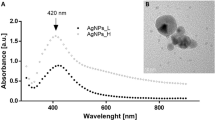
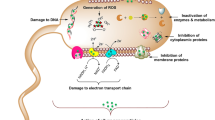
Abstract
Unlike conventional antimicrobials, the study of bacterial resistance to silver nanoparticles (AgNPs) remains in its infancy and the mechanism(s) through which it evolves are limited and inconclusive. The central question remains whether bacterial resistance is driven by the AgNPs, released Ag(I) ions or a combination of these and other factors. Here, we show a specific resistance in an Escherichia coli K-12 MG1655 strain to subinhibitory concentrations of AgNPs, and not Ag(I) ions, as indicated by a statistically significant greater-than-twofold increase in the minimum inhibitory concentration occurring after eight repeated passages that was maintained after the AgNPs were removed and reintroduced. Whole-population genome sequencing identified a cusS mutation associated with the heritable resistance that possibly increased silver ion efflux. Finally, we rule out the effect of particle aggregation on resistance and suggest that the mechanism of resistance may be enhanced or mediated by flagellum-based motility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Figures 1–3, Tables 1–3 and all compiled supplementary figures and tables were composed from raw data that are available from the corresponding author. Whole-population genome sequencing data is available through NCBI SRA BioProject ID PRJNA729974.
References
Mijnendonckx, K., Leys, N., Mahillon, J., Silver, S. & Van Houdt, R. Antimicrobial silver: uses, toxicity and potential for resistance. BioMetals 26, 609–621 (2013).
Molling, J. W., Seezink, J. W., Teunissen, B. E., Muijrers-Chen, I. & Borm, P. J. Comparative performance of a panel of commercially available antimicrobial nanocoatings in Europe. Nanotechnol. Sci. Appl. 7, 97–104 (2014).
Silver nanoparticles market by application (electronics & electrical, healthcare, food & beverages, textiles) and segment forecasts to 2022. GrandView Research (2015); https://www.grandviewresearch.com/industry-analysis/silver-nanoparticles-market
Panacek, A. et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 110, 16248–16253 (2006).
Franci, G. et al. Silver nanoparticles as potential antibacterial agents. Molecules 20, 8856–8874 (2015).
Duran, N. et al. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine 12, 789–799 (2016).
Lara, H. H., Ayala-Nunez, N. V., Turrent, L. & Padilla, C. R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 26, 615–621 (2010).
Graves, J. L. et al. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 6, 42 (2015).
Gunawan, C., Teoh, W. Y., Marquis, C. P. & Amal, R. Induced adaptation of Bacillus sp. to antimicrobial nanosilver. Small 9, 3554–3560 (2013).
Panacek, A. et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nano. 13, 65–71 (2018).
Losasso, C. et al. Antibacterial activity of silver nanoparticles: sensitivity of different Salmonella serovars. Front. Microbiol. 5, 227 (2014).
Valentin, E. et al. Heritable nanosilver resistance in priority pathogen: a unique genetic adaptation and comparison with ionic silver and antibiotics. Nanoscale 12, 2384–2392 (2020).
Gunawan, C. et al. Widespread and indiscriminate nanosilver use: genuine potential for microbial resistance. ACS Nano 11, 3438–3445 (2017).
Haefeli, C., Franklin, C. & Hardy, K. Plasmid-determined silver resistance in Pseudomonas stutzeri isolated from a silver mine. J. Bacteriol. 158, 389–392 (1984).
Li, X. Z., Nikaido, H. & Williams, K. E. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J. Bacteriol. 179, 6127–6132 (1997).
Gupta, A., Matsui, K., Lo, J. F. & Silver, S. Molecular basis for resistance to silver cations in Salmonella. Nat. Med. 5, 183–188 (1999).
Silver, S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 27, 341–353 (2003).
Pelgrift, R. Y. & Friedman, A. J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 65, 1803–1815 (2013).
Wang, L., Hu, C. & Shao, L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J. Nanomed. 12, 1227–1249 (2017).
Johnston, K. A. et al. Impacts of broth chemistry on silver ion release, surface chemistry composition, and bacterial cytotoxicity of silver nanoparticles. ES: Nano 5, 304–312 (2018).
Johnston, K. A., Smith, A. M., Marbella, L. E. & Millstone, J. E. Impact of as-synthesized ligands and low-oxygen conditions on silver nanoparticle surface functionalized. Langmuir 32, 3820–3826 (2016).
Bastus, N. G., Merkoci, F., Piella, J. & Puntes, V. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: kinetic control and catalytic properties. Chem. Mater. 26, 2836–2846 (2014).
Ivanova, E. P., Bazaka, K. & Crawford, R. J. in New Functional Biomaterials for Medicine and Healthcare Ch. 3, 71–99 (Woodhead, 2014).
Olofsson, S. K. & Cars, O. Optimizing drug exposure to minimize selection of antibiotic resistance. Clin. Infect. Dis. 45, S129–S136 (2007).
Gullberg, E. et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 7, e1002158 (2011).
Adam, M., Murali, B., Glenn, N. O. & Potter, S. S. Epigenetic inheritance based evolution of antibiotic resistance in bacteria. BMC Evol. Biol. 8, 52 (2008).
George, A. M. & Levy, S. B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J. Bacteriol. 155, 531–540 (1983).
Zhao, Y. et al. Small molecule-capped gold nanoparticles as potent antibacterial agents that target Gram-negative bacteria. J. Am. Chem. Soc. 132, 12349–12356 (2010).
Ji, F. et al. Tetrabromobisphenol A (TBBPA) exhibits specific antimicrobial activity against Gram-positive bacteria without detectable resistance. Chem. Commun. 53, 3512–3515 (2017).
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement Vol. 30(1) (Approved Standard M100-S20, Clinical and Laboratory Standards Institute, 2010).
Silver, S. in Molecular Biology, Pathogenicity, and Ecology of Bacterial Plasmids (eds Levy, S. B. et al.) 179–189 (Plenum, 1981).
Flynn, K. M., Cooper, T. F., Moore, F. B. G. & Cooper, V. S. The environment affects epistatic interactions to alter the topology of an empirical fitness landscape. PLoS Genet. 9, e1003426 (2013).
Hall, A. E. et al. Environment changes epistasis to alter trade-offs along alternative evolutionary paths. Evolution 73, 2094–2105 (2019).
Stabryla, L. M., Johnston, K. A., Millstone, J. E. & Gilbertson, L. M. Emerging investigator series: it’s not all about the ion: support for particle-specific contributions to silver nanoparticle antimicrobial activity. ES: Nano 5, 2047–2068 (2018).
Sandoval-Motta, S. & Aldana, M. Adaptive resistance to antibiotics in bacteria: a systems biology perspective. WIREs Syst. Biol. Med 8, 253–267 (2016).
Normark, B. H. & Normark, S. Evolution and spread of antibiotic resistance. J. Intern. Med. 252, 91–106 (2002).
Lok, C.-N. et al. Proteomic identification of the cus system as a major determinant of constitutive Escherichia coli silver resistance of chromosomal origin. J. Proteome Res. 7, 2351–2356 (2008).
Knetsch, M. L. & Koole, L. H. New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymers 3, 340–366 (2011).
Chopra, I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J. Antimicrob. Chemother. 59, 587–590 (2007).
Bridges, K., Kidson, A., Lowbury, E. J. L. & Wilkins, M. D. Gentamicin- and silver-resistant Pseudomonas in a burns unit. Brit. Med. J. 1, 446–449 (1979).
Gudipaty, S. A. & McEvoy, M. M. The histidine kinase CusS senses silver ions through direct binding by its sensor domain. Chem. Biochem. 1844, 1656–1661 (2014).
Affandi, T. & McEvoy, M. M. Mechanism of metal ion-induced activation of a two-component sensor kinase. Biochem. J. 476, 115–135 (2019).
Cooper, V. S. Experimental evolution as a high-throughput screen for genetic adaptations. mSphere 3, e00121–18 (2018).
Tajkarimi, M. et al. Selection for ionic- confers silver nanoparticle resistance in Escherichia coli. JSM Nanotechnol. Nanomed. 5, 1047 (2017).
Randall, C. P., Gupta, A., Jackson, N., Busse, D. & O’Neill, A. J. Silver resistance in Gram-negative bacteria: a dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 70, 1037–1046 (2015).
Koskella, B., Taylor, T. B., Bates, J. & Buckling, A. Using experimental evolution to explore natural patterns between bacterial motility and resistance to bacteriophages. ISME J. 5, 1809–1817 (2011).
Samad, T. et al. Swimming bacteria promote dispersal of non-motile Staphylococcal species. ISME J. 11, 1933–1937 (2017).
Gauger, E. J. et al. Role of motility and the flhDC operon in Escherichia coli MG1655 colonization of the mouse intestine. Infect. Immun. 75, 3315–3324 (2007).
Barker, C. S., Prub, B. M. & Matsumura, P. Increased motility of Escherichia coli by insertion sequence element integration into the regulatory region of the flhD operon. J. Bacteriol. 186, 7529–7537 (2004).
Sanchez-Torres, V., Hu, H. & Wood, T. K. GGDEF proteins YeaI, YedQ, and YfiN reduce early biofilm formation and swimming motility in Escherichia coli. Appl. Microbiol. Biotechnol. 90, 651–658 (2011).
Butler, M. T., Wang, Q. & Harshey, R. M. Cell density and mobility protect swarming bacteria against antibiotics. Proc. Natl Acad. Sci. USA 107, 3776–3781 (2010).
Lai, S., Tremblay, J. & Deziel, E. Swarming motility: a multicellular behaviour conferring antimicrobial resistance. Environ. Microbiol. 11, 126–136 (2009).
Sun, E. et al. Broad-spectrum adaptive antibiotic resistance associated with Pseudomonas aeruginosa mucin-dependent surfing motility. Antimicrob. Agents Chemother. 62, e00848–18 (2018).
Zhang, H. et al. Stress resistance, motility and biofilm formation mediated by a 25kb plasmid pLMSZ08 in Listeria monocytogenes. Food Control 94, 345–352 (2018).
Asadishad, B., Hidalgo, G. & Tufenkji, N. Pomegranate materials inhibit flagellin gene expression and flagellar-propelled motility of uropathogenic Escherichia coli strain CFT073. FEMS Microbiol. Lett. 334, 87–94 (2012).
Paramelle, D. et al. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 139, 4855–4861 (2014).
Zhang, W., Crittenden, J., Li, K. & Chen, Y. Attachment efficiency of nanoparticle aggregation in aqueous dispersions: modeling and experimental validation. Environ. Sci. Technol. 46, 7054–7062 (2012).
Li, X., Lenhart, J. J. & Walker, H. W. Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir 26, 16690–16698 (2010).
Ma, R. et al. Size-controlled dissolution of organic-coated silver nanoparticles. Environ. Sci. Technol. 46, 752–759 (2012).
Biggest Threats and Data (Centers for Disease Control and Prevention, 2020); https://www.cdc.gov/drugresistance/biggest-threats.html
Demirdjian, S. et al. Phosphatidylinositol-(3,4,5)-trisphosphate induces phagocytosis of nonmotile Pseudomonas aeruginosa. Infect. Immun. 86, 215–218 (2018).
Deatherage, D. E. & Barrick, J. E. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 1151, 165–188 (2014).
Narayanaswamy, V. P. et al. In vitro activity of novel glycopolymer against clinical isolates of multidrug-resistant Staphylococcus aureus. PLoS One 13, e0191522 (2018).
Li, X. et al. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 8, 10682–10686 (2014).
Landman, D., Salamera, J. & Quale, J. Irreproducible and uninterpretable polymyxin B MICs for Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 51, 4106–4111 (2013).
El-Halfawy, O. M. & Valvano, M. A. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin. Microbiol. Rev. 28, 191–207 (2015).
Baalousha, M. et al. The concentration-dependent behavior of nanoparticles. Environ. Chem. 13, 1–3 (2015).
Lecture 11: Antimicrobial Susceptibility Testing—Broth Dilution. Online video clip. Technical Univ. Denmark (n.d); https://www.coursera.org/lecture/antimicrobial-resistance/lecture-11-antimicrobial-susceptibility-testing-broth-dilution-VeNw0
Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically 9th edn, Vol. 32(2) (Approved Standard M7-A9, Clinical and Laboratory Standards Institute, 2012).
Riley, M. et al. Escherichia coli K-12: a cooperatively developed annotation snapshot—2005. Nucleic Acids Res. 34, 1–9 (2006).
Acknowledgements
L.M.S. and L.M.G. acknowledge the generous support from the Department of Defense (DoD) through the National Defense Science and Engineering Graduate Fellowship (NDSEG) Program, the 3M Non-Tenured Faculty Award and the Department of Civil and Environmental Engineering at the University of Pittsburgh. Additionally, K.A.J., N.A.D. and J.E.M. thank the Research Corporation for Science Advancement and the Department of Chemistry at the University of Pittsburgh for their support in carrying out this research. We thank M. J. Fritz for her assistance with the DNA extractions prior to whole-population genome sequencing.
Author information
Authors and Affiliations
Contributions
L.M.S. and L.M.G. conceived the project and designed the evolutionary experiments dealing with bacteria resistance to AgNPs and aggregation. L.M.S. carried out the microbiological and evolutionary experiments, the synthesis and long-term stability study of AgNPs, the aggregation experiments, the microbial growth kinetics analyses and statistical analyses. K.A.J. and N.A.D. were responsible for the characterizations of the AgNPs (that is, ICP-MS for Ag atom concentration and NMR for ligand density and TEM for particle size, respectively). V.S.C. conducted the genomic variant analysis. S.-J.H. contributed guidance on the microbiological and evolutionary experiments, the analysis of microbial growth kinetics and the statistical analyses. J.E.M. aided in discussion and data interpretation of the AgNP characterization and stability experiments as well as making edits to the manuscript. L.M.S. and L.M.G. wrote the paper with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Joseph Graves, Cindy Gunawan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, Discussions 1–3 and Tables 1–5.
Rights and permissions
About this article
Cite this article
Stabryla, L.M., Johnston, K.A., Diemler, N.A. et al. Role of bacterial motility in differential resistance mechanisms of silver nanoparticles and silver ions. Nat. Nanotechnol. 16, 996–1003 (2021). https://doi.org/10.1038/s41565-021-00929-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-021-00929-w
This article is cited by
-
Comparative antibacterial and anti-virulence effects of silver ions from electrolysis, silver nanoparticles, and silver nitrate against Pseudomonas aeruginosa and Staphylococcus aureus
Scientific Reports (2026)
-
Comparative Evaluation of Boron, Aluminum, and Silicon Nanoparticles for Antibiofilm and Antimicrobial Applications: Insights into Alternative Strategies
Journal of Cluster Science (2026)
-
Biological activities of optimized biosynthesized selenium nanoparticles using Proteus mirabilis PQ350419 alone or combined with chitosan and ampicillin against common multidrug-resistant bacteria
Microbial Cell Factories (2025)
-
Green synthesis of a lactoferrin-infused silver nanoparticle gel for enhanced wound healing
Scientific Reports (2025)
-
Bacterial cell wall-specific nanomedicine for the elimination of Staphylococcus aureus and Pseudomonas aeruginosa through electron-mechanical intervention
Nature Communications (2025)