotide sequence motifs of 82 known or potential 5’TOP genes were generated using weblogo.berkely.edu. b. Log2FC changes in 5’TOP mRNAs contained in 80S or polysome fractions from mock infected or infected samples from Fig. 1. Red and blue bars indicate increases and decreases in Log2FC enrichment over total RNA, respectively. c. Log2FC comparison of total mock versus infected or 80S mock versus infected samples. While also downregulated at the transcript level, more robust loss from 80S samples was detected in infected samples. DESeq2 uses Wald tests to evaluate the significance of differential expression and applies Benjamini-Hochberg (BH) correction to control the false discovery rate (FDR). Adjusted p-values (padj) identify significantly differentially expressed genes after multiple testing correction. The adjusted p-value cutoff was set above 0.01 and the Log2FC threshold was set to 1.1. For a–c, data is derived from 3 biologically independent experiments.
Extended Data Fig. 3 Induction of JUN expression in orthopoxvirus-infected cells.
a. PCR analysis of RNA levels of JUN and DNAJB1, together with GAPDH and late viral RNA (A14) in HAP1 cells infected with VacV at MOI 10 for the indicated times in hours post-infection (h.p.i.). b. qPCR analysis of JUN (left) and DNAJB1 (right) expression in samples as described in A, normalized to β-actin. The body of the box plot represents the minima and maxima of the distribution and the median line. The whiskers extend from the minimum and maximum values of the data points no outliers shown n = 4 per group, one-way ANOVA with two-sided, Tukey’s multiple comparison test. Data are presented as mean values +/− SEM. n.s. = no significance, ****padjust ≤0.0001. Statistical values are shown for JUN expression at 1 h.p.i., illustrating that although not significant statistically using multiple sample comparisons, there is a statistically significant increase over uninfected samples using paired t-tests. c. PCR analysis of RNA levels for representative host genes induced during infection, together with GAPDH isolated from mock or VacV-infected (MOI 10) total lysate, 80S or polysome (poly) fractions of HAP1 cells at 24 h.p.i. qPCR analysis associated with this panel is shown in Fig. 2b of the main manuscript. d. MRC5 or 293 A cells were mock infected or infected with VacV at MOI 10 for 24 h. Whole cell lysates were analyzed by WB using the indicated antibodies. e. Differentiated THP1 cells (macrophages) were infected with VacV at MOI 20 or 40 for 1 or 2 days, as indicated. Whole cell lysates were analyzed by WB using the indicated antibodies. Data in D-E is representative of 3 independent experiments. f. Murine B6/WT19 fibroblasts or human HeLa cells were infected with Ectromelia Virus (ECTV) at MOI 10 for 24 h. Whole cell lysates were analyzed by WB using the indicated antibodies. Note that HSP70 is not detectable in mouse cells, due either to low protein abundance or species specificity of the antibody used. Data in F is representative of 2 independent experiments per cell type. Source data are provided as a Source Data file.
Extended Data Fig. 4 Comparisons of predicted gene expression changes with published proteomic resources.
The interactive ‘Plotter’ worksheet from proteomic studies by Soday et al., 2019 was used to compare changes in the levels of proteins in our predicted protein output analysis and in prior ribosome profiling studies. Blue lines show protein levels in uninfected samples. Red line show protein levels in VacV-infected samples. a. Expression levels of proteins at the top of our finalized ‘protein output’ predictions. b. Expression levels of proteins that top our list of largest increase in TE without filtering out low read counts. In line with these being too low to be accurate or reliable predictors, none of these mRNAs are associated with proteins that increase in abundance during infection. c. Expression levels of proteins that our analysis suggests are encoded by mRNAs whose TE increase the most but whose total mRNA levels decline so much that they would not be expected to increase protein production. d. Expression levels of proteins predicted to be translationally increased by Dhungel et al., 2017. Note that increases are small and transient but align with the early-to-mid stage phase of infection in their study. For information on replicates and statistical analyses, please refer to the relevant cited publication in each panel.
Extended Data Fig. 5 Effects of eIF3 depletion on JUN versus late viral proteins in primary NHDFs infected with VacV.
HAP1 cells were treated with control non-targeting siRNAs (con) or one of two independent siRNAs targeting eIF3d or eIF3a, followed by infection with VacV at MOI 10 for 24 h. Samples were analyzed by WB for the indicated proteins. Blots are representative of 3 independent experiments. Source data are provided as a Source Data file.
Extended Data Fig. 6 Transcriptomic and translational analysis of host genes during VacV infection of HAP1 cells lacking RACK1.
RACK1 knockout HAP1 cells (shown in Fig. 3g) were infected with VacV at MOI 25 for 24 h. Cell lysates were prepared and fractionated. PolyA-mRNA was isolated from total, 80S and polysome (pols) fractions and sequenced on a Novaseq S4. Differential gene expression between mock infected WT HAP1 cells and VacV-infected RACK1 knockout cells using DEseq comparisons of total, 80S and polysome samples. DESeq2 uses Wald tests to evaluate the significance of differential expression and applies Benjamini-Hochberg (BH) correction to control the false discovery rate (FDR). Adjusted p-values (padj) identify significantly differentially expressed genes after multiple testing correction. p-adjusted cutoff was set to above 0.01 and Log2FC to 1.1. Results show that host genes are increased or decreased to the same extent even in the absence of RACK1, demonstrating that RACK1 and its phosphorylation are not essential for host transcript and translational reprogramming during infection. Data is derived from 3 biologically independent experiments.
Extended Data Fig. 7 Consensus structures of eIF3-bound and free 40S particles from mock-infected and VacV-infected cells.
a. mock-infected samples. b. VacV-infected samples. Resolution of each structure is indicated.
Extended Data Fig. 8 Cryo-EM data processing workflow and classification of 40S head classes by 3DVA for mock-infected and VacV-infected samples.
a. Representative cryo-EM micrographs of purified ribosomes from mock-infected (left, out of 18,975 curated micrographs) and VacV-infected (right, out of 10,764 curated micrographs) cells. b. Reference-free 2D class averages of extracted particles. c. Data processing workflow. 40S particles were used for downstream 3DVA processing. d. CryoSPARC 3DVA workflow. The mask used in focused classification is shown in purple. Maps that displayed the greatest degree of 40S head rotation swiveling are indicated by red boxes. e. Gold standard FSC curves for eIF3-bound 40S particles, state 1 (least rotated), state 2 (most rotated) and of free 40S, state 1 (least rotated), state 2 (most rotated).
Extended Data Fig. 9 Extent of 40S head swivel on eIF3-bound 40S subunits is greater in ribosomes from infected cells (state 1; least rotated).
a–c. 40S head swivel on eIF3-bound 40S particles from mock-infected cells. a. Fitting of the eIF3-bound 40S state (PDB 6ZVJ) into cryo-EM reconstruction of eIF3-bound 40S ribosomal subunit (state 1) isolated from mock-infected cells. Insets show focused views of good fits of the 40S body (RPS7, RPS13), but the model is slightly displaced in the 40S head (RPS25, RPS18, RPS5, RPS16). b. Focused views of RPS5 and RPS25 before (eIF3-bound 40S, PDB 6ZVJ) and after (best fit) separate rigid-body fitting of the 40S head into the cryo-EM density. Right, overlay of the before-and-after fitting reveals the trajectory of motion (tan, before; green, after). c. Separate rigid-body fitting of the 40S head into cryo-EM reconstruction of ribosomes from mock-infected cells reveals a 1.4° rotation of the head (green ribbon). Tan ribbon, standard fit from PDB 6ZVJ based on 40S body components. Rotation axis depicted as gray rod. d-f. 40S head swivel on eIF3-bound 40S particles from VacV-infected cells. d. Fitting of the eIF3-bound 40S state (PDB 6ZVJ) into cryo-EM reconstruction of eIF3-bound 40S ribosomal subunit (state 1) isolated from VacV-infected cells. Insets show focused views of good fits of the 40S body (RPS8), but the model is slightly displaced in the 40S head (RPS18, RPS12, RPS27a). e. Focused views of RPS18 before (eIF3-bound 40S, PDB 6ZVJ) and after (best fit) separate rigid-body fitting of the 40S head into the cryo-EM density. Right, overlay of the before-and-after fitting reveals the trajectory of motion (tan, before; green, after). f. Separate rigid-body fitting of the 40S head into cryo-EM reconstruction of virus-modified ribosomes reveals a 3.8° rotation of the head (green ribbon). Tan ribbon, standard fit from PDB 6ZVJ based on 40S body components. Rotation axis depicted as gray rod. g. Comparison of extent of 40S head swivel between mock-infected and VacV-infected cells. Structures of the 40S head in the least rotated state (state 1) after rigid body fitting (mock infected: teal, VacV infected: pink) overlayed against a previously published structure of eIF3-bound 40S particles (PDB 6ZVJ, tan), highlighting the increase in movement from VacV infected samples. Rotation axes of movement indicated by black rods.
Extended Data Fig. 10 Effects of JUN depletion or HSP70 inhibition on VacV Replication.
a. HAP1 cells were treated with control non-targeting siRNAs or one of two independent siRNAs targeting JUN (to deplete the Jun protein) followed by infection with VacV at MOI 10 for 24 h. Representative WB’s are shown demonstrating the relative efficacy of each JUN-targeting siRNA and the lack of effects of viral protein accumulation at high MOI. b. Measurement of viral titers from HAP1 cells treated and infected as described in A. n = 3, no statistical significance was found for any sample. c. Measurement of viral titers from NHDF cells treated with the HSP70 inhibitors, PES-CI (2.5 mM) or VER155008 (20 mM) followed by infection with VacV at MOI 0.01 for 72 h. n = 9 independent samples from 3 biologically independent experiments, one-way ANOVA, two-sided Tukey’s multiple comparison test. Data are presented as mean values +/− SEM. n.s. = no significance, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. Source data are provided as a Source Data file.
Supplementary information
Supplementary Tables 1–5
1. Datasets for genes enriched in 80S polysomes. 2. Datasets for TE calculations for uninfected cells from 3 biological replicate experiments. 3. Datasets for TE calculations for infected cells from 3 biological replicate experiments. 4. Datasets for relative TE, relative total RNA abundance and predicted protein output calculations for uninfected versus infected cells using base means from 3 biological replicate experiments shown in Supplementary Tables 2 and 3. Tab 1 provides finalized datasets wherein genes with low read counts and artificially polyA-enriched histone mRNAs were removed as they produce unreliable TE calculations. Tab 2 provides raw unfiltered datasets. 5. Details of primers used for PCR and cloning in this study.
Supplementary Video 1
Maps were generated in cryoSPARC 3DVA ‘simple’ mode. The first component (component 0) is shown. Compare 40S head motion with Video 2.
Supplementary Video 2
Maps were generated in cryoSPARC 3DVA ‘simple’ mode. The first component (component 0) is shown. Compare 40S head motion with Video 1.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed PCR gels and western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed PCR gels and western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Extended Data Fig. 10
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, C., Ferrell, A.J., Meade, N. et al. Distinct non-canonical translation initiation modes arise for specific host and viral mRNAs during poxvirus-induced shutoff. Nat Microbiol 10, 1535–1549 (2025). https://doi.org/10.1038/s41564-025-02009-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41564-025-02009-4