Abstract
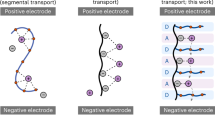
Solid-state lithium-metal (Li0) batteries are gaining traction for electric vehicle applications because they replace flammable liquid electrolytes with a safer, solid-form electrolyte that also offers higher energy density and better resistance against Li dendrite formation. Solid polymer electrolytes (SPEs) are highly promising candidates because of their tuneable mechanical properties and easy manufacturability; however, their electrochemical instability against lithium-metal (Li0), mediocre conductivity and poorly understood Li0/SPE interphases have prevented extensive application in real batteries. In particular, the origin of the low Coulombic efficiency (CE) associated with SPEs remains elusive, as the debate continues as to whether it originates from unfavoured interfacial reactions or lithium dendritic growth and dead lithium formation. In this work, we use state-of-the-art cryo-EM imaging and spectroscopic techniques to characterize the structure and chemistry of the interface between Li0 and a polyacrylate-based SPE. Contradicting the conventional knowledge, we find that no protective interphase forms, owing to the sustained reactions between deposited Li dendrites and polyacrylic backbones and succinonitrile plasticizer. Due to the reaction-induced volume change, large amounts of cracks form inside the Li dendrites with a stress–corrosion–cracking behaviour, indicating that Li0 cannot be passivated in this SPE system. On the basis of this observation, we then introduce additive engineering, leveraging from knowledge of liquid electrolytes, and demonstrate that the Li0 surface can be effectively protected against corrosion using fluoroethylene carbonate, leading to densely packed Li0 domes with conformal and stable solid–electrolyte interphase films. Owing to the high room-temperature ionic conductivity of 1.01 mS cm−1, the high transference number of 0.57 and the stabilized lithium–electrolyte interface, this improved SPE delivers an excellent lithium plating/stripping CE of 99% and 1,800 hours of stable cycling in Li||Li symmetric cells (0.2 mA cm−2, 1 mAh cm−2). This improved cathodic stability, along with the high anodic stability, enables a record high cycle life of >2,000 cycles for Li||LiFePO4 and >400 cycles for Li||LiCoO2 full cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information. Any other data are available from the corresponding author on request.
Change history
12 August 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41565-022-01211-3
References
Lopez, J., Mackanic, D. G., Cui, Y. & Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 4, 312–330 (2019).
Mindemark, J., Lacey, M. J., Bowden, T. & Brandell, D. Beyond PEO—alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 81, 114–143 (2018).
Cui, G. Reasonable design of high-energy-density solid-state lithium-metal batteries. Matter 2, 805–815 (2020).
Yue, L. et al. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 5, 139–164 (2016).
Angell, C. A., Liu, C. & Sanchez, E. Rubbery solid electrolytes with dominant cationic transport and high ambient conductivity. Nature 362, 137–139 (1993).
Alarco, P. J., Abu-Lebdeh, Y., Abouimrane, A. & Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat. Mater. 3, 476–481 (2004).
Croce, F., Appetecchi, G. B., Persi, L. & Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998).
Wang, Y. et al. Solid-state rigid-rod polymer composite electrolytes with nanocrystalline lithium ion pathways. Nat. Mater. 20, 1255–1263 (2021).
Khurana, R., Schaefer, J. L., Archer, L. A. & Coates, G. W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 136, 7395–7402 (2014).
Hu, P. et al. Progress in nitrile-based polymer electrolytes for high performance lithium batteries. J. Mater. Chem. A 4, 10070–10083 (2016).
Wang, C. et al. High polymerization conversion and stable high-voltage chemistry underpinning an in situ formed solid electrolyte. Chem. Mater. 32, 9167–9175 (2020).
Li, S. et al. A superionic conductive, electrochemically stable dual-salt polymer electrolyte. Joule 2, 1838–1856 (2018).
Lin, D. et al. A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv. Mater. 30, e1802661 (2018).
Fang, C. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019).
Ju, Z. et al. Biomacromolecules enabled dendrite-free lithium metal battery and its origin revealed by cryo-electron microscopy. Nat. Commun. 11, 488 (2020).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017).
Liu, Y. et al. Visualizing the sensitive lithium with atomic precision: cryogenic electron microscopy for batteries. Acc. Chem. Res. 54, 2088–2099 (2021).
Liu, Y. et al. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries. Science 375, 739–745 (2022).
Sheng, O. et al. In situ construction of a LiF-enriched interface for stable all-solid-state batteries and its origin revealed by cryo-TEM. Adv. Mater. 32, 2000223 (2020).
Zachman, M. J., Tu, Z., Choudhury, S., Archer, L. A. & Kourkoutis, L. F. Cryo-STEM mapping of solid–liquid interfaces and dendrites in lithium-metal batteries. Nature 560, 345–349 (2018).
Zhang, Z. et al. Capturing the swelling of solid-electrolyte interphase in lithium metal batteries. Science 375, 66–70 (2022).
Xu, Y. et al. Atomic to nanoscale origin of vinylene carbonate enhanced cycling stability of lithium metal anode revealed by cryo-transmission electron microscopy. Nano Lett. 20, 418–425 (2020).
Zhang, X.-Q., Cheng X.-B, Chen, X., Yan, C. & Zhang, Q. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv. Funct. Mater 27, 1605989 (2017).
Philippe, B. et al. Photoelectron spectroscopy for lithium battery interface studies. J. Electrochem. Soc. 163, A178–A191 (2015).
Ding, J. F. et al. Non-solvating and low-dielectricity cosolvent for anion-derived solid electrolyte interphases in lithium metal batteries. Angew. Chem. Int. Ed. Engl. 60, 11442–11447 (2021).
Han, L. et al. Modulating single-atom palladium sites with copper for enhanced ambient ammonia electrosynthesis. Angew. Chem. Int. Ed. Engl. 60, 345–350 (2021).
Xu, C. et al. Interface layer formation in solid polymer electrolyte lithium batteries: an XPS study. J. Mater. Chem. A 2, 7256–7264 (2014).
Xu, H. et al. High-performance all-solid-state batteries enabled by salt bonding to perovskite in poly(ethylene oxide). Proc. Natl Acad. Sci. USA 116, 18815–18821 (2019).
Farhat, D. et al. Towards high-voltage Li-ion batteries: reversible cycling of graphite anodes and Li-ion batteries in adiponitrile-based electrolytes. Electrochim. Acta 281, 299–311 (2018).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J. G. Accurate determination of Coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Deng, T. et al. In situ formation of polymer-inorganic solid-electrolyte interphase for stable polymeric solid-state lithium-metal batteries. Chem 7, 3052–3068 (2021).
Lee, Y.-G. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 5, 299–308 (2020).
Ye, L. & Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 593, 218–222 (2021).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017).
Gadim, T. D. et al. Nanostructured bacterial cellulose-poly(4-styrene sulfonic acid) composite membranes with high storage modulus and protonic conductivity. ACS Appl. Mater. Interfaces 6, 7864–7875 (2014).
Ma, J. et al. A strategy to make high voltage LiCoO2 compatible with polyethylene oxide electrolyte in all-solid-state lithium ion batteries. J. Electrochem. Soc. 164, A3454–A3461 (2017).
Chen, R. et al. An investigation of functionalized electrolyte using succinonitrile additive for high voltage lithium-ion batteries. J. Power Sources 306, 70–77 (2016).
Evans, J., Vincent, C. A. & Bruce, P. G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28, 2324–2328 (1987).
Acknowledgements
This research was primarily supported by the Early Career Research Program, Materials Science and Engineering Divisions, Office of Basic Energy Sciences of the US Department of Energy, under award DE-SC0021204, and additional support for synthesis was received from start-up funding to H.L.X. provided by UC Irvine. R.L., E.H. and X.-Q.Y. were supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technology Office of the US Department of Energy through the Advanced Battery Materials Research (BMR) Program, under contract DE-SC0012704. K.X. thanks the Joint Center of Energy Storage Research, an energy hub funded by US DOE Basic Energy Science, for support. This research used resources of the Center for Functional Nanomaterials (CFN), which is a US Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under contract no. DE-SC0012704. This work made use of facilities and instrumentation at the UC Irvine Materials Research Institute (IMRI), which is supported in part by the National Science Foundation through the UC Irvine Materials Research Science and Engineering Center (DMR-2011967). XPS work was performed using instrumentation funded in part by the National Science Foundation Major Research Instrumentation Program under grant CHE-1338173.
Author information
Authors and Affiliations
Contributions
R.L., X.-Q.Y., K.X. and H.L.X. conceived the idea. R.L., E.H. and Y.H. contributed to the experimental design. R.L. and C.W. performed the cryo-EM study and related electrochemical cycling. Y.H. performed optimization and performance tests of the electrolytes and electrodes. P.Z. performed SEM and XPS studies. R.L. and Y.H. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Peer review information
Nature Nanotechnology thanks Guanglei Cui, Xinyong Tao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1 and Figs. 1−30.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, R., He, Y., Wang, C. et al. Characterization of the structure and chemistry of the solid–electrolyte interface by cryo-EM leads to high-performance solid-state Li-metal batteries. Nat. Nanotechnol. 17, 768–776 (2022). https://doi.org/10.1038/s41565-022-01148-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41565-022-01148-7
This article is cited by
-
Chemical-sensitive Electron Tomography for Nanomaterials
Chemical Research in Chinese Universities (2025)
-
A self-healing plastic ceramic electrolyte by an aprotic dynamic polymer network for lithium metal batteries
Nature Communications (2024)
-
Single-phase local-high-concentration solid polymer electrolytes for lithium-metal batteries
Nature Energy (2024)
-
g-C3N4@COF heterojunction filler for polymer electrolytes enables fast Li+ transport and high mechanical strength
Ionics (2024)
-
A Cross-Linked Gel Polymer Electrolyte Composed of PEGDA and Acrylonitrile for High-Voltage Lithium Metal Batteries
Journal of Electronic Materials (2024)