Abstract
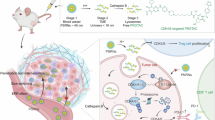
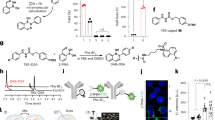
The forward design of biosensors that implement Boolean logic to improve detection precision primarily relies on programming genetic components to control transcriptional responses. However, cell- and gene-free nanomaterials programmed with logical functions may present lower barriers for clinical translation. Here we report the design of activity-based nanosensors that implement AND-gate logic without genetic parts via bi-labile cyclic peptides. These actuate by releasing a reporter if and only if cleaved by a specific pair of proteases. AND-gated nanosensors that detect the concomitant activity of the granzyme B protease secreted by CD8 T cells and matrix metalloproteinases overexpressed by cancer cells identify the unique condition of cytotoxic T cell killing of tumour cells. In preclinical mouse models, AND-gated nanosensors discriminate tumours that are responsive to immune checkpoint blockade therapy from B2m–/– tumours that are resistant to it, minimize signals from tissues without co-localized protease expression including the lungs during acute influenza infection, and release a reporter locally in tissue or distally in the urine for facile detection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The source data underlying the main text figures (Figs. 1–6) and Supplementary figures are provided as source data files. All raw data and calculations used to generate plotted data are provided with this paper in the source data files. Image source data for Supplementary figures are provided at the end of the Supplementary Information file. Source data are provided with this paper.
References
Kitada, T., DiAndreth, B., Teague, B. & Weiss, R. Programming gene and engineered-cell therapies with synthetic biology. Science 359, eaad1067 (2018).
Xie, M. & Fussenegger, M. Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nat. Rev. Mol. Cell Biol. 19, 507–525 (2018).
Cubillos-Ruiz, A. et al. Engineering living therapeutics with synthetic biology. Nat. Rev. Drug Discov. 20, 941–960 (2021).
Courbet, A., Endy, D., Renard, E., Molina, F. & Bonnet, J. Detection of pathological biomarkers in human clinical samples via amplifying genetic switches and logic gates. Sci. Transl. Med. 7, 289ra283 (2015).
Chang, H.-J. et al. Programmable receptors enable bacterial biosensors to detect pathological biomarkers in clinical samples. Nat. Commun. 12, 5216 (2021).
Kwong, G. A. et al. Synthetic biomarkers: a twenty-first century path to early cancer detection. Nat. Rev. Cancer 21, 655–668 (2021).
Danino, T. et al. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 7, 289ra284–289ra284 (2015).
Panteli, J. T., Van Dessel, N. & Forbes, N. S. Detection of tumors with fluoromarker-releasing bacteria. Int. J. Cancer 146, 137–149 (2020).
Cho, J. H., Collins, J. J. & Wong, W. W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438.e1411 (2018).
Roybal, K. T. et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 164, 770–779 (2016).
Fedorov, V. D., Themeli, M. & Sadelain, M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 5, 215ra172 (2013).
Kaseniit, K. E. et al. Modular, programmable RNA sensing using ADAR editing in living cells. Nat. Biotechnol. 41, 482–487 (2023).
Kawasaki, S., Fujita, Y., Nagaike, T., Tomita, K. & Saito, H. Synthetic mRNA devices that detect endogenous proteins and distinguish mammalian cells. Nucleic Acids Res. 45, e117–e117 (2017).
Wagner, T. E. et al. Small-molecule-based regulation of RNA-delivered circuits in mammalian cells. Nat. Chem. Biol. 14, 1043–1050 (2018).
Mc Cafferty, S. et al. In vivo validation of a reversible small molecule-based switch for synthetic self-amplifying mRNA regulation. Mol. Ther. 29, 1164–1173 (2021).
Vlahos, A. E. et al. Protease-controlled secretion and display of intercellular signals. Nat. Commun. 13, 912 (2022).
Wang, X. et al. A programmable protease-based protein secretion platform for therapeutic applications. Nat. Chem. Biol. 20, 432–442 (2023).
Gao, X. J., Chong, L. S., Kim, M. S. & Elowitz, M. B. Programmable protein circuits in living cells. Science 361, 1252–1258 (2018).
Chen, Z. et al. De novo design of protein logic gates. Science 368, 78 (2020).
Holt, B. A. & Kwong, G. A. Protease circuits for processing biological information. Nat. Commun. 11, 5021 (2020).
Holt, B. A. et al. Dimensionless parameter predicts bacterial prodrug success. Mol. Syst. Biol. 18, e10495 (2022).
Widen, J. C. et al. AND-gate contrast agents for enhanced fluorescence-guided surgery. Nat. Biomed. Eng. 5, 264–277 (2021).
Holt, B. A. et al. Embracing enzyme promiscuity with activity-based compressed biosensing. Cell Rep. Methods 3, 100372 (2023).
Zhuang, Q., Holt, B. A., Kwong, G. A. & Qiu, P. Deconvolving multiplexed protease signatures with substrate reduction and activity clustering. PLoS Comput. Biol. 15, e1006909 (2019).
Mac, Q. D. et al. Non-invasive early detection of acute transplant rejection via nanosensors of granzyme B activity. Nat. Biomed. Eng. 3, 281–291 (2019).
Mac, Q. D. et al. Urinary detection of early responses to checkpoint blockade and of resistance to it via protease-cleaved antibody-conjugated sensors. Nat. Biomed. Eng. 6, 310–324 (2022).
Werle, M. & Bernkop-Schnurch, A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 30, 351–367 (2006).
Diao, L. & Meibohm, B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin. Pharmacokinet. 52, 855–868 (2013).
Jokerst, J. V., Lobovkina, T., Zare, R. N. & Gambhir, S. S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 6, 715–728 (2011).
Kwong, G. A. et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat. Biotechnol. 31, 63–70 (2013).
Dall, E. & Brandstetter, H. Mechanistic and structural studies on legumain explain its zymogenicity, distinct activation pathways, and regulation. Proc. Natl Acad. Sci. USA 110, 10940–10945 (2013).
Aggarwal, S. et al. Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry 47, 1076–1086 (2008).
Joo, S. H. Cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther. (Seoul) 20, 19–26 (2012).
Nielsen, D. S. et al. Orally absorbed cyclic peptides. Chem. Rev. 117, 8094–8128 (2017).
McKay, C. S. & Finn, M. G. Polyvalent catalysts operating on polyvalent substrates: a model for surface-controlled reactivity. Angew. Chem. Int. Ed. 55, 12643–12649 (2016).
Algar, W. R. et al. Proteolytic activity at quantum dot-conjugates: kinetic analysis reveals enhanced enzyme activity and localized interfacial “hopping”. Nano Lett. 12, 3793–3802 (2012).
Chen, P. L. et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 6, 827–837 (2016).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558 (2018).
Kessenbrock, K., Plaks, V. & Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
Morad, G., Helmink, B. A., Sharma, P. & Wargo, J. A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184, 5309–5337 (2021).
Yap, T. A. et al. Development of immunotherapy combination strategies in cancer. Cancer Discov. 11, 1368–1397 (2021).
Nguyen, A. et al. Granzyme B nanoreporter for early monitoring of tumor response to immunotherapy. Sci. Adv. 6, eabc2777 (2020).
Zhao, N. et al. In vivo measurement of granzyme proteolysis from activated immune cells with PET. ACS Cent. Sci. 7, 1638–1649 (2021).
Kalbasi, A. & Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 20, 25–39 (2020).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Smith, B. L. et al. Feasibility study of a novel protease-activated fluorescent imaging system for real-time, intraoperative detection of residual breast cancer in breast conserving surgery. Ann. Surg. Oncol. 27, 1854–1861 (2020).
Whitley, M. J. et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med. 8, 320ra324 (2016).
Steinkamp, P. J. et al. A standardized framework for fluorescence-guided margin assessment for head and neck cancer using a tumor acidosis sensitive optical imaging agent. Mol. Imaging Biol. 23, 809–817 (2021).
Lord, S. J., Rajotte, R. V., Korbutt, G. S. & Bleackley, R. C. Granzyme B: a natural born killer. Immunol. Rev. 193, 31–38 (2003).
Trapani, J. A. & Sutton, V. R. Granzyme B: pro-apoptotic, antiviral and antitumor functions. Curr. Opin. Immunol. 15, 533–543 (2003).
Sun, Q. et al. Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduct. Target. Ther. 8, 320 (2023).
Vaddepally, R. K., Kharel, P., Pandey, R., Garje, R. & Chandra, A. B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) 12, 738 (2020).
Tune, B. X. J. et al. Matrix metalloproteinases in chemoresistance: regulatory roles, molecular interactions, and potential inhibitors. J. Oncol. 2022, 3249766 (2022).
Vyas, D., Laput, G. & Vyas, A. K. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. OncoTargets Ther. 7, 1015–1023 (2014).
Goodwin, R. A. & Asmis, T. R. Overview of systemic therapy for colorectal cancer. Clin. Colon Rectal Surg. 22, 251–256 (2009).
Zhang, X. et al. Hepatitis B virus reactivation in cancer patients with positive hepatitis B surface antigen undergoing PD-1 inhibition. J. Immunother. Cancer 7, 322 (2019).
Hutchinson, J. A. et al. Virus-specific memory T cell responses unmasked by immune checkpoint blockade cause hepatitis. Nat. Commun. 12, 1439 (2021).
Esfahani, K. et al. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 17, 504–515 (2020).
Kyi, C., Hellmann, M. D., Wolchok, J. D., Chapman, P. B. & Postow, M. A. Opportunistic infections in patients treated with immunotherapy for cancer. J. Immunother. Cancer 2, 19 (2014).
Del Castillo, M. et al. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin. Infect. Dis. 63, 1490–1493 (2016).
Zhu, I. et al. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell 185, 1431–1443.e1416 (2022).
Depil, S., Duchateau, P., Grupp, S. A., Mufti, G. & Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
Mo, F. et al. Engineered off-the-shelf therapeutic T cells resist host immune rejection. Nat. Biotechnol. 39, 56–63 (2021).
Su, F.-Y. et al. In vivo mRNA delivery to virus-specific T cells by light-induced ligand exchange of MHC class I antigen-presenting nanoparticles. Sci. Adv. 8, eabm7950 (2022).
Smith, T. T. et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 12, 813–820 (2017).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Fridman, W. H., Zitvogel, L., Sautès–Fridman, C. & Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717–734 (2017).
He, S., Cheng, P. & Pu, K. Activatable near-infrared probes for the detection of specific populations of tumour-infiltrating leukocytes in vivo and in urine. Nat. Biomed. Eng. 7, 281–297 (2023).
Kratochwil, C. et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 60, 801–805 (2019).
Galluzzi, L., Guilbaud, E., Schmidt, D., Kroemer, G. & Marincola, F. M. Targeting immunogenic cell stress and death for cancer therapy. Nat. Rev. Drug Discov. 23, 445–460 (2024).
Yatim, N., Cullen, S. & Albert, M. L. Dying cells actively regulate adaptive immune responses. Nat. Rev. Immunol. 17, 262–275 (2017).
Park, J.-H. et al. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv. Mater. 20, 1630–1635 (2008).
Acknowledgements
This work was funded in part by National Institutes of Health (NIH) grants 5U01CA265711 (G.A.K., P.Q. and M.G.F.), 5R01CA237210 (G.A.K.), 1DP2HD091793 (G.A.K.) and 5DP1CA280832 (G.A.K.). The authors were supported by the National Science Foundation (NSF) Graduate Research Fellowships Program (grant DGE-2039655, A.S. and A.D.S.T.) and the National Institutes of Health Cell and Tissue Engineering Training Program T32GM145735 (A.D.S.T.). This work was performed in part at the Georgia Tech Institute for Electronics and Nanotechnology, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (grant ECCS-1542174). This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the staff at Georgia Tech’s Systems Mass Spectrometry Core, Cellular Analysis and Cytometry Core, Organic Materials Characterization Laboratory and Department of Animal Resources for their assistance in performing our studies.
Author information
Authors and Affiliations
Contributions
A.S. and G.A.K. conceived the idea. A.S., H.P., Q.D.M., M.X., P.Q., M.G.F. and G.A.K. designed the experiments and interpreted the results. A.S., H.P., H.R., L.C.R., A.D.S.T., S.S.B., R.H., Z.L., S.V. and I.L. synthesized the materials and carried out the experiments. A.S. and G.A.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
G.A.K. is an equity shareholder of, and consults for, Sunbird Bio and Port Therapeutics. This study could affect his personal financial status. The terms of this arrangement have been reviewed and approved by Georgia Tech in accordance with its conflict-of-interest policies. A.S., Q.D.M. and G.A.K. are listed as inventors on patent application (PCT/US2020/030132) pertaining to the results of the paper. The patent applicant is the Georgia Tech Research Corporation. The patent is published (WO2020191416A3). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–30 (including image source data for Supplementary figures) and Table 1.
Supplementary Data 1
Numerical source data for Supplementary Figs. 2–4, 6–10 and 12–22.
Source data
Source Data Fig. 1
Numerical source data.
Source Data Fig. 2
Numerical source data.
Source Data Fig. 3
Numerical source data.
Source Data Fig. 4
Numerical source data.
Source Data Fig. 4
Image source data.
Source Data Fig. 5
Numerical source data.
Source Data Fig. 5
Image source data.
Source Data Fig. 6
Numerical source data.
Source Data Fig. 6
Image source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sivakumar, A., Phuengkham, H., Rajesh, H. et al. AND-gated protease-activated nanosensors for programmable detection of anti-tumour immunity. Nat. Nanotechnol. 20, 441–450 (2025). https://doi.org/10.1038/s41565-024-01834-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-024-01834-8