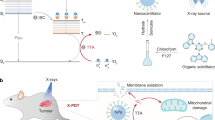
Abstract
Leveraging X-rays to initiate prolonged luminescence (radio-afterglow) and stimulate radiodynamic 1O2 production from optical agents provides opportunities for diagnosis and therapy at tissue depths inaccessible to light. However, X-ray-responsive organic luminescent materials are rare due to their intrinsic low X-ray conversion efficiency. Here we report a cascade X-ray energy converting approach to develop organic radio-afterglow nanoprobes (RANPs) for cancer theranostics. RANPs comprise a radiowave absorber that down-converts X-ray energy to emit radioluminescence, which is transferred to a radiosensitizer to produce singlet oxygen (1O2). 1O2 then reacts with a radio-afterglow substrate to generate an active intermediate that simultaneously decomposes to emit radio-afterglow. Through finetuning such a cascade, intraparticle radioluminescence energy transfer and the 1O2 transfer process, RANPs possess tunable wavelengths and long half-lives, and generate radio-afterglow and 1O2 at tissue depths of up to 15 cm. Moreover, we developed a biomarker-activatable nanoprobe (tRANP) that produces a tumour-specific radio-afterglow signal, leading to ultrasensitive detection and the possibility of surgical removal of diminutive tumours (1 mm3) under an X-ray dosage 20 times lower than inorganic materials. The efficient radiodynamic 1O2 generation of tRANP permits complete tumour eradication at an X-ray dosage lower than clinical radiotherapy and a drug dosage one to two orders of magnitude lower than most existing inorganic agents, leading to prolonged survival rates with minimized radiation-related adverse effects. Thus, our work reveals a generic approach to address the lack of organic radiotheranostic materials and provides molecular design towards precision cancer radiotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study are available within the Article and its Supplementary Information, or from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976 (2004).
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Waterhouse, D. J., Fitzpatrick, C. R. M., Pogue, B. W., O’Connor, J. P. B. & Bohndiek, S. E. A roadmap for the clinical implementation of optical-imaging biomarkers. Nat. Biomed. Eng. 3, 339–353 (2019).
So, M. K., Xu, C., Loening, A. M., Gambhir, S. S. & Rao, J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat. Biotechnol. 24, 339–343 (2006).
Jiang, Y. & Pu, K. Molecular probes for autofluorescence-free optical imaging. Chem. Rev. 121, 13086–13131 (2021).
Miao, Q. et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 35, 1102–1110 (2017).
le Masne de Chermont, Q. et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl Acad. Sci. USA 104, 9266–9271 (2007).
Jiang, Y. et al. A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 10, 2064 (2019).
Wu, L. et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun. 11, 446 (2020).
Qu, R. et al. Afterglow/photothermal bifunctional polymeric nanoparticles for precise postbreast-conserving surgery adjuvant therapy and early recurrence theranostic. Nano Lett. 23, 4216–4225 (2023).
Chen, W. et al. Near-infrared afterglow luminescence of chlorin nanoparticles for ultrasensitive in vivo imaging. J. Am. Chem. Soc. 144, 6719–6726 (2022).
Ni, X. et al. Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumour-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 19, 318–330 (2019).
Wei, X. et al. Leveraging long-distance singlet-oxygen transfer for bienzyme-locked afterglow imaging of intratumoral granule enzymes. J. Am. Chem. Soc. 146, 17393–17403 (2024).
Maldiney, T. et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 13, 418–426 (2014).
Chen, H. et al. LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumours. Mater. Horiz. 4, 1092–1101 (2017).
Pei, P. et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 16, 1011–1018 (2021).
Zhang, C. et al. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. 54, 1770–1774 (2015).
Li, J., Cheng, F., Huang, H., Li, L. & Zhu, J. J. Nanomaterial-based activatable imaging probes: from design to biological applications. Chem. Soc. Rev. 44, 7855–7880 (2015).
Wang, X. & Pu, K. Molecular substrates for the construction of afterglow imaging probes in disease diagnosis and treatment. Chem. Soc. Rev. 52, 4549–4566 (2023).
Huang, J. et al. Molecular radio afterglow probes for cancer radiodynamic theranostics. Nat. Mater. 22, 1421–1429 (2023).
Huang, J. et al. Chemiluminescent probes with long-lasting high brightness for in vivo imaging of neutrophils. Angew. Chem. Int. Ed. 61, e202203235 (2022).
Wei, X. et al. Highly bright near-infrared chemiluminescent probes for cancer imaging and laparotomy. Angew. Chem. Int. Ed. 62, e202213791 (2023).
Yang, Z. et al. Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 46, 915–1016 (2017).
Ma, W. et al. Thermally activated delayed fluorescence (TADF) organic molecules for efficient X-ray scintillation and imaging. Nat. Mater. 21, 210–216 (2022).
Hong, X. et al. TADF molecules with π-extended acceptors for simplified high-efficiency blue and white organic light-emitting diodes. Chem 8, 1705–1719 (2022).
Gorrini, C., Harris, I. S. & Mak, T. W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 (2013).
Lippert, A. R., Van de Bittner, G. C. & Chang, C. J. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 44, 793–804 (2011).
Chen, Z. Z. et al. Low dose of X-ray-excited long-lasting luminescent concave nanocubes in highly passive targeting deep-seated hepatic tumours. Adv. Mater. 31, e1905087 (2019).
Lo, S. S. et al. Stereotactic body radiation therapy: a novel treatment modality. Nat. Rev. Clin. Oncol. 7, 44–54 (2010).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Hymes, S. R., Strom, E. A. & Fife, C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J. Am. Acad. Dermatol. 54, 28–46 (2006).
Acknowledgements
Y.Z. thanks the National Natural Science Foundation of China (22322406) for financial support. D.D. thanks the National Natural Science Foundation of China (52225310) for financial support. J.S. thanks the National Natural Science Foundation of China (21874024, U21A20377, U22A20348), the Fundamental Research Funds for the Central Universities (buctrc202235) and the National Key Research and Development Plan (2023YFB3810002). K.P. thanks the Singapore National Research Foundation (NRF) (NRF-NRFI07-2021-0005) and the Singapore Ministry of Education Academic Research Fund Tier2 (MOE-T2EP30220-0010 and MOE-T2EP30221-0004) for financial support. We thank D. Shiye, L. Qingqing and L. Xing for assistance in operating the equipment.
Author information
Authors and Affiliations
Contributions
K.P. conceived the study. K.P., Y.Z. and C.X. designed the experiments. C.X. and X.Q. prepared the nanomaterials and conducted the in vitro characterization. X.W. conducted the chemical syntheses. C.X., J.Y. and Y.Z. conducted the cell experiments. C.X. performed the in vivo experiments. K.P., J.S., Y.Z., D.D. and C.X. analysed the data and drafted the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Peer review
Peer review information
Nature Nanotechnology thanks Marc Vendrell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39, Tables 1–3, Scheme 1 and Spectroscopic data
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Qin, X., Wei, X. et al. A cascade X-ray energy converting approach toward radio-afterglow cancer theranostics. Nat. Nanotechnol. 20, 286–295 (2025). https://doi.org/10.1038/s41565-024-01809-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-024-01809-9
This article is cited by
-
Organic afterglow luminescence for disease diagnosis and treatment
Nature Reviews Bioengineering (2025)
-
Sonoafterglow nanoprobes for deep-tissue imaging of peroxynitrite
Nature Protocols (2025)
-
Afterglow nanoparticle-assisted PLK1-targeted antisense oligonucleotide delivery for cancer gene therapy
Science China Chemistry (2025)