Abstract
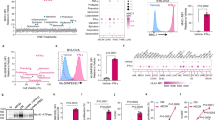
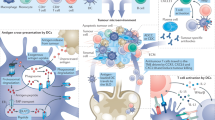
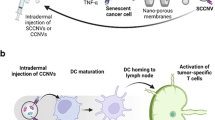
The strategy of combining a vaccine with immune checkpoint inhibitors has been widely investigated in cancer management, but the complete response rate for this strategy is still unresolved. We describe a genetically engineered cell membrane nanovesicle that integrates antigen self-presentation and immunosuppression reversal (ASPIRE) for cancer immunotherapy. The ASPIRE nanovaccine is derived from recombinant adenovirus-infected dendritic cells in which specific peptide-major histocompatibility complex class I (pMHC-I), anti-PD1 antibody and B7 co-stimulatory molecules are simultaneously anchored by a programmed process. ASPIRE can markedly improve antigen delivery to lymphoid organs and generate broad-spectrum T-cell responses that eliminate established tumours. This work presents a powerful vaccine formula that can directly activate both native T cells and exhausted T cells, and suggests a general strategy for personalized cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that data supporting the findings of this study are available within the article and its Supplementary Information. All the relevant data can be provided by the authors upon reasonable request
References
Couzin-Frankel, J. Cancer immunotherapy. Science 342, 1432–1433 (2013).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Huppa, J. B. & Davis, M. M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3, 973–983 (2003).
Fuertes, M. B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Steinman, R. M. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30, 1–22 (2012).
Heath, W. R. & Carbone, F. R. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19, 47–64 (2001).
Heath, W. R. & Carbone, F. R. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 1, 126–134 (2001).
Albert, M. L. & Bhardwaj, N. Resurrecting the dead: DCs cross-present antigen derived from apoptotic cells on MHC I. Immunologist 6, 194–198 (1998).
Palucka, A. K. et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J. Immunother. 29, 545–557 (2006).
Steinman, R. M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
Palucka, K. & Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 12, 265–277 (2012).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Nopora, A. & Brocker, T. Bcl-2 controls dendritic cell longevity in vivo. J. Immunol. 169, 3006–3014 (2002).
Park, D., Lapteva, N., Seethammagari, M., Slawin, K. M. & Spencer, D. M. An essential role for Akt1 in dendritic cell function and tumor immunotherapy. Nat. Biotechnol. 24, 1581–1590 (2006).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Hui, E. F. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017).
Kamphorst, A. O. et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355, 1423–1427 (2017).
Chen, Z. W., Wang, Z. J. & Gu, Z. Bioinspired and biomimetic nanomedicines. Acc. Chem. Res. 52, 1255–1264 (2019).
Zhang, P. F. et al. Genetically engineered liposome-like nanovesicles as active targeted transport platform. Adv. Mater. 30, 1705350 (2018).
Hu, C. M. J. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Liu, X. et al. Vesicular antibodies: a bioactive multifunctional combination platform for targeted therapeutic delivery and cancer immunotherapy. Adv. Mater. 31, 1808294 (2019).
Zhang, P. F. et al. Virus-mimetic nanovesicles as a versatile antigen-delivery system. Proc. Natl Acad. Sci. USA 112, E6129–E6138 (2015).
Liu, X. et al. Bioinspired artificial nanodecoys for hepatitis B virus. Angew. Chem. Int. Ed. 57, 12499–12503 (2018).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Munro, J. M. Endothelial-leukocyte adhesive interactions in inflammatory diseases. Eur. Heart J. 14, 72–77 (1993).
Chen, L. & Flies, D. B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13, 227–242 (2013).
Worbs, T., Hammerschmidt, S. I. & Forster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 17, 30–48 (2017).
Li, H. Y., Li, Y. H., Jiao, J. & Hu, H. M. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat. Nanotechnol. 6, 645–650 (2011).
Flach, T. L. et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat. Med. 17, 479–487 (2011).
Wheeler, C. M. et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect. Dis. 16, 1154–1168 (2016).
Zhu, F. C. et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 376, 895–902 (2010).
Leslie, M. Solution to vaccine mystery starts to crystallize. Science 341, 26–27 (2013).
Verdegaal, E. M. E. et al. Neoantigen landscape dynamics during human melanoma–T cell interactions. Nature 536, 91–95 (2016).
Bertrand, F. et al. TNF alpha blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 8, 2256 (2017).
Li, H. Y. et al. The tumor microenvironment regulates sensitivity of murine lung tumors to PD-1/PD-L1 antibody blockade. Cancer Immunol. Res. 5, 767–777 (2017).
Yadav, M. et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515, 572–576 (2014).
Chen, L. M. et al. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. 8, 1156–1175 (2018).
Verma, V. et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 20, 1555–1555 (2019).
Bulik-Sullivan, B. et al. Deep learning using tumor HLA peptide mass spectrometry datasets improves neoantigen identification. Nat. Biotechnol. 37, 55–63 (2019).
Robbins, P. F. et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19, 747–752 (2013).
Liu, X. S. & Mardis, E. R. Applications of immunogenomics to cancer. Cell 168, 600–612 (2017).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Blackburn, S. D. et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37 (2009).
Acknowledgements
This work was supported by the Major State Basic Research Development Program of China (2017YFA0205201) (G.L.), the National Natural Science Foundation of China (81925019, 81422023 and U1705281) (G.L.), the National University of Singapore Startup Fund (NUHSRO/2020/133/Startup/08) (X.C.), the Nanomedicine Translational Research Programme (NUHSRO/2021/034/TRP/09/Nanomedicine) (X.C.), the Fundamental Research Funds for the Central Universities (20720190088 and 20720200019) (G.L.), the China Postdoctoral Science Foundation (2021M702738) (C.L.) and (2021M700115) (Xue Liu) and the Program for New Century Excellent Talents in University, China (NCET-13-0502) (G.L.).
Author information
Authors and Affiliations
Contributions
C.L. and G.L. conceived and designed the experiments. C.L., Xue Liu, X.X., X.P., S.C., L.Z. and X.W. performed the experiments. C.L., Xue Liu, Xuan Liu, Y.Z., E.R., P.L., X.C. and G.L. analysed the data. C.L., Xue Liu, X.C. and G.L. wrote the manuscript. G.L. supervised the entire project. All the authors discussed the results and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
G.L. and C.L. are inventors on a patent (No. ZL202010070724.9) related to this study. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Michele De Palma, Lana Kandalaft and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24.
Rights and permissions
About this article
Cite this article
Liu, C., Liu, X., Xiang, X. et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat. Nanotechnol. 17, 531–540 (2022). https://doi.org/10.1038/s41565-022-01098-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-022-01098-0
This article is cited by
-
Granzyme B-mimetic nanozyme for nanovesicle targeted anticancer applications
Nature Communications (2026)
-
Biomimetic vesicles engineered from modified tumour cells act as personalized vaccines for post-surgical cancer immunotherapy
Nature Nanotechnology (2026)
-
Synthesis, preclinical evaluation and clinical application of a novel heterodimeric tracer 68Ga-pentixafor-c(RGDfK) for PET-CT imaging
European Journal of Nuclear Medicine and Molecular Imaging (2026)
-
CircRNAs: functions and emerging roles in cancer and immunotherapy
BMC Medicine (2025)
-
Genetically engineered Magnesium/Manganese nanoparticles for cancer radioimmunotherapy
Journal of Nanobiotechnology (2025)