Abstract
Polymer electrolytes hold great promise for safe and high-energy batteries comprising solid or semi-solid electrolytes. Multiphase polymer electrolytes, consisting of mobile and rigid phases, exhibit fast ion conduction and desired mechanical properties. However, fundamental challenges exist in understanding and regulating interactions at the electrode|electrolyte interface, especially when using high-potential layered oxide active materials at the positive electrode. Here we demonstrate that depletion of the mobile conductive phase at the interface contributes to battery performance degradation. Molecular ionic composite electrolytes, composed of a rigid-rod ionic polymer with nanometric mobile cations and anions, serve as a multiphase platform to investigate the evolution of ion conductive domains at the interface. Chemical and structural characterizations enable the visualization of concentration heterogeneity and spatially resolve the interfacial chemical states over a statistically significant field of view for buried interfaces. We report that concentration and chemical heterogeneities prevail at electrode|electrolyte interfaces, leading to phase separation in polymer electrolytes. Understanding the hidden roles of interfacial chemomechanics in polymer electrolytes enables us to design an interphase tailoring strategy based on electrolyte additives to mitigate the interfacial heterogeneity and improve battery performance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the published article and its Supplementary Information. Source data are provided with this paper.
References
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 1–16 (2017).
Wan, J. et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 14, 705–711 (2019).
Choudhury, S. Solid-state polymer electrolytes for high-performance lithium metal batteries. Nat. Commun. 10, 4398 (2019).
Christie, A. M., Lilley, S. J., Staunton, E., Andreev, Y. G. & Bruce, P. G. Increasing the conductivity of crystalline polymer electrolytes. Nature 433, 50–53 (2005).
Dong, T. et al. A multifunctional polymer electrolyte enables ultra-long cycle-life in a high-voltage lithium metal battery. Energy Environ. Sci. 11, 1197–1203 (2018).
Zhao, Q., Liu, X., Stalin, S., Khan, K. & Archer, L. A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 4, 365–373 (2019).
Hatzell, K. B. et al. Challenges in lithium metal anodes for solid-state batteries. ACS Energy Lett. 5, 922–934 (2020).
Wang, X. et al. Toward high-energy-density lithium metal batteries: opportunities and challenges for solid organic electrolytes. Adv. Mater. 32, 1905219 (2020).
Glynos, E., Pantazidis, C. & Sakellariou, G. Designing all-polymer nanostructured solid electrolytes: advances and prospects. ACS Omega 5, 2531–2540 (2020).
Lu, G. et al. Trade-offs between ion-conducting and mechanical properties: the case of polyacrylate electrolytes. Carbon Energy 5, e287 (2023).
Gu, Y. et al. High toughness, high conductivity ion gels by sequential triblock copolymer self-assembly and chemical cross-linking. J. Am. Chem. Soc. 135, 9652–9655 (2013).
Cho, B. K., Jain, A., Gruner, S. M. & Wiesner, U. Mesophase structure-mechanical and ionic transport correlations in extended amphiphilic dendrons. Science 305, 1598–1601 (2004).
Grundy, L. S. et al. Inaccessible polarization-induced phase transitions in a block copolymer electrolyte: an unconventional mechanism for the limiting current. Macromolecules 55, 7637–7649 (2022).
Galluzzo, M. D., Loo, W. S., Schaible, E., Zhu, C. & Balsara, N. P. Dynamic structure and phase behavior of a block copolymer electrolyte under dc polarization. ACS Appl. Mater. Interfaces 12, 57421–57430 (2020).
Virgili, J. M., Nedoma, A. J., Segalman, R. A. & Balsara, N. P. Ionic liquid distribution in ordered block copolymer solutions. Macromolecules 43, 3750–3756 (2010).
Gomez, E. D. et al. Effect of ion distribution on conductivity of block copolymer electrolytes. Nano Lett. 9, 1212–1216 (2009).
Choi, J. H., Ye, Y., Elabd, Y. A. & Winey, K. I. Network structure and strong microphase separation for high ion conductivity in polymerized ionic liquid block copolymers. Macromolecules 46, 5290–5300 (2013).
Koerver, R. et al. Chemo-mechanical expansion of lithium electrode materials—on the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 11, 2142–2158 (2018).
Lewis, J. A. et al. Interphase morphology between a solid-state electrolyte and lithium controls cell failure. ACS Energy Lett. 4, 591–599 (2019).
Lewis, J. A. et al. Linking void and interphase evolution to electrochemistry in solid-state batteries using operando X-ray tomography. Nat. Mater. 20, 503–510 (2021).
Tippens, J. et al. Visualizing chemomechanical degradation of a solid-state battery electrolyte. ACS Energy Lett. 4, 1475–1483 (2019).
Lewis, J. A., Tippens, J., Cortes, F. J. Q. & McDowell, M. T. Chemo-mechanical challenges in solid-state batteries. Trends Chem. 1, 845–857 (2019).
Sharon, D. et al. Molecular level differences in ionic solvation and transport behavior in ethylene oxide-based homopolymer and block copolymer electrolytes. J. Am. Chem. Soc. 143, 3180–3190 (2021).
Chintapalli, M. et al. Structure and ionic conductivity of polystyrene-block-poly(ethylene oxide) electrolytes in the high salt concentration limit. Macromolecules 49, 1770–1780 (2016).
Shen, K. H. & Hall, L. M. Ion conductivity and correlations in model salt-doped polymers: effects of interaction strength and concentration. Macromolecules 53, 3655–3668 (2020).
Lee, Y., Ma, B. & Bai, P. Overlimiting ion transport dynamic toward Sand’s time in solid polymer electrolytes. Mater. Today Energy 27, 101037 (2022).
Lee, Y., Ma, B. & Bai, P. Concentration polarization and metal dendrite initiation in isolated electrolyte microchannels. Energy Environ. Sci. 13, 3504–3513 (2020).
Cheng, Q. et al. Operando and three-dimensional visualization of anion depletion and lithium growth by stimulated Raman scattering microscopy. Nat. Commun. 9, 2942 (2018).
Devaux, D. et al. Failure mode of lithium metal batteries with a block copolymer electrolyte analyzed by X-ray microtomography. J. Electrochem. Soc. 162, A1301–A1309 (2015).
Kaboli, S. et al. Behavior of solid electrolyte in Li-polymer battery with NMC cathode via in-situ scanning electron microscopy. Nano Lett. 20, 1607–1613 (2020).
Harry, K. J., Hallinan, D. T., Parkinson, D. Y., MacDowell, A. A. & Balsara, N. P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 13, 69–73 (2013).
Golozar, M. et al. In situ scanning electron microscopy detection of carbide nature of dendrites in Li-polymer batteries. Nano Lett. 18, 7583–7589 (2018).
Maslyn, J. A. et al. Growth of lithium dendrites and globules through a solid block copolymer electrolyte as a function of current density. J. Phys. Chem. C 122, 26797–26804 (2018).
Harry, K. J., Liao, X., Parkinson, D. Y., Minor, A. M. & Balsara, N. P. Electrochemical deposition and stripping behavior of lithium metal across a rigid block copolymer electrolyte membrane. J. Electrochem. Soc. 162, A2699–A2706 (2015).
Andersson, E. K. W. et al. Early-stage decomposition of solid polymer electrolytes in Li-metal batteries. J. Mater. Chem. A 9, 22462–22471 (2021).
Zhang, X. et al. Multi-scale characterization techniques for polymer-based solid-state lithium batteries. Macromol. Chem. Phys. 224, 2200351 (2023).
Bostwick, J. E. et al. Ionic interactions control the modulus and mechanical properties of molecular ionic composite electrolytes. J. Mater. Chem. C 10, 947–957 (2022).
Yu, D. et al. Room temperature to 150 °C lithium metal batteries enabled by a rigid molecular ionic composite electrolyte. Adv. Energy Mater. 11, 2003559 (2021).
Fox, R. J. et al. Nanofibrillar ionic polymer composites enable high-modulus ion-conducting membranes. ACS Appl. Mater. Interfaces 11, 40551–40563 (2019).
Wang, Y. et al. Highly conductive and thermally stable ion gels with tunable anisotropy and modulus. Adv. Mater. 28, 2571–2578 (2016).
Bostwick, J. E. et al. Ion transport and mechanical properties of non-crystallizable molecular ionic composite electrolytes. Macromolecules 53, 1405–1414 (2020).
Wang, Y. et al. Solid-state rigid-rod polymer composite electrolytes with nanocrystalline lithium ion pathways. Nat. Mater. 20, 1255–1263 (2021).
Wang, Y. Double helical conformation and extreme rigidity in a rodlike polyelectrolyte. Nat. Commun. 10, 801 (2019).
Yu, Z., He, Y., Wang, Y., Madsen, L. A. & Qiao, R. Molecular structure and dynamics of ionic liquids in a rigid-rod polyanion-based ion gel. Langmuir 33, 322–331 (2017).
Forsyth, M., Porcarelli, L., Wang, X., Goujon, N. & Mecerreyes, D. Innovative electrolytes based on ionic liquids and polymers for next-generation solid-state batteries. Acc. Chem. Res. 52, 686–694 (2019).
Hasanpoor, M. et al. Morphological evolution and solid-electrolyte interphase formation on LiNi0.6Mn0.2Co0.2O2 cathodes using highly concentrated ionic liquid electrolytes. ACS Appl. Mater. Interfaces 14, 13196–13205 (2022).
Yu, D., Zanelotti, C. J., Fox, R. J., Dingemans, T. J. & Madsen, L. A. Solvent-cast solid electrolyte membranes based on a charged rigid-rod polymer and ionic liquids. ACS Appl. Energy Mater. 4, 6599–6605 (2021).
Dong, Q. et al. Insights into the dual role of lithium difluoro(oxalato)borate additive in improving the electrochemical performance of NMC811||graphite cells. ACS Appl. Energy Mater. 3, 695–704 (2020).
Gao, H., Maglia, F., Lamp, P., Amine, K. & Chen, Z. Mechanistic study of electrolyte additives to stabilize high-voltage cathode-electrolyte interface in lithium-ion batteries. ACS Appl. Mater. Interfaces 9, 44542–44549 (2017).
Swiderska-Mocek, A. & Gabryelczyk, A. Interfacial stabilizing effect of lithium borates and pyrrolidinium ionic liquid in gel polymer electrolytes for lithium-metal batteries. J. Phys. Chem. C 127, 18875–18890 (2023).
Yu, X. et al. Direct observation of the redistribution of sulfur and polysulfides in Li-S batteries during first cycle by in situ X-ray fluorescence microscopy. Adv. Energy Mater. 5, 1500072 (2015).
Freiberg, A. T. S. et al. Species in lithium-sulfur batteries using spatially resolved operando X-ray absorption spectroscopy and X-ray fluorescence mapping. J. Phys. Chem. C 122, 5303–5316 (2018).
Sun, B. et al. At the polymer electrolyte interfaces: the role of the polymer host in interphase layer formation in Li-batteries. J. Mater. Chem. A 3, 13994–14000 (2015).
Vairavamurthy, A. Using X-ray absorption to probe sulfur oxidation states in complex molecules. Spectrochim. Acta A 54, 2009–2017 (1998).
Lin, Z. et al. High-performance lithium/sulfur cells with a bi-functionally immobilized sulfur cathode. Nano Energy 9, 408–416 (2014).
Pickering, I. J., Prince, R. C., Divers, T. & George, G. N. Sulfur K-edge X-ray absorption spectroscopy for determining the chemical speciation of sulfur in biological systems. FEBS Lett. 441, 11–14 (1998).
Dey, A. et al. Sulfur K-edge XAS and DFT calculations on nitrile hydratase: geometric and electronic structure of the non-heme iron active site. J. Am. Chem. Soc. 128, 533–541 (2006).
Dezarnaud, C., Tronc, M. & Hitchcock, A. P. Inner shell spectroscopy of the carbon—sulfur bond. Chem. Phys. 142, 455–462 (1990).
Jalilehvand, F. Sulfur: not a “silent” element any more. Chem. Soc. Rev. 35, 1256–1268 (2006).
Acknowledgements
This work was primarily supported by the US Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE) under award number DE-EE0008860 (F.L. and L.A.M.). Part of the work was also supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy through the Advanced Battery Materials Research (BMR) Program (Battery500 Consortium) under contract number 683639 (F.L. and L.A.M.). F.L. and L.A.M. also acknowledge the seedling support from the Virginia Tech College of Science Strategic Initiative in Energy (03400). This work used shared facilities at the Virginia Tech Nanoscale Characterization and Fabrication Laboratory (NCFL) and Surface Analysis Laboratory, supported by the National Science Foundation (NSF) under grant number CHE-1531834. This research used 8-BM of the National Synchrotron Light Source II (NSLS-II), which is a US Department of Energy Office of Science User Facility at Brookhaven National Laboratory under contract number DE-SC0012704. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract number DE-AC02-06CH11357. NMC811 was produced at the US Department of Energy’s (DOE) CAMP (Cell Analysis, Modeling, and Prototyping) Facility, Argonne National Laboratory. The CAMP Facility is fully supported by the DOE Vehicle Technologies Program (VTP) within the core funding of the Applied Battery Research (ABR) for Transportation Program. We thank M. Hedge and T. J. Dingemans (University of North Carolina-Chapel Hill) and D. Yu (Virginia Tech) for discussions. We also thank M. Ashraf-Khorasani for chromatography analysis and discussions.
Author information
Authors and Affiliations
Contributions
F.L. conceived and led the project. F.L. and J.M. designed the experiments. J.M. performed the materials synthesis, electrochemical measurements and characterizations. J.M., S.-M.B. and Y.D. performed the synchrotron X-ray characterization. Y.Z. and M.Y. assisted with the membrane processing. D.X. and L.T. helped with data analysis. J.A.R. and H.X. conducted ex situ atomic force microscopy. N.F.P. and L.A.M. conducted the pulsed-field-gradient NMR diffusiometry and participated in scientific discussions. Z.D. and L.L. conducted synchrotron X-ray micro-computed tomography. J.M. and F.L. analysed all the data and wrote the paper with the assistance of L.A.M. and S.-M.B. All authors approved the paper for publication.
Corresponding author
Ethics declarations
Competing interests
Part of the results in this paper is included in a patent application (application no. 63/734,312) filed by some co-authors (J.M., L.A.M. and F.L.).
Peer review
Peer review information
Nature Nanotechnology thanks Xin Guo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
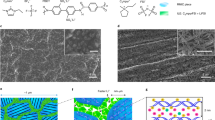
Extended Data Fig. 1 Graphic representation of synchrotron X-ray measurements to investigate interfacial degradation in polymer electrolyte-based cells.
Schematic showing synchrotron X-ray measurements of a solid-state battery cross-section, which combines X-ray fluorescence (XRF) microscopy and X-ray absorption spectroscopy (XAS) measurements to visualize ionic concentration and probe chemical states across buried interfaces of solid-state battery components. Sulfur species from IL (TFSI−) and PBDT polymer (-SO3−) are present in the MIC electrolyte. Tracking the sulfur species of polymer electrolytes with XRF mapping reveals local ionic concentration heterogeneities, and spatially resolved XAS analysis informs the evolution of new sulfur species from interfacial side reactions by probing the changes in the oxidation states of sulfur elements therein. From the right panel of the XRF map, the green area represents the regions containing sulfur species, the black areas indicate regions without sulfur species, and the red area represents the sample holder (see also Spatially resolved XRF/XAS measurement and Sample preparation for synchrotron measurements from Methods). The point scanning XAS on the cross-sectional sample probes sulfur chemical states across electrode|electrolyte interfaces with a spatial resolution of a few micrometers (Right part, point 1).
Supplementary information
Supplementary Information
Supplementary Figs. 1–33 and Notes 1–10.
Source data
Source Data Figs. 2–6
Statistical source data of Figs. 2–6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Min, J., Bak, SM., Zhang, Y. et al. Investigating the effect of heterogeneities across the electrode|multiphase polymer electrolyte interfaces in high-potential lithium batteries. Nat. Nanotechnol. 20, 787–797 (2025). https://doi.org/10.1038/s41565-025-01885-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-01885-5
This article is cited by
-
Engineering thin 3D Li-composite foil negative electrodes with high mechanical toughness
Nature Communications (2026)
-
Uncovering interfacial instability: How phase separation in polymer electrolytes undermines battery performance?
Science China Materials (2025)