Abstract
Room-temperature non-aqueous sodium metal batteries are viable candidates for cost-effective and safe electrochemical energy storage. However, they show low specific energy and poor cycle life as the use of conventional organic-based non-aqueous electrolyte solutions enables the formation of interphases that cannot prevent degradations at the positive and negative electrodes. Here, to promote the formation of inorganic NaF-rich interphases on both negative and positive electrodes, we propose the salt-in-presalt (SIPS) electrolyte formulation strategy. In SIPS, sodium bis(fluorosulfonyl)imide (NaFSI) salt is dissolved in the liquid precursor of the sodium bis(trifluoromethylsulfonyl)imide (NaTFSI) salt, that is, N,N-dimethyltrifluoromethane-sulfonamide, called PreTFSI. The prepared 0.5 M NaFSI in PreTFSI (SIPS5) electrolyte solution shows an electrochemical stability up to 6.7 V versus Na|Na+ and enables a Na stripping/plating average Coulombic efficiency of 99.7% at 2.0 mA cm−2 and 4.0 mAh cm−2 in Na||Al cell configuration. By testing SIPS5 in Na metal and ‘anode-less’ coin and pouch cell configurations using NaNi0.6Mn0.2Co0.2O2 or sulfurized polyacrylonitrile as positive electrode active materials, we demonstrate the ability of the SIPS strategy to deliver improved specific discharge capacity and capacity retentions at high cell potentials and moderate applied specific currents for cell cycle life up to 1,000 cycles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2298530 (neat NaFSI) and CCDC 2298531 (NaFSI/PreTFSI, 3:1). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. Source data are provided with this paper.
References
Choi, J. W. et al. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016).
Vaalma, C. et al. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 3, 18013 (2018).
Lee, B. et al. Sodium metal anodes: emerging solutions to dendrite growth. Chem. Rev. 119, 5416–5460 (2019).
Lu, X. et al. Advanced intermediate-temperature Na–S battery. Energy Environ. Sci. 6, 299–306 (2013).
Li, G. et al. Advanced intermediate temperature sodium-nickel chloride batteries with ultra-high energy density. Nat. Commun. 7, 10683 (2016).
Jin, T. et al. Realizing complete solid-solution reaction in high sodium content P2-type cathode for high-performance sodium-ion batteries. Angew. Chem. 132, 14619–14624 (2020).
Usiskin, R. et al. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 6, 1020–1035 (2021).
Zhao, C. et al. Rational design of layered oxide materials for sodium-ion batteries. Science 370, 708–711 (2020).
Lu, Z. et al. Building a beyond concentrated electrolyte for high-voltage anode-free rechargeable sodium batteries. Angew. Chem. 134, e202200410 (2022).
Li, Y. et al. Interfacial engineering to achieve an energy density of over 200 Wh kg–1 in sodium batteries. Nat. Energy 7, 511–519 (2022).
Ni, Q. et al. Anode-free rechargeable sodium-metal batteries. Batteries 8, 272 (2022).
Yang, T. et al. Anode-free sodium metal batteries as rising stars for lithium-ion alternatives. iScience 26, 105982 (2023).
Suo, L. et al. “Water-in-salt” electrolyte makes aqueous sodium-ion battery safe, green, and long-lasting. Adv. Energy Mater. 7, 1701189 (2017).
Xu, G. L. et al. Challenges in developing electrodes, electrolytes, and diagnostics tools to understand and advance sodium-ion batteries. Adv. Energy Mater. 8, 1702403 (2018).
Che, H. et al. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci. 10, 1075–1101 (2017).
Zheng, X. et al. Critical effects of electrolyte recipes for Li and Na metal batteries. Chem 7, 2312–2346 (2021).
Xu, J. et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 614, 694–700 (2023).
Xiang, Y. et al. Visualizing the growth process of sodium microstructures in sodium batteries by in-situ 23Na MRI and NMR spectroscopy. Nat. Nanotechnol. 15, 883–890 (2020).
Han, B. et al. Probing the Na metal solid electrolyte interphase via cryo-transmission electron microscopy. Nat. Commun. 12, 3066 (2021).
Seh, Z. W. et al. A highly reversible room-temperature sodium metal anode. ACS Cent. Sci. 1, 449–455 (2015).
Cao, R. et al. Enabling room temperature sodium metal batteries. Nano Energy 30, 825–830 (2016).
Zhuang, R. et al. Fluorinated porous frameworks enable robust anode-less sodium metal batteries. Sci. Adv. 9, eadh8060 (2023).
Wang, C. et al. Robust anode-free sodium metal batteries enabled by artificial sodium formate interface. Adv. Energy Mater. 13, 2204125 (2023).
Choudhury, S. et al. Designing solid–liquid interphases for sodium batteries. Nat. Commun. 8, 898 (2017).
Zheng, X. et al. Bridging the immiscibility of an all-fluoride fire extinguishant with highly-fluorinated electrolytes toward safe sodium metal batteries. Energy Environ. Sci. 13, 1788–1798 (2020).
Zheng, X. et al. Knocking down the kinetic barriers towards fast-charging and low-temperature sodium metal batteries. Energy Environ. Sci. 14, 4936–4947 (2021).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Shkrob, I. A. et al. Why bis(fluorosulfonyl)imide is a “magic anion” for electrochemistry. J. Phys. Chem. C 118, 19661–19671 (2014).
Zheng, J. et al. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes. ACS Energy Lett. 3, 315–321 (2018).
Chen, J. et al. High energy density Na-metal batteries enabled by a tailored carbonate-based electrolyte. Energy Environ. Sci. 15, 3360–3368 (2022).
Ignat’ev, N. V. et al. Comparative fluorination of N,N-dialkylamidosulfonyl halides. J. Fluor. Chem. 74, 181–184 (1995).
Fu, S.-T. et al. N,N-Dialkyl perfluoroalkanesulfonamides: synthesis, characterization and properties. J. Fluor. Chem. 147, 56–64 (2013).
Xue, W. et al. FSI-inspired solvent and “full fluorosulfonyl” electrolyte for 4 V class lithium-metal batteries. Energy Environ. Sci. 13, 212–220 (2020).
Xue, W. et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 6, 495–505 (2021).
Cao, X. et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 4, 796–805 (2019).
Suo, L. et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Suo, L. et al. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 4, 1481 (2013).
Murphy, S. et al. Acyclic and cyclic alkyl and ether-functionalised sulfonium ionic liquids based on the [TFSI]− and [FSI]− anions as potential electrolytes for electrochemical applications. ChemPhysChem 19, 3226–3236 (2018).
Shin, W. et al. A facile potential hold method for fostering an inorganic solid-electrolyte interphase for anode-free lithium-metal batteries. Angew. Chem. 61, e202115909 (2022).
Shi, Q. et al. High-performance sodium metal anodes enabled by a bifunctional potassium salt. Angew. Chem. 57, 9069–9072 (2018).
Gao, L. et al. The chemical evolution of solid electrolyte interface in sodium metal batteries. Sci. Adv. 8, eabm4606 (2022).
Holoubek, J. et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy 6, 303–313 (2021).
Yao, Y. X. et al. Regulating interfacial chemistry in lithium-ion batteries by a weakly solvating electrolyte. Angew. Chem. 60, 4090–4097 (2021).
Zheng, X. et al. Toward a stable sodium metal anode in carbonate electrolyte: a compact, inorganic alloy interface. J. Phys. Chem. Lett. 10, 707–714 (2019).
Song, J. et al. Controlling surface phase transition and chemical reactivity of O3-layered metal oxide cathodes for high-performance Na-ion batteries. ACS Energy Lett. 5, 1718–1725 (2020).
Xue, W. et al. Stabilizing electrode–electrolyte interfaces to realize high-voltage Li||LiCoO2 batteries by a sulfonamide-based electrolyte. Energy Environ. Sci. 14, 6030–6040 (2021).
Jin, Y. et al. Low-solvation electrolytes for high-voltage sodium-ion batteries. Nat. Energy 7, 718–725 (2022).
Pu, X. et al. Building the robust fluorinated electrode–electrolyte interface in rechargeable batteries: from fundamentals to applications. Electrochem. Energy Rev. 7, 21 (2024).
Liu, H. et al. Ultrahigh Coulombic efficiency electrolyte enables Li||SPAN batteries with superior cycling performance. Mater. Today 42, 17–28 (2021).
Xu, X. et al. A room-temperature sodium-sulfur battery with high capacity and stable cycling performance. Nat. Commun. 9, 3870 (2018).
Wu, J. et al. Non-flammable electrolyte for dendrite-free sodium-sulfur battery. Energy Storage Mater. 23, 8–16 (2019).
Zhang, C.-P. et al. Determination of pKa values of fluoroalkanesulfonamides and investigation of their nucleophilicity. J. Fluor. Chem. 131, 761–766 (2010).
Willcott, M. R. MestRe Nova. JACS 131, 13180 (2009).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Acknowledgements
This work is supported by the US Department of Energy (DOE) Office of Electricity (OE) under contract DE-AC06-76LO1830 through the Pacific Northwest National Laboratory (70247) (C.W. and X.L.).
Author information
Authors and Affiliations
Contributions
A.-M.L. and C.W. conceived the idea for the project and wrote the paper. P.Y.Z. helped with the single-crystal data collection and analysis. F.O. and X.L. helped with the NMC622-based positive electrode preparation and casting.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Seung Woo Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
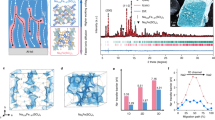
Extended Data Fig. 1 The solid crystal structure of NaFSI salt and NaFSI/PreTFSI (3:1 by mole) solid and liquid solvation of 0.5 M NaFSI in PreTFSI liquid electrolyte solution.
(A) The asymmetry unit of NaFSI crystal shows the mono-capped octahedron, where six oxygen atoms facilitate the octahedral geometry with one nitrogen atom capped to the top. The O-η2- solvation mode of the FSI anion is highlighted in a red circle. (B) The close-packing structure of NaFSI crystal, in which the cationic layer with fluorine atoms is pushed aside. (C) The asymmetry unit of NaFSI/PreTFSI (3:1) solid structure with two octahedral solvated Na+ centers and one distorted trigonal bipyramidal site. All the FSI anion oxygen atoms adapt the O-η1- solvation mode as highlighted in the red circle. (D) The scattered packing layer in NaFSI/PreTFSI (3:1) crystal structure. (E–F) Solvation of SIPS5 electrolyte. (E) Proposed solvation cluster {Na3(FSI)4(PreTFSI)4} in SIPS5 electrolyte, where four NaFSI is solvated by four PreTFSI molecules to have the dispersed cluster rather than the infinite sheets in the isolated crystals showing in B and D. (F) 2D {7Li–1H} (orange-cyan, below) HOESY contour plot of SIPS5 electrolyte, the inset shows the chemical shifts of 19F-NMR of SIPS5 electrolyte and PreTFSI molecule.
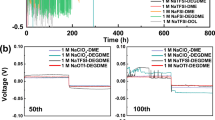
Extended Data Fig. 2 Linear sweep voltammetry (LSV) anodic scanning in Na||Al cells at a scan rate of 0.5 mV s-1 for SIPS5 (magenta) and EE (blue) electrolytes (23 ± 3 ˚C).
The LSV scan data was shown with different current density resolutions for comparison, note that the current density unit on the y-axis for (A) is mA cm-2, and for (B) is μA cm-2.
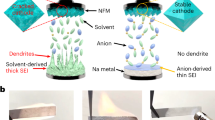
Extended Data Fig. 3 Morphology and composition of CEI formed in the NMC622-based positive electrode after 100 charge/discharge cycles in SIPS5 and EE electrolytes.
(A, B) Ex situ HRTEM images of NMC622-based positive electrode after 100 cycles at 23 ± 3 ˚C (50 mA g-1) in EE (2.0–4.2 V) (A) and SIPS5 (2.0–4.7 V) (B) electrolytes. Yellow dashed lines indicate the interfaces between the CEI and NMC622 bulk phase or the surface transition region of the NMC622 particle. (C–D) Ex situ C1s XPS spectra of the cycled NMC622-based positive electrodes cycled in (C) EE or (D) SIPS5 electrolyte solutions. The XPS raw data were fitted with CasaXPS software with binding energy calibrated at 284.8 eV of C1s. Shirley BG type was used for background subtraction and GL(30) line shape was used for peak fitting. (E–H) Ex situ SEM micrographs of NMC622-based positive electrodes cycled in EE (E) and SIPS5 (F) electrolyte solutions; Schematic representation of the CEI role on the stability of positive electrode active material particles (G, H). Thick, organic-rich EE-derived CEI cracks at the NMC622 particle surface during the sodiation/desodiation process (G), while thinner NaF-rich SIPS5-derived CEI is stable and effectively protects NMC622 particles from degradation (H).
Supplementary information
Supplementary Information
Supplementary Notes 1–7, Tables 1–7, Figs. 1–35 and references.
Supplementary Data 1
Crystal data for NaFSI.
Supplementary Data 2
Crystal data for NaFSI/PreTFSI (3:1).
Source data
Source Data Fig. 3
Statistical source data for Fig. 3a.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, AM., Zavalij, P.Y., Omenya, F. et al. Salt-in-presalt electrolyte solutions for high-potential non-aqueous sodium metal batteries. Nat. Nanotechnol. 20, 388–396 (2025). https://doi.org/10.1038/s41565-024-01848-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41565-024-01848-2
This article is cited by
-
All-anion electrolyte solutions for high-potential sodium batteries
Nature Nanotechnology (2025)
-
High-entropy sulfoselenide as negative electrodes with fast kinetics and high stability for sodium-ion batteries
Nature Communications (2025)