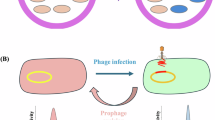
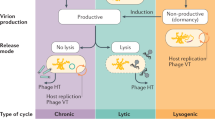
Abstract
Phages are diverse and abundant within microbial communities, where they play major roles in their evolution and adaptation. Phage replication, and multiplication, is generally thought to be restricted within a single or narrow host range. Here we use published and newly generated proximity-ligation-based metagenomic Hi-C (metaHiC) data from various environments to explore virus–host interactions. We reconstructed 4,975 microbial and 6,572 phage genomes of medium quality or higher. MetaHiC yielded a contact network between genomes and enabled assignment of approximately half of phage genomes to their hosts, revealing that a substantial proportion of these phages interact with multiple species in environments as diverse as the oceanic water column or the human gut. This observation challenges the traditional view of a narrow host spectrum of phages by unveiling that multihost associations are common across ecosystems, with implications for how they might impact ecology and evolution and phage therapy approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Sequence data as well as raw assemblies generated in this study have been deposited in the NCBI under the BioProject number PRJNA1169672 (mock community) and PRJNA1169674 (metagenomic datasets). Publicly available datasets as well as newly produced data used in the present study are all listed in Supplementary Table 2 with their associated BioProject ID. All MAG and MGEMAG data are provided as supplementary datasets and are available on Zenodo at https://doi.org/10.5281/zenodo.14851637 (ref. 63).
Code availability
Open-access versions of the programmes and pipelines used (Hicstuff, MetaTOR, HiContacts) are available online on GitHub: Hicstuff v.3.1.2 (https://github.com/koszullab/hicstuff), MetaTOR v.1.3.2 (https://github.com/koszullab/metaTOR) and HiContacts v.1.7.1 (https://github.com/js2264/HiContacts). Other mandatory programmes are also available online: Bowtie2 v.2.4.5 (http://bowtie-bio.sourceforge.net/bowtie2/), SAMtools v.1.9 (http://www.htslib.org/) and Cooler v.0.8.7–0.8.11 (https://cooler.readthedocs.io/en/latest/). The pipeline used in the present study to generate the data is available on GitHub at https://github.com/mmarbout/MetaHiC_pipeline (ref. 64).
References
Salmond, G. P. C. & Fineran, P. C. A century of the phage: past, present and future. Nat. Rev. Microbiol. 13, 777–786 (2015).
Paez-Espino, D. et al. Uncovering Earth’s virome. Nature 536, 425–430 (2016).
Nayfach, S. et al. Metagenomic compendium of 189,680 DNA viruses from the human gut microbiome. Nat. Microbiol. 6, 960–970 (2021).
Modi, S. R., Lee, H. H., Spina, C. S. & Collins, J. J. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature 499, 219–222 (2013).
Shousha, A. et al. Bacteriophages isolated from chicken meat and the horizontal transfer of antimicrobial resistance genes. Appl. Environ. Microbiol. 81, 4600–4606 (2015).
Edwards, R. A., McNair, K., Faust, K., Raes, J. & Dutilh, B. E. Computational approaches to predict bacteriophage–host relationships. FEMS Microbiol. Rev. https://doi.org/10.1093/femsre/fuv048 (2015).
Hwang, Y., Roux, S., Coclet, C., Krause, S. J. E. & Girguis, P. R. Viruses interact with hosts that span distantly related microbial domains in dense hydrothermal mats. Nat. Microbiol. 8, 946–957 (2023).
Hedžet, S., Rupnik, M. & Accetto, T. Broad host range may be a key to long-term persistence of bacteriophages infecting intestinal Bacteroidaceae species. Sci. Rep. 12, 21098 (2022).
Göller, P. C. et al. Multi-species host range of staphylococcal phages isolated from wastewater. Nat. Commun. 12, 6965 (2021).
Quince, C., Walker, A. W., Simpson, J. T., Loman, N. J. & Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 35, 833–844 (2017).
Gounot, J.-S. et al. Genome-centric analysis of short and long read metagenomes reveals uncharacterized microbiome diversity in Southeast Asians. Nat. Commun. 13, 6044 (2022).
Nayfach, S. et al. CheckV assesses the quality and completeness of metagenome-assembled viral genomes. Nat. Biotechnol. 39, 578–585 (2021).
Arisdakessian, C. G., Nigro, O. D., Steward, G. F., Poisson, G. & Belcaid, M. CoCoNet: an efficient deep learning tool for viral metagenome binning. Bioinformatics 37, 2803–2810 (2021).
Johansen, J. et al. Genome binning of viral entities from bulk metagenomics data. Nat. Commun. 13, 965 (2022).
Camargo, A. P. et al. Identification of mobile genetic elements with geNomad. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01953-y (2023).
Roux, S. et al. iPHoP: an integrated machine learning framework to maximize host prediction for metagenome-derived viruses of archaea and bacteria. PLoS Biol. 21, e3002083 (2023).
Marbouty, M. et al. Metagenomic chromosome conformation capture (meta3C) unveils the diversity of chromosome organization in microorganisms. eLife 3, e03318 (2014).
Beitel, C. W. et al. Strain- and plasmid-level deconvolution of a synthetic metagenome by sequencing proximity ligation products. PeerJ 2, e415 (2014).
Burton, J. N., Liachko, I., Dunham, M. J. & Shendure, J. Species-level deconvolution of metagenome assemblies with Hi-C-based contact probability maps. G3 4, 1339–1346 (2014).
Marbouty, M. & Koszul, R. Metagenome analysis exploiting high-throughput chromosome conformation capture (3C) data. Trends Genet. 31, 673–682 (2015).
Stalder, T., Press, M. O., Sullivan, S., Liachko, I. & Top, E. M. Linking the resistome and plasmidome to the microbiome. ISME J. https://doi.org/10.1038/s41396-019-0446-4 (2019).
Marbouty, M., Thierry, A., Millot, G. A. & Koszul, R. MetaHiC phage–bacteria infection network reveals active cycling phages of the healthy human gut. eLife 10, e60608 (2021).
Marbouty, M., Baudry, L., Cournac, A. & Koszul, R. Scaffolding bacterial genomes and probing host–virus interactions in gut microbiome by proximity ligation (chromosome capture) assay. Sci. Adv. 3, e1602105 (2017).
Chen, Y., Wang, Y., Paez-Espino, D., Polz, M. F. & Zhang, T. Prokaryotic viruses impact functional microorganisms in nutrient removal and carbon cycle in wastewater treatment plants. Nat. Commun. 12, 5398 (2021).
Chevallereau, A. et al. Next-generation ‘-omics’ approaches reveal a massive alteration of host RNA metabolism during bacteriophage infection of Pseudomonas aeruginosa. PLoS Genet. 12, e1006134 (2016).
Baudry, L., Foutel-Rodier, T., Thierry, A., Koszul, R. & Marbouty, M. MetaTOR: a computational pipeline to recover high-quality metagenomic bins from mammalian gut proximity-ligation (meta3C) libraries. Front. Genet. 10, 753 (2019).
Pan, S., Zhu, C., Zhao, X.-M. & Coelho, L. P. A deep Siamese neural network improves metagenome-assembled genomes in microbiome datasets across different environments. Nat. Commun. 13, 2326 (2022).
Du, Y., Fuhrman, J. A. & Sun, F. ViralCC retrieves complete viral genomes and virus–host pairs from metagenomic Hi-C data. Nat. Commun. 14, 502 (2023).
Marie-Nelly, H. et al. High-quality genome (re)assembly using chromosomal contact data. Nat. Commun. 5, 5695 (2014).
Bickhart, D. M. et al. Assignment of virus and antimicrobial resistance genes to microbial hosts in a complex microbial community by combined long-read assembly and proximity ligation. Genome Biol. 20, 153 (2019).
Yaffe, E. & Relman, D. A. Tracking microbial evolution in the human gut using Hi-C reveals extensive horizontal gene transfer, persistence and adaptation. Nat. Microbiol. https://doi.org/10.1038/s41564-019-0625-0 (2019).
Press, M. O. et al. Hi-C deconvolution of a human gut microbiome yields high-quality draft genomes and reveals plasmid-genome interactions. Preprint at bioRxiv https://doi.org/10.1101/198713 (2017).
Kalmar, L. et al. HAM-ART: an optimised culture-free Hi-C metagenomics pipeline for tracking antimicrobial resistance genes in complex microbial communities. PLoS Genet. 18, e1009776 (2022).
Varona, N. S. et al. Host-specific viral predation network on coral reefs. ISME J. 18, wrae240 (2024).
DeMaere, M. Z. et al. Metagenomic Hi-C of a healthy human fecal microbiome transplant donor. Microbiol. Resour. Announc. 9, e01523-19 (2020).
Rojas, C. A., Gardy, J., Eisen, J. A. & Ganz, H. H. Recovery of 52 bacterial genomes from the fecal microbiome of the domestic cat (Felis catus) using Hi-C proximity ligation and shotgun metagenomics. Microbiol. Resour. Announc. 12, e0060123 (2023).
Kent, A. G., Vill, A. C., Shi, Q., Satlin, M. J. & Brito, I. L. Widespread transfer of mobile antibiotic resistance genes within individual gut microbiomes revealed through bacterial Hi-C. Nat. Commun. 11, 4379 (2020).
Ivanova, V. et al. Hi-C metagenomics in the ICU: exploring clinically relevant features of gut microbiome in chronically critically ill patients. Front. Microbiol. 12, 770323 (2022).
Bickhart, D. M. et al. Generating lineage-resolved, complete metagenome-assembled genomes from complex microbial communities. Nat. Biotechnol. 40, 711–719 (2022).
Piligrimova, E. G. et al. Putative plasmid prophages of Bacillus cereus sensu lato may hold the key to undiscovered phage diversity. Sci. Rep. 11, 7611 (2021).
Pfeifer, E., Moura de Sousa, J. A., Touchon, M. & Rocha, E. P. C. Bacteria have numerous distinctive groups of phage-plasmids with conserved phage and variable plasmid gene repertoires. Nucleic Acids Res. 49, 2655–2673 (2021).
Bouras, G. et al. Pharokka: a fast scalable bacteriophage annotation tool. Bioinformatics 39, btac776 (2022).
Al-Shayeb, B. et al. Clades of huge phages from across Earth’s ecosystems. Nature 578, 425–431 (2020).
Du, Y. & Sun, F. HiCBin: binning metagenomic contigs and recovering metagenome-assembled genomes using Hi-C contact maps. Genome Biol. 23, 63 (2022).
Nishimura, Y. et al. ViPTree: the viral proteomic tree server. Bioinformatics 33, 2379–2380 (2017).
Shkoporov, A. N. et al. Long-term persistence of crAss-like phage crAss001 is associated with phase variation in Bacteroides intestinalis. BMC Biol. 19, 163 (2021).
Guerin, E. et al. Biology and taxonomy of crAss-like bacteriophages, the most abundant virus in the human gut. Cell Host Microbe 24, 653–664.e6 (2018).
Schmidtke, D. T. et al. The prototypic crAssphage is a linear phage-plasmid. Cell Host Microbe 33, 1347–1362.e5 (2025).
Beaulaurier, J. et al. Metagenomic binning and association of plasmids with bacterial host genomes using DNA methylation. Nat. Biotechnol. 36, 61–69 (2018).
Ravi, A., Valdés-Varela, L., Gueimonde, M. & Rudi, K. Transmission and persistence of IncF conjugative plasmids in the gut microbiota of full-term infants. FEMS Microbiol. Ecol. 94, fix158 (2018).
Brödel, A. K. et al. In situ targeted base editing of bacteria in the mouse gut. Nature 632, 877–884 (2024).
Lamy-Besnier, Q. et al. Chromosome folding and prophage activation reveal specific genomic architecture for intestinal bacteria. Microbiome 11, 111 (2023).
Li, D., Liu, C.-M., Luo, R., Sadakane, K. & Lam, T.-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Gurevich, A., Saveliev, V., Vyahhi, N. & Tesler, G. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075 (2013).
Matthey-Doret, C. et al. koszullab/hicstuff: use miniconda layer for docker and improved P(s) normalisation. Zenodo https://doi.org/10.5281/zenodo.4066363 (2020).
Cournac, A., Marie-Nelly, H., Marbouty, M., Koszul, R. & Mozziconacci, J. Normalization of a chromosomal contact map. BMC Genomics 13, 436 (2012).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Bowers, R. M. et al. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat. Biotechnol. 35, 725–731 (2017).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Abdennur, N. & Mirny, L. A. Cooler: scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics 36, 311–316 (2019).
Serizay, J., Matthey-Doret, C., Bignaud, A., Baudry, L. & Koszul, R. Orchestrating chromosome conformation capture analysis with Bioconductor. Nat. Commun. 15, 1072 (2024).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44, W242–W245 (2016).
Marbouty, M. Phages with a broad host range are common across ecosystems. Zenodo https://doi.org/10.5281/zenodo.14851637 (2025).
Bignaud, A., Serizay, J., Baudry, L., Matthey-Doret, C. & Marbouty, M. Metagenomic Tridimensional Organisation-based Reassembly. GitHub https://github.com/mmarbout/MetaHiC_pipeline (2024).
Acknowledgements
We thank P. Moreau for assistance during experimental work, the different teams of the Microbiology department from Institut Pasteur, and especially J. Czarnecki for providing bacterial pellets; D. d’Alelio from the Stazione Zoologica Anton Dohrm for help with the oceanic sample; and the hacienda Vergel de Gaudalupe for allowing us to sample their fermenter. This research was funded, in whole or in part, by Agence nationale pour la recherche ANR-20-CE92-0048 to M.M. and L.D. and ANR-16-JPEC-0003–05 to R.K. and by a grant from the French government, managed by the Agence Nationale de la Recherche under the France 2030 programme (ANR-23-CHBS-0002) to R.K. The Biomics Platform, C2RT, Institut Pasteur, Paris, France, is supported by France Génomique (ANR-10-INBS-09) and IBISA. A.B. was supported by an ENS fellowship from the French Ministry of Higher Education, Research and Innovation. D.E.C. is supported by a PhD grant from the PhastGut project. A.B. and D.E.C. belong to Ecole Doctorale Complexité du vivant ED515 of Sorbonne Université. L.M., M.G.-O. and M.C.-G. were supported by funding from Programa de Apoyo a Proyectos de Investigacion e Innovacion Technologica (DGAPA-UNAM – IN212524). Sequencing and library preparation was supported by Agencia Nacional de Investigación e Innovación (ANII-Uruguay) grant number FSGSK_1_2019_1_159735 and Fondo para la Convergencia Estructural del MERCOSUR (FOCEM). Illustrations used in the present publication (Figs. 1 and 3 and Extended Data Fig. 2) were obtained from the Internet under a Creative Commons CC0 license (https://svgsilh.com/fr/). A CC-BY public copyright license has been applied by the authors to the present document and on all subsequent versions up to the author-accepted manuscript version arising from this submission, in accordance with the grants’ open-access conditions.
Author information
Authors and Affiliations
Contributions
A.B., R.K. and M.M. conceptualized the study. A.B., R.K. and M.M. designed the methodology. A.B. and M.M. designed software. M.M. performed validation. M.M. conducted investigations, with contributions from J.S., G.L.T., O.C., N.R., D.E.C., A.T., M.G.-O., M.C.-G., J.P. and K.L. A.B. and M.M. conducted formal analysis, with contributions from A.P., G.A.M. and J.S. M.M. and A.B. curated data. D.E.C., G.I., N.R., M.M., K.L., L.M., A.T., A.B., J.P., P.H., D.E.C., G.L.T., M.G.-O. and M.C.-G. procured resources. M.M. and R.K. performed visualization. M.M., R.K. and A.B. wrote the original draft. All authors edited the paper. M.M., R.K., S.H., L.M., G.I., L.D., G.L.T. and O.C. supervised the project. M.M., G.I., L.D. and R.K. acquired funding. M.M. and R.K. administered the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Louis-Patrick Haraoui and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 contact map of the mock community.
Normalized contact map (bin = 16 kb) of the mock community. Black lines delineate the different DNA molecules. Genomic coordinates are indicated on the sides of the contact map and the scale bar is indicated under. Some bacteria exhibit an abundance below our detection threshold (~0.1 %) and therefore appear as white squares in the contact map.
Extended Data Fig. 2 datasets and pipeline used in the present study.
Datasets used in the present study. The number of each type of sample is indicated next to each drawing. The different softwares used to generate and analyze the data are indicated in diamond. (SG : Shotgun). Figure adapted from SVG Silh under a CC 1.0 license.
Extended Data Fig. 3 Phylogenetic tree of the MAGs.
Phylogenetic tree of medium and high-quality MAGs obtained across all datasets using MetaTOR. The tree is decorated with different annotations. From inner to outer: sample data (1- sample type: environment or gut; 2- ecosystem: wastewater, hydrothermal mat, fermenter, ocean, mammal, bird; 3- geographic location: Europe, North America, South America, Asia), MAG taxonomy (1- Phylum, 2- Class, 3- Order, 4- Family, 5- Genus), MAG quality (completion - green, contamination - red), MAG size in bp. The different legends for the annotations are indicated above the tree.
Extended Data Fig. 4 Contigs contact map of different large vMAGs.
Raw contact map of six vMAGs. Black lines delineate the boundaries of the different contigs. Sample (environment), vMAG ID, taxonomic annotation, quality and size are indicated above contact maps while scale bars are present on the left.
Extended Data Fig. 5 Contact maps of MGEMAGs and their different hosts.
Intra- and inter- contact maps obtained of vMAG exhibiting multiple hosts (50kb bins). Sample (environmental) origin and vMAG references are indicated above and below each contact map. Dark lines indicate boundaries of the different genomic entities. Black triangles point at vMAGS. Scale bars (raw scores) are indicated aside each contact map. a. vMAGs with clear contact with multiple microbial MAGs that do not display noise signals between them. b. vMAGs with clear contact with multiple microbial MAGs exhibiting noise signals between them. c. vMAGs with low contact signal with multiple microbial MAGs.
Extended Data Fig. 6 Multihost vMAGs features.
a. Violin plot of the log(size) of vMAGs as a function of their host number. b. Violin plot of the log(RPKM) of vMAGs as a function of their host number (RPKM: Reads Per Kilobase Million). c. Bar plot of vMAGs proportion as a function of their host assignment for the different processed environments encompassing a sufficient number of vMAGs (gut, ocean, hydrothermal mat and wastewater). The different categories are indicated by colors (white = no host assigned, orange = multiple host assigned, red = one contaminated [conta] host assigned, grey = one LQ host assigned, blue = one MQ characterized host assigned, darkblue = one HQ host assigned).
Extended Data Fig. 7 Proteomic tree of the Crassphages family and related phages infecting Bacteroidetes.
Proteomic tree of the different Crassvirales and related phages characterized in the present study. The Tree also encompasses different representative reference Crass genomes from Guerin et al.57, indicated by a black star. The different crass families are indicated by colored areas over the branches. The tree is decorated with different informations (from the inside to the outside): i) sample type, ii) reference genomes (black stars), iii) geNomad annotation (blue = virus; red = plasmid), iv) vMAG taxonomy (order, genus), v) host taxonomy (phylum, class, order, family, genus) white if no host or multiple host attributed), vi) vMAG exhibiting multiple hosts (red circles), vii) vMAG genome size (scale bar = 100 kb).
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Supplementary Table 1
Reference genomes of the different species present in the mock community. NA, not applicable.
Supplementary Table 2
Metagenomic datasets used in the present study. Grey colours delimit the different studies.
Supplementary Table 3
Metadata associated with the newly generated samples. ND, not determined.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bignaud, A., Conti, D.E., Thierry, A. et al. Phages with a broad host range are common across ecosystems. Nat Microbiol 10, 2537–2549 (2025). https://doi.org/10.1038/s41564-025-02108-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-025-02108-2
This article is cited by
-
Benchmarking alignment strategies for Hi-C reads in metagenomic Hi-C data
Genome Biology (2026)
-
Revisiting giant virus-host dynamics in brown algae: old stories and new perspectives
The EMBO Journal (2026)
-
Long-read metagenomics reveals phage dynamics in the human gut microbiome
Nature (2026)
-
Licence to knockdown — the phage gene silencer
Nature Reviews Microbiology (2025)