Abstract
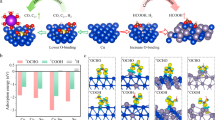
The electrochemical CO reduction reaction (CORR) has attracted a surge of research interest in sustainably producing high-value multi-carbon products, such as acetate. Nevertheless, most current CORR catalysts exhibit low acetate current densities, poor longevity and limited acetate selectivity. Here we present a Zeolite Socony Mobil-confined Cu single-atom cluster (CuZSM SACL) for CORR, in which Cu SAs are chemically anchored via robust Cu–O–Si bonds while Cu CLs are physically trapped within the porous framework of zeolite cavities. Consequently, the CuZSM SACL-containing membrane electrode assembly enables a remarkable CO-to-acetate current density of 1.8 A cm−2 with a high acetate Faraday efficiency of 71 ± 3%. More importantly, we demonstrate that the Cu-based membrane electrode assembly can stably catalyse CO to acetate at an industrial current density of 1 A cm−2 at 2.7 V (Faraday efficiency 61 ± 5%) beyond 1,000 h at atmospheric pressure. This milestone sheds light on high-performing Cu-type catalysts for practical CORR applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the provided Source data and the Supplementary Information. Additional data are also available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
The computational codes used in this work are available from the corresponding authors upon reasonable request.
References
Overa, S. et al. Enhancing acetate selectivity by coupling anodic oxidation to carbon monoxide electroreduction. Nat. Catal. 5, 738–745 (2022).
Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
Wang, H. et al. CO2 electrolysis towards acetate: a review. Curr. Opin. Electrochem. 39, 101253 (2023).
Qian, Q., Zhang, J., Cui, M. & Han, B. Synthesis of acetic acid via methanol hydrocarboxylation with CO2 and H2. Nat. Commun. 7, 11481 (2016).
Jouny, M., Hutchings, G. S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2, 1062–1070 (2019).
Zheng, T. et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 5, 288–396 (2022).
Rong, Y. et al. Directing the selectivity of CO electrolysis to acetate by constructing metal-organic interfaces. Angew. Chem. Int. Ed. 62, e202309893 (2023).
Wang, X. et al. Site-selective protonation enables efficient carbon monoxide electroreduction to acetate. Nat. Commun. 15, 616 (2024).
Yang, T. et al. Interfacial synergy between the Cu atomic layer and CeO2 promotes CO electrocoupling to acetate. ACS Nano 17, 8521–8529 (2023).
Yan, X. et al. Synergy of Cu/C3N4 interface and Cu nanoparticles dual catalytic regions in electrolysis of CO to acetic acid. Angew. Chem. Int. Ed. 62, e202301507 (2023).
Hendrik, H. H. et al. The mechanism for acetate formation in electrochemical CO(2) reduction on Cu: selectivity with potential, pH, and nanostructuring. Energy Environ. Sci. 15, 3978 (2022).
Kim, J. Y. T., Sellers, C., Hao, S., Senftle, T. P. & Wang, H. Different distributions of multi-carbon products in CO2 and CO electroreduction under practical reaction conditions. Nat. Catal. 6, 1115–1124 (2023).
Wei, P. et al. Coverage-driven selectivity switch from ethylene to acetate in high-rate CO2/CO electrolysis. Nat. Nanotechnol. 18, 299–306 (2023).
Chang, B. et al. Electrochemical reduction of carbon dioxide to multicarbon (C2+) products: challenges and perspectives. Energy Environ. Sci. 16, 4714 (2023).
Li, J. et al. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. Nat. Catal. 2, 1124–1131 (2019).
Niu, W. et al. Pb-rich Cu grain boundary sites for selective CO-to-n-propanol electroconversion. Nat. Commun. 14, 4882 (2023).
Song, Y., Zhang, X., Xie, K., Wang, G. & Bao, X. High-temperature CO2 electrolysis in solid oxide electrolysis cells: developments, challenges, and prospects. Adv. Mater. 31, 1902033 (2019).
Wu, Y., Jiang, Z., Lu, X., Liang, Y. & Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 575, 639–642 (2019).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
Choi, C. et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat. Catal. 3, 804–812 (2020).
Wu, Z. Z. et al. Gerhardtite as a precursor to an efficient CO-to-acetate electroreduction catalyst. J. Am. Chem. Soc. 145, 24338–24348 (2023).
Ji, Y. et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 5, 251–258 (2022).
Jin, J. et al. Constrained C2 adsorbate orientation enables CO-to-acetate electroreduction. Nature 617, 724–729 (2023).
Ma, G. et al. A hydrophobic Cu/Cu2O sheet catalyst for selective electroreduction of CO to ethanol. Nat. Commun. 14, 501 (2023).
Fang, M. et al. Hydrophobic, ultrastable Cuδ+ for robust CO2 electroreduction to C2 products at ampere-current levels. J. Am. Chem. Soc. 145, 11323–11332 (2023).
Jin, Z. et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 367, 193–197 (2020).
Yang, T. et al. Coordination tailoring of Cu single sites on C3N4 realizes selective CO2 hydrogenation at low temperature. Nat. Commun. 12, 6022 (2021).
Zheng, T. et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 16, 1386–1393 (2021).
Li, J. et al. Electrokinetic and in situ spectroscopic investigations of CO electrochemical reduction on copper. Nat. Commun. 12, 3264 (2021).
Chen, N. et al. High-performance anion exchange membrane water electrolyzers with a current density of 7.68 A cm−2 and a durability of 1000 hours. Energy Environ. Sci. 14, 6338–6348 (2021).
Delmo, E. P. et al. In situ infrared spectroscopic evidence of enhanced electrochemical CO2 reduction and C–C coupling on oxide-derived copper. J. Am. Chem. Soc. 46, 1935–1945 (2024).
Li, J. et al. Selective CO2 electrolysis to CO using isolated antimony alloyed copper. Nat. Commun. 14, 340 (2023).
Wen, Y. et al. Cu7S4 nanosheets enriched with Cu–S bond for highly active and selective CO2 electroreduction to formate. J. Mater. Chem. A 11, 10823–10827 (2023).
Jiang, H. et al. Light-driven CO2 methanation over Au-grafted Ce0.95Ru0.05O2 solid-solution catalysts with activities approaching the thermodynamic limit. Nat. Catal. 6, 519–530 (2023).
Cai, J. et al. Highly selective electrochemical reduction of CO2 into methane on nanotwinned Cu. J. Am. Chem. Soc. 145, 9136–9143 (2023).
Zhuansun, M. et al. Promoting CO2 electroreduction to multi-carbon products by hydrophobicity-induced electro-kinetic retardation. Angew. Chem. Int. Ed. 62, e202309875 (2023).
Qi, G. et al. Au-ZSM-5 catalyses the selective oxidation of CH4 to CH3OH and CH3COOH using O2. Nat. Catal. 5, 45–54 (2022).
Wang, P. et al. Phase and structure engineering of copper tin heterostructures for efficient electrochemical carbon dioxide reduction. Nat. Commun. 9, 4933 (2018).
Ren, W., Ma, W. & Hu, X. Tailored water and hydroxide transport at a quasi-two-phase interface of membrane electrode assembly electrolyzer for CO electroreduction. Joule 7, 2349–2360 (2023).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251 (1994).
Kresse, G. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2022YFA1504500 and 2024YFA1509500 to X.H.), the National Natural Science Foundation of China (22025108, U21A20327 and 22121001 to X.H.), the National Natural Science Foundation of China (22475181 to N.C.), the Fundamental Research Funds for the Central Universities of China (20720240059 to N.C.), the Postdoctoral Fellowship Program of CPSF (GZB20240393 to C.Z.), the start-up funding from Xiamen University National Key Research, the National Key Research and Development Program of China (2021YFB2500303 to D.S.), the National Natural Science Foundation of China (22075317, U21A20328, 22105220 and 52101277 to D.S.), the Strategic Priority Research Program (B) (XDB33030200 to D.S.) and the Project Funded by China Postdoctoral Science Foundation (2021M703457 to X.L.). We thank beamline BL14W1 (Shanghai Synchrotron Radiation Facility) for providing the beam time. We acknowledge support from the Max Planck-POSTECH-Hsinchu Center for Complex Phase Materials.
Author information
Authors and Affiliations
Contributions
X.H. and N.C. conceived and supervised the research. Y.W., C.Z. and J.L. designed and conducted the experiments. N.C., Y.W., C.Z. and J.L. wrote the draft. N.C. and X.H. edited the paper. J.H. conducted the DFT calculations. D.S., X.L. and C.Z. performed the AC-HAADF-STEM measurements. C.Z., S.L., J.L., T.Y. and W.L. helped for the materials synthesis. C.Z., C.-W.K., Y.-C.H., T.-S.C. and Z.H. performed the XAS analysis. X.Z. performed in situ ATR-FTIR testing. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–37 and Tables 1–13.
Supplementary Data 1
1H NMR spectrum of the electrolyte to analyse liquid products after CORR testing in a flow cell in 1 M KOH.
Supplementary Data 2
1H NMR spectrum of the electrolyte to analyse the liquid products after CORR in MEA.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, Y., Zhan, C., Liu, J. et al. Zeolite-confined Cu single-atom clusters stably catalyse CO to acetate at 1 A cm−2 beyond 1,000 h. Nat. Nanotechnol. 20, 656–663 (2025). https://doi.org/10.1038/s41565-025-01892-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-01892-6