Abstract
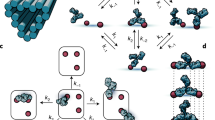
Although repetitive patterns of antigens are crucial for certain immune responses, an understanding of how antibodies bind and dynamically interact with various spatial arrangements of molecules is lacking. Hence, we introduced a new method in which molecularly precise nanoscale patterns of antigens are displayed using DNA origami and immobilized in a surface plasmon resonance set-up. Using antibodies with identical antigen-binding domains, we found that all the subclasses and isotypes studied bind bivalently to two antigens separated at distances that range from 3 to 17 nm. The binding affinities of these antibodies change with the antigen distances, with a distinct preference for antigens separated by approximately 16 nm, and considerable differences in spatial tolerance exist between IgM and IgG and between low- and high-affinity antibodies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Code availability
All the code used for the computational results is available upon request.
Data availability
The raw data that support the plots within this paper and other findings of this study are provided in Supplementary Information and are available from the authors upon reasonable request.
Change history
19 February 2019
In the Supplementary Information file originally published with this Article, the Supplementary references 48–62 were missing; the amended file has now been uploaded.
References
Bachmann, M. F. & Jennings, G. T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796 (2010).
Martinez-Murillo, P. et al. Particulate array of well-ordered HIV clade C Env trimers elicits neutralizing antibodies that display a unique V2 cap approach. Immunity 46, 804–817.e7 (2017).
Kanekiyo, M. et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499, 102–106 (2013).
Preiner, J. et al. IgGs are made for walking on bacterial and viral surfaces. Nat. Commun. 5, 4394 (2014).
Maity, P. C. et al. B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Sci. Signal. 8, ra93 (2015).
Diebolder, C. A. et al. Complement is activated by IgG hexamers assembled at the cell surface. Science 343, 1260–1263 (2014).
Sondermann, P., Huber, R., Oosthuizen, V. & Jacob, U. The 3.2-Å crystal structure of the human IgG1 Fc fragment–FγRIII complex. Nature 406, 267–273 (2000).
Baker, K. et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8–CD11b+ dendritic cells. Proc. Natl Acad. Sci. USA 108, 9927–9932 (2011).
Qiao, S.-W. et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc. Natl Acad. Sci. USA 105, 9337–9342 (2008).
Saphire, E. O. Crystal structure of a neutralizing human IgG against HIV-1: a template for vaccine design. Science 293, 1155–1159 (2001).
Bruhns, P. et al. Specificity and affinity of human Fc receptors and their polymorphic variants for human IgG subclasses. Blood 113, 3716–3725 (2009).
Tian, X. et al. In-depth analysis of subclass-specific conformational preferences of IgG antibodies. IUCrJ 2, 9–18 (2015).
Smith, T. J., Olson, N. H., Cheng, R. H., Chase, E. S. & Baker, T. S. Structure of a human rhinovirus-bivalently bound antibody complex: implications for viral neutralization and antibody flexibility. Proc. Natl Acad. Sci. USA 90, 7015–7018 (1993).
Zhang, X. et al. 3D structural fluctuation of IgG1 antibody revealed by individual particle electron tomography. Sci. Rep. 5, 9803 (2015).
Luedtke, R., Owen, C. S. & Karush, F. Proximity of antibody binding sites studied by fluorescence energy transfer. Biochemistry 19, 1182–1192 (1980).
Seeman, N. C. & Sleiman, H. F. DNA nanotechnology. Nat. Rev. Mater. 3, 17068 (2017).
Rothemund, P. W. K. Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302 (2006).
Douglas, S. M. et al. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459, 414–418 (2009).
Benson, E. et al. DNA rendering of polyhedral meshes at the nanoscale. Nature 523, 441–444 (2015).
Steinhauer, C., Jungmann, R., Sobey, T. L., Simmel, F. C. & Tinnefeld, P. DNA origami as a nanoscopic ruler for superresolution microscopy. Angew. Chem. Int. Ed. 48, 8870–8873 (2009).
Nickels, P. C. et al. Molecular force spectroscopy with a DNA origami-based nanoscopic force clamp. Science 354, 305–307 (2016).
Fu, J. et al. Multi-enzyme complexes on DNA scaffolds capable of substrate channelling with an artificial swinging arm. Nat. Nanotech. 9, 531–536 (2014).
Wollman, A. J. M., Sanchez-Cano, C., Carstairs, H. M. J., Cross, R. A. & Turberfield, A. J. Transport and self-organization across different length scales powered by motor proteins and programmed by DNA. Nat. Nanotech. 9, 44–47 (2013).
Funke, J. J. & Dietz, H. Placing molecules with Bohr radius resolution using DNA origami. Nat. Nanotech. 11, 47–52 (2015).
Rinker, S., Ke, Y., Liu, Y., Chhabra, R. & Yan, H. Self-assembled DNA nanostructures for distance-dependent multivalent ligand–protein binding. Nat. Nanotech. 3, 418–422 (2008).
McKinney, S. A., Joo, C. & Ha, T. Analysis of single-molecule FRET trajectories using hidden Markov modeling. Biophys. J. 91, 1941–1951 (2006).
Müller, K. M., Arndt, K. M. & Plückthun, A. Model and simulation of multivalent binding to fixed ligands. Anal. Biochem. 261, 149–158 (1998).
Natkanski, E. et al. B cells use mechanical energy to discriminate antigen affinities. Science 340, 1587–1590 (2013).
Mack, E. T., Snyder, P. W., Perez-Castillejos, R. & Whitesides, G. M. Using covalent dimers of human carbonic anhydrase II to model bivalency in immunoglobulins. J. Am. Chem. Soc. 133, 11701–11715 (2011).
Sela-Culang, I., Kunik, V. & Ofran, Y. The structural basis of antibody–antigen recognition. Front. Immunol. 4, 1–13 (2013).
Übelhart, R. et al. Responsiveness of B cells is regulated by the hinge region of IgD. Nat. Immunol. 16, 534–543 (2015).
Cole, D., Young, G., Weigel, A., Sebesta, A. & Kukura, P. Label-free single-molecule imaging with numerical-aperture-shaped interferometric scattering microscopy. ACS Photon. 4, 211–216 (2017).
Douglas, S. M. et al. Rapid prototyping of 3D DNA-origami shapes with caDNAno. Nucleic Acids Res. 37, 5001–5006 (2009).
Shaw, A. et al. Spatial control of membrane receptor function using ligand nanocalipers. Nat. Methods 11, 841–846 (2014).
Zhao, Y.-X. et al. DNA origami delivery system for cancer therapy with tunable release properties. ACS Nano 6, 8684–8691 (2012).
Reuss, M. et al. Measuring true localization accuracy in super resolution microscopy with DNA-origami nanostructures. New J. Phys. 19, 025013 (2017).
Gillespie, D. T. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 81, 2340–2361 (1977).
Kurtz, T. G. The relationship between stochastic and deterministic models for chemical reactions. J. Chem. Phys. 57, 2976–2978 (1972).
Moore, E. F. in Proceedings of the International Symposium on the Theory of Switching 285–292 (Harvard Univ. Press, 1959).
Lee, C. Y. An algorithm for path connections and its applications. IRE Trans. Electron. Comput. EC 10, 346–365 (1961).
Fox, B. L. & Glynn, P. W. Computing Poisson probabilities. Commun. ACM 31, 440–445 (1988).
Gross, D. & Miller, D. R. The randomization technique as a modeling tool and solution procedure for transient Markov processes. Oper. Res. 32, 343–361 (1984).
Michaelsen, T. E., Garred, P. & Aase, A. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur. J. Immunol. 21, 11–16 (1991).
Norderhaug, L., Olafsen, T., Michaelsen, T. E. & Sandlie, I. Versatile vectors for transient and stable expression of recombinant antibody molecules in mammalian cells. J. Immunol. Methods 204, 77–87 (1997).
Berntzen, G. et al. Prolonged and increased expression of soluble Fc receptors, IgG and a TCR-Ig fusion protein by transiently transfected adherent 293E cells. J. Immunol. Methods 298, 93–104 (2005).
Michaelsen, T. E. et al. One disulfide bond in front of the second heavy chain constant region is necessary and sufficient for effector functions of human IgG3 without a genetic hinge. Proc. Natl Acad. Sci. USA 91, 9243–9247 (1994).
Brekke, O. H., Michaelsen, T. E., Sandin, R. & Sandlie, I. Activation of complement by an IgG molecule without a genetic hinge. Nature 363, 628–630 (1993).
Acknowledgements
This work was funded through grants from the Swedish Research Council (grant no. 2013–5883 to B.H.), the Swedish Foundation for Strategic Research (grant FFL12–0219 to B.H.), the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 724872 to B.H.) and the Knut and Alice Wallenberg foundation (Academy Fellow grant KAW2014.0241 to B.H.). The SPR instrument was funded by the SFO initiative StatRegen at Karolinska Institutet. J.T.A. was in part supported by the Research Council of Norway through its Centres of Excellence funding scheme (project no. 179573) and the Research Council of Norway (grant no. 230526/F20 and no. 274993/O70). We thank S. Foss for help with schematic antibody figures. We thank Y. Bryceson and S. Chiang for work on the experimental testing and discussions, and G. Karlsson Hedestam and A. Teixeira for discussions.
Author information
Authors and Affiliations
Contributions
A.S., J.T.A. and B.H. conceived the study and wrote the paper. J.T.A., I.Sa., A.G. and T.E.M. helped conceive the human antibody panel study. A.S. performed most of the experimental work on origami and SPR. I.T.H. performed most of the theoretical and modelling work. I.Sm. performed a significant proportion of the experimental work on origami and SPR. J.R. performed origami experiments and design. J.T.A., D.B., T.E.M. and A.G. performed the production and purification of the human antibody variants. All the authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes 1–4, Supplementary Figures 1–31, Supplementary Tables 1–5
Rights and permissions
About this article
Cite this article
Shaw, A., Hoffecker, I.T., Smyrlaki, I. et al. Binding to nanopatterned antigens is dominated by the spatial tolerance of antibodies. Nature Nanotech 14, 184–190 (2019). https://doi.org/10.1038/s41565-018-0336-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41565-018-0336-3
This article is cited by
-
The molecular reach of antibodies crucially underpins their viral neutralisation capacity
Nature Communications (2025)
-
Spatial and stoichiometric in situ analysis of biomolecular oligomerization at single-protein resolution
Nature Communications (2025)
-
DNA origami signal amplification in lateral flow immunoassays
Nature Communications (2025)
-
Towards a unifying model for B-cell receptor triggering
Nature Reviews Immunology (2025)
-
Resolving the structural basis of therapeutic antibody function in cancer immunotherapy with RESI
Nature Communications (2025)