Abstract
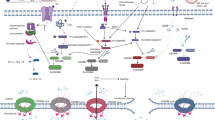
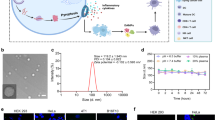
Pyroptosis has emerged as a promising approach for cancer immunotherapy. However, current pyroptosis inducers lack specificity for cancer cells and have a weak antitumour immune response. Here we report a tumour-specific nanoparticle (NP-NH-D5) that activates pyroptosis by disrupting lysosomes for cancer immunotherapy. NP-NH-D5 undergoes negative-to-positive charge reversal and nanoparticle-to-nanofibre transformation within tumour cell lysosomes through tandem response to extracellular matrix metallopeptidase-2 and intracellular reducing agents. The as-formed non-peptide nanofibres efficiently break the lysosomes and trigger gasdermin-D-mediated pyroptosis, leading to strong immunogenic cell death and alleviation of the immunosuppressive tumour microenvironment. In vivo, NP-NH-D5 inhibits orthotopic 4T1 breast tumours, prevents metastasis and recurrence, and prolongs survival without systemic side effects. Furthermore, it greatly enhances the effectiveness of PD-L1 antibody immunotherapy in the 4T1 late-stage lung metastasis and aggressive orthotopic Pan02 pancreatic tumour models. Our research may open pathways for developing stimuli-responsive pyroptosis inducers for precise cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data that support the findings of this study are available within the paper and the Supplementary Information. Other raw and relevant data from the study are available for research purposes from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Broz, P., Pelegrín, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 43–157 (2020).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Wang, Q. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 (2020).
Zhou, B. et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 28, 1171–1185 (2018).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Li, F. et al. mRNA lipid nanoparticle-mediated pyroptosis sensitizes immunologically cold tumors to checkpoint immunotherapy. Nat. Commun. 14, 4223 (2023).
Ploetz, E. et al. Metal–organic framework nanoparticles induce pyroptosis in cells controlled by the extracellular pH. Adv. Mater. 32, 1907267 (2020).
Ngai, W. S. C. et al. Bioorthogonally activatable base editing for on-demand pyroptosis. J. Am. Chem. Soc. 144, 5411–5417 (2022).
Li, M. et al. Photoredox catalysis may be a general mechanism in photodynamic therapy. Proc. Natl Acad. Sci. USA 119, e2210504 (2022).
Yu, L. et al. Photocatalytic superoxide radical generator that induces pyroptosis in cancer cells. J. Am. Chem. Soc. 144, 11326–11337 (2022).
Chen, B. et al. A pyroptosis nanotuner for cancer therapy. Nat. Nanotechnol. 17, 788–798 (2022).
Su, X. et al. Disruption of zinc homeostasis by a novel platinum(IV)-terthiophene complex for antitumor immunity. Angew. Chem. Int. Ed. 135, e202216917 (2022).
Zhang, W. et al. Bioorthogonal disruption of pyroptosis checkpoint for high-efficiency pyroptosis cancer therapy. J. Am. Chem. Soc. 145, 16658–16668 (2023).
Liu, X. et al. Apoptosis-amplified assembly of porphyrin nanofiber enhances photodynamic therapy of oral tumor. J. Am. Chem. Soc. 145, 7918–7930 (2023).
Li, M. et al. Photon-controlled pyroptosis activation (PhotoPyro): an emerging trigger for antitumor immune response. J. Am. Chem. Soc. 145, 6007–6023 (2023).
Chauhan, V. & Jain, R. Strategies for advancing cancer nanomedicine. Nat. Mater. 12, 958–962 (2013).
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020).
Tu, Y. et al. Mimicking the cell: bio-inspired functions of supramolecular assemblies. Chem. Rev. 116, 2023–2078 (2016).
Cademartiri, L. & Bishop, K. Programmable self-assembly. Nat. Mater. 14, 2–9 (2015).
Wang, Y., Hu, Y. & Ye, D. Activatable multimodal probes for in vivo imaging and theranostics. Angew. Chem. Int. Ed. 61, e202209512 (2022).
Chagri, S., Ng, D. Y. W. & Weil, T. Designing bioresponsive nanomaterials for intracellular self-assembly. Nat. Rev. Chem. 6, 320–338 (2022).
Kim, J. et al. In situ self-assembly for cancer therapy and imaging. Nat. Rev. Mater. 8, 710–725 (2023).
Liang, G., Ren, H. & Rao, J. A biocompatible condensation reaction for controlled assembly of nanostructures in living cells. Nat. Chem. 2, 54–60 (2010).
Zhang, Y. et al. Tuning the autophagy-inducing activity of lanthanide-based nanocrystals through specific surface-coating peptides. Nat. Mater. 11, 817–826 (2012).
Jeena, M. T. et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat. Commun. 8, 26 (2017).
Yan, R. et al. Activatable NIR fluorescence/MRI bimodal probes for in vivo imaging by enzyme-mediated fluorogenic reaction and self-assembly. J. Am. Chem. Soc. 141, 10331–10341 (2019).
Feng, Z. et al. Enzyme-instructed peptide assemblies selectively inhibit bone tumors. Chem. 5, 2442–2449 (2019).
Li, J. et al. New power of self-assembling carbonic anhydrase inhibitor: short peptide–constructed nanofibers inspire hypoxic cancer therapy. Sci. Adv. 5, eaax0937 (2019).
An, H. et al. A tumour-selective cascade activatable self-detained system for drug delivery and cancer imaging. Nat. Commun. 10, 4861 (2019).
Gong, N. et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 15, 1053–1064 (2020).
Pieszka, M. et al. Controlled supramolecular assembly inside living cells by sequential multistaged chemical reactions. J. Am. Chem. Soc. 142, 15780–15789 (2020).
Zhang, L. et al. Transformable peptide nanoparticles arrest HER2 signalling and cause cancer cell death in vivo. Nat. Nanotechnol. 15, 145–153 (2020).
Borkowska, M. et al. Targeted crystallization of mixed-charge nanoparticles in lysosomes induces selective death of cancer cells. Nat. Nanotechnol. 15, 331–341 (2020).
He, H., Liu, S., Wu, D. & Xu, B. Enzymatically formed peptide assemblies sequestrate proteins and relocate inhibitors to selectively kill cancer cells. Angew. Chem. Int. Ed. 59, 16445 (2020).
Song, Y. et al. Self-amplifying assembly of peptides in macrophages for enhanced inflammatory treatment. J. Am. Chem. Soc. 144, 6907–6917 (2022).
Wen, X. et al. Controlled sequential in situ self-assembly and disassembly of a fluorogenic cisplatin prodrug for cancer theranostics. Nat. Commun. 14, 800 (2023).
Guo, J. et al. Hierarchical assembly of intrinsically disordered short peptides. Chem 9, 2530–2546 (2023).
Ji, S. et al. Targeted enrichment of enzyme-instructed assemblies in cancer cell lysosomes turns immunologically cold tumors hot. Angew. Chem. Int. Ed. 60, 26994 (2021).
Li, W. et al. Molecular engineering of pH-responsive NIR oxazine assemblies for evoking tumor ferroptosis via triggering lysosomal dysfunction. J. Am. Chem. Soc. 145, 3736–3747 (2023).
Zhu, S. et al. Lysosomal quality control of cell fate: a novel therapeutic target for human diseases. Cell Death Dis. 11, 817 (2020).
Iulianna, T., Kuldeep, N. & Eric, F. The Achilles’ heel of cancer: targeting tumors via lysosome-induced immunogenic cell death. Cell Death Dis. 13, 509 (2022).
Wang, J. et al. Intracellular condensates of oligopeptide for targeting lysosome and addressing multiple drug resistance of cancer. Adv. Mater. 34, 2104704 (2022).
Wu, C. et al. Lysosome-targeted and fluorescence-turned ‘on’ cytotoxicity induced by alkaline phosphatase-triggered self-assembly. Adv. Healthc. Mater. 11, 2101346 (2022).
Jana, B. et al. Intra-lysosomal peptide assembly for the high selectivity index against cancer. J. Am. Chem. Soc. 145, 18414–18431 (2023).
Peng, J. et al. Weakly perturbative imaging of interfacial water with submolecular resolution by atomic force microscopy. Nat. Commun. 9, 122 (2018).
Egeblad, M. & Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2, 161–174 (2002).
Gamcsik, M. P., Kasibhatla, M. S., Teeter, S. D. & Colvin, O. M. Glutathione levels in human tumors. Biomarkers. 17, 671–691 (2012).
Xie, Z. et al. Cathepsin B in programmed cell death machinery: mechanisms of execution and regulatory pathways. Cell Death Dis. 14, 255 (2023).
Bertheloot, D., Latz, E. & Franklin, B. S. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol. Immunol. 18, 1106–1121 (2021).
Martens, S. et al. MLKL in cancer: more than a necroptosis regulator. Cell Death Differ. 28, 1757–1772 (2021).
Yu, Y. et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 7, 193 (2021).
Bussi, C. et al. Lysosomal damage drives mitochondrial proteome remodelling and reprograms macrophage immunometabolism. Nat. Commun. 13, 7338 (2022).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Curiel, T. et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 9, 562–567 (2003).
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFA0910003, 2020YFA0713801), the National Natural Science Foundation of China (22137003, 22274074), the Natural Science Foundation of Jiangsu Province (BK20232020), the State Key Laboratory of Analytical Chemistry for Life Science (5431ZZXM2408) and the Excellent Research Program of Nanjing University (ZYJH004).
Author information
Authors and Affiliations
Contributions
D.Y. and Junya Zhang conceived the research plan. Junya Zhang and Y.H. synthesized the probes. X.W. established the tumour model. Z.Y. performed the AFM analysis. Z.W. and Z.Y. analysed the AFM data. H.B. and Junya Zhang performed the TEM analysis. Z.F. and Junya Zhang performed the CRISPR–Cas9-mediated knockout experiments. Junya Zhang, Q.X. and T.T. performed the flow cytometric analysis. Junya Zhang and Y.M. performed the in vivo experiments. L.Q. performed the molecular simulation. Junya Zhang, J.L., P.Z., Jingjing Zhang., J.-J.X. and D.Y. analysed the data. Junya Zhang. and D.Y. wrote the paper. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Yuan Gao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–147, Tables 1–7, Materials and Instruments, Methods and References.
Supplementary Data
Source data for quantitative data of western blots.
Source data
Source Data Fig. 2
All numerical source data for statistical analysis, unmodified gels and images.
Source Data Fig. 3
All numerical source data for statistical analysis, unmodified gels and images.
Source Data Fig. 4
All numerical source data for statistical analysis, unmodified gels and images.
Source Data Fig. 5
All numerical source data for statistical analysis, unmodified gels and images.
Source Data Fig. 6
All numerical source data for statistical analysis, unmodified gels and images.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Hu, Y., Wen, X. et al. Tandem-controlled lysosomal assembly of nanofibres induces pyroptosis for cancer immunotherapy. Nat. Nanotechnol. 20, 563–574 (2025). https://doi.org/10.1038/s41565-025-01857-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-01857-9
This article is cited by
-
Targeting pyroptosis for cancer immunotherapy: mechanistic insights and clinical perspectives
Molecular Cancer (2025)
-
Reprogramming of cancer metabolism via photoresponsive nano-PROTAC enhances pyroptosis-mediated immunotherapy
Signal Transduction and Targeted Therapy (2025)
-
Lysosome-dependent cell death: disease implications and potential therapeutic targets
Molecular Biology Reports (2025)