Abstract
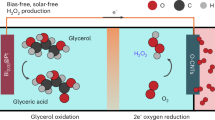
Selective production of valuable glycerol chemicals, such as glycerate (which serves as an important chemical intermediate), poses a significant challenge due to the facile cleavage of C–C bonds and the presence of multiple reaction pathways. This challenge is more severe in the electro-oxidation of glycerol, which requires the development of desirable electrocatalysts. To facilitate the glycerol electro-oxidation reaction to glycerate, here we present an approach utilizing a high-entropy PtCuCoNiMn nanosurface. It exhibits exceptional activity (~200 mA cm−2 at 0.75 V versus a reversible hydrogen electrode) and selectivity (75.2%). In situ vibrational measurements and theoretical calculations reveal that the exceptional glycerol electro-oxidation selectivity and activity can be attributed to the unique characteristics of the high-entropy surface, which effectively modifies the electronic structure of the exposed Pt sites. The catalyst is successfully applied in an electrolyser for long-term glycerol electro-oxidation reaction, demonstrating excellent performance (~200 mA cm−2 at 1.2Vcell) over 210 h. The present study highlights that tailoring the catalytic sites at the catalyst–electrolyte interface by constructing a high-entropy surface is an effective strategy for electrochemical catalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the results and discussions of this study are available in this Article and its Supplementary Information or from the corresponding authors upon reasonable request.
References
Da Silva Ruy, A. D., Ferreira, A. L. F., Bresciani, A. É., de Brito Alves, R. M. & Pontes, L. A. M. Market prospecting and assessment of the economic potential of glycerol from biodiesel. In Biotechnological Applications of Biomass (eds Basso, T. P. et al.) Ch. 11 (IntechOpen Press, 2021).
Yan, Y. et al. Electrocatalytic upcycling of biomass and plastic wastes to biodegradable polymer monomers and hydrogen fuel at high current densities. J. Am. Chem. Soc. 145, 6144–6155 (2023).
Schichtl, Z. G., Conlin, S. K., Mehrabi, H., Nielander, A. C. & Coridan, R. H. Characterizing sustained solar-to-hydrogen electrocatalysis at low cell potentials enabled by crude glycerol oxidation. ACS Appl. Energy Mater. 5, 3863–3875 (2022).
de Souza, M. B. C. et al. Bi-modified Pt electrodes toward glycerol electrooxidation in alkaline solution: effects on activity and selectivity. ACS Catal. 9, 5104–5110 (2019).
Houache, M. S. E. et al. Electrochemical valorization of glycerol on Ni-rich bimetallic NiPd nanoparticles: insight into product selectivity using in situ polarization modulation infrared-reflection absorption spectroscopy. ACS Sustain. Chem. Eng. 7, 14425–14434 (2019).
Wang, Y., Xiao, Y. & Xiao, G. Sustainable value-added C3 chemicals from glycerol transformations: a mini review for heterogeneous catalytic processes. Chinese J. Chem. Eng. 27, 1536–1542 (2019).
Valter, M., dos Santos, E. C., Pettersson, L. G. M. & Hellman, A. Partial electrooxidation of glycerol on close-packed transition metal surfaces: insights from first-principles calculations. J. Phys. Chem. C 124, 17907–17915 (2020).
Dodekatos, G., Schünemann, S. & Tüysüz, H. Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS Catal. 8, 6301–6333 (2018).
Dai, C. et al. Electrochemical production of lactic acid from glycerol oxidation catalyzed by AuPt nanoparticles. J. Catal. 356, 14–21 (2017).
Huang, B. et al. Seeded synthesis of hollow PdSn intermetallic nanomaterials for highly efficient electrocatalytic glycerol oxidation. Adv. Mater. 35, 2302233 (2023).
Yu, X. et al. Electrocatalytic glycerol oxidation with concurrent hydrogen evolution utilizing an efficient MoOx/Pt Catalyst. Small 17, 2104288 (2021).
Sheng, H. et al. Linear paired electrochemical valorization of glycerol enabled by the electro-Fenton process using a stable NiSe2 cathode. Nat. Catal. 5, 716–725 (2022).
Wu, J. et al. Ligand hybridization for electro-reforming waste glycerol into isolable oxalate and hydrogen. Angew. Chem. Int. Ed. 62, e202216083 (2023).
Ma, Y. et al. Reaction mechanism and kinetics for Pt/CNTs catalyzed base-free oxidation of glycerol. Chem. Eng. Sci. 203, 228–236 (2019).
Holade, Y., Morais, C., Servat, K., Napporn, T. W. & Kokoh, K. B. Toward the electrochemical valorization of glycerol: Fourier transform infrared spectroscopic and chromatographic studies. ACS Catal. 3, 2403–2411 (2013).
Jeffery, D. Z. & Camara, G. A. The formation of carbon dioxide during glycerol electrooxidation in alkaline media: first spectroscopic evidences. Electrochem. Commun. 12, 1129–1132 (2010).
Terekhina, I. & Johnsson, M. Improving glycerol electrooxidation performance on nanocubic PtCo Catalysts. ACS Appl. Mater. Interfaces 16, 56987–56996 (2024).
Chen, W. et al. High-entropy intermetallic PtRhBiSnSb nanoplates for highly efficient alcohol oxidation electrocatalysis. Adv. Mater. 34, 2206276 (2022).
Yang, C.-L. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 374, 459–464 (2021).
Cao, G. et al. Liquid metal for high-entropy alloy nanoparticles synthesis. Nature 619, 73–77 (2023).
Feng, G. et al. Engineering structurally ordered high-entropy intermetallic nanoparticles with high-activity facets for oxygen reduction in practical fuel cells. J. Am. Chem. Soc. 145, 11140–11150 (2023).
Xing, F., Ma, J., Shimizu, K. I. & Furukawa, S. High-entropy intermetallics on ceria as efficient catalysts for the oxidative dehydrogenation of propane using CO2. Nat. Commun. 13, 5065 (2022).
Ren, J.-T., Chen, L., Wang, H.-Y. & Yuan, Z.-Y. High-entropy alloys in electrocatalysis: from fundamentals to applications. Chem. Soc. Rev. 52, 8319–8373 (2023).
Li, H. et al. The self-complementary effect through strong orbital coupling in ultrathin high-entropy alloy nanowires boosting pH-universal multifunctional electrocatalysis. Appl. Catal. B 312, 121431 (2022).
Liu, G. et al. Hydrogen-intercalation-induced lattice expansion of Pd@Pt core–shell nanoparticles for highly efficient electrocatalytic alcohol oxidation. J. Am. Chem. Soc. 143, 11262–11270 (2021).
Liu, T. et al. Origin of structural degradation in Li-rich layered oxide cathode. Nature 606, 305–312 (2022).
Liu, L. et al. Structure and performance relationship of silica-supported platinum-tungsten catalysts in selective C-O hydrogenolysis of glycerol and 1,4-anhydroerythritol. Appl. Catal. B 292, 120164 (2021).
Russell, A. E. & Rose, A. X-ray absorption spectroscopy of low temperature fuel cell catalysts. Chem. Rev. 104, 4613–4636 (2004).
Luo, H. et al. Role of Ni in PtNi bimetallic electrocatalysts for hydrogen and value-added chemicals coproduction via glycerol electrooxidation. ACS Catal. 12, 14492–14506 (2022).
Xing, Z., Li, J., Wang, S., Su, C. & Jin, H. Structure engineering of PtCu3/C catalyst from disordered to ordered intermetallic compound with heat-treatment for the methanol electrooxidation reaction. Nano Res. 15, 3866–3871 (2022).
Jia, Q. et al. Improved oxygen reduction activity and durability of dealloyed PtCox catalysts for proton exchange membrane fuel cells: strain, ligand, and particle size effects. ACS Catal. 5, 176–186 (2015).
Jia, Q. et al. Roles of Mo surface dopants in enhancing the ORR performance of octahedral PtNi nanoparticles. Nano Lett. 18, 798–804 (2018).
Liu, Y. et al. Promoting n-butane dehydrogenation over PtMn/SiO2 through structural evolution induced by a reverse water-gas shift reaction. ACS Catal. 12, 13506–13512 (2022).
Reier, T., Oezaslan, M. & Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal. 2, 1765–1772 (2012).
Sheng, W. et al. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 6, 5848 (2015).
Takimoto, D. et al. Platinum nanosheets synthesized via topotactic reduction of single-layer platinum oxide nanosheets for electrocatalysis. Nat. Commun. 14, 19 (2023).
Wu, J., Yang, X. & Gong, M. Recent advances in glycerol valorization via electrooxidation: catalyst, mechanism and device. Chinese J. Catal. 43, 2966–2986 (2022).
Simões, M., Baranton, S. & Coutanceau, C. Electro-oxidation of glycerol at Pd based nano-catalysts for an application in alkaline fuel cells for chemicals and energy cogeneration. Appl. Catal. B 93, 354–362 (2010).
Zhang, W.-Y., Zou, S.-Z. & Cai, W.-B. Recent advances in glycerol electrooxidation on Pt and Pd: from reaction mechanisms to catalytic materials. J. Electrochem. 27, 233–256 (2021).
Vo, T.-G., Ho, P.-Y. & Chiang, C.-Y. Operando mechanistic studies of selective oxidation of glycerol to dihydroxyacetone over amorphous cobalt oxide. Appl. Catal. B 300, 120723 (2022).
Liu, C. et al. Selective electro-oxidation of glycerol to dihydroxyacetone by a non-precious electrocatalyst—CuO. Appl. Catal. B 265, 118543 (2020).
Kuhl, K. P. et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 136, 14107–14113 (2014).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Pang, X. et al. In situ electrochemical reconstitution of CF–CuO/CeO2 for efficient active species generation. Inorg. Chem. 61, 8940–8954 (2022).
Li, Y., Wei, X., Han, S., Chen, L. & Shi, J. MnO2 electrocatalysts coordinating alcohol oxidation for ultra-durable hydrogen and chemical productions in acidic solutions. Angew. Chem. Int. Ed. 60, 21464–21472 (2021).
Vo, T.-G. et al. Au@NiSx yolk@shell nanostructures as dual-functional electrocatalysts for concomitant production of value-added tartronic acid and hydrogen fuel. Adv. Funct. Mater. 33, 2209386 (2023).
Chang, Z., Huo, S., Zhang, W., Fang, J. & Wang, H. The tunable and highly selective reduction products on Ag@Cu bimetallic catalysts toward CO2 electrochemical reduction reaction. J. Phys. Chem. C 121, 11368–11379 (2017).
Bu, L. et al. PtPb/PtNi intermetallic core/atomic layer shell octahedra for efficient oxygen reduction electrocatalysis. J. Am. Chem. Soc. 139, 9576–9582 (2017).
Acknowledgements
We acknowledge financial support from the National Key Research and Development Program of China (no. 2023YFB4005900); National Natural Science Foundation of China (nos. 52271232 and 52171022); the Ministry of Industry and Information Technology of the People's Republic of China (no. 2024ZD0607700); Ningbo Youth Science and Technology Leading Talents Project (no. 2023QL026); Natural Science Foundation of Zhejiang Province (no. LY21E020008); the Ningbo S&T Innovation 2025 Major Special Program (no. 2022Z205); Youth Innovation Promotion Association, CAS (no. 2020300); and Natural Science Foundation of Ningbo City (no. 2023J253).
Author information
Authors and Affiliations
Contributions
Y. Lin, H. Yin and L.C. conceived the project design and supervised the research. Shuibo Wang and Y. Lin prepared the samples; measured their electrochemical properties; carried out the X-ray diffraction, X-ray photoelectron spectroscopy, TEM and in situ attenuated total reflectance FTIR characterizations; and analysed the data. Y.W. performed the XAS measurements. B.N. and K.J. performed the in situ Raman measurements. Y. Li and Z.T. performed the DFT calculations. Shuibo Wang and Y. Lin wrote the manuscript. L.C., Z.T. and H. Yin provided helpful suggestions, and revised the manuscript. Z.L., H. Yu and Shiwei Wang helped with the discussion of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Yan Chen, Hui Luo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
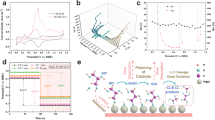
Extended Data Fig. 1 Decay mechanism investigation.
a, A multi-steps CA testing curve for probing the GEOR activity decay. b, Chronoamperometric measurements of PtCuCoNiMn-EC using interval CA strategies with intermittent potential at 0.8 V vs. RHE in 1 M KOH with 0.1 M glycerol at r.t. over 6 h. c, Conversion rate of glycerol at different potentials in 1 M KOH with 0.1 M glycerol over Pt-EC and PtCuCoNiMn-EC. d, Faradaic efficiency for glycerate at multiple potentials over PtCuCoNiMn-EC. e, Conversion of glycerol during GEOR with time in 1 M KOH with 0.01 M glycerol over PtCuCoNiMn-EC.
Supplementary information
Supplementary Information
Supplementary Figs. 1–82, Tables 1–7, Notes 1–8, Diagram 1, characterization and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Lin, Y., Li, Y. et al. Nanoscale high-entropy surface engineering promotes selective glycerol electro-oxidation to glycerate at high current density. Nat. Nanotechnol. 20, 646–655 (2025). https://doi.org/10.1038/s41565-025-01881-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-01881-9