Abstract
Cancer immunotherapy utilizing cytotoxic T lymphocytes has demonstrated significant promise in clinical applications, but cancer immunosuppressive mechanisms hamper further progress in T cell immunotherapy. Here we show a correlation between cancer cell mitochondrial content and their resistance to immunotherapy. Observing that cancer cells with higher mitochondrial content show increased resistance to CD8+ T cells, we developed mitochondrial nanoinducers designed to selectively target and degrade mitochondria within autophagosomes. The direct degradation of mitochondria not only enhances the recognition and activation of CD8+ T cells but also increases the susceptibility of cancer cells to CD8+ T cell-mediated cytotoxicity. We demonstrated the feasibility and efficacy of this strategy in multiple in vitro and in vivo tumour therapeutic models. This nanoinducer, designed to manipulate cellular mitochondrial degradation, holds promise as a versatile tool for enhancing adoptive T cell therapy, CAR-T cell therapy and tumour-vaccine-based immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. There are no data from third-party or publicly available datasets. Raw data of non-targeted metabolomics analysis are deposited in MetaboLights and are accessible through accession number MTBLS12284. Due to the very large file sizes and volume of data, the remaining raw data are available from the corresponding author on reasonable request. Source data are available with this paper.
References
Nagata, S. & Tanaka, M. Programmed cell death and the immune system. Nat. Rev. Immunol. 17, 333–340 (2017).
Lu, B. & Finn, O. T-cell death and cancer immune tolerance. Cell Death Differ. 15, 70–79 (2008).
Philip, M. & Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Morotti, M. et al. Promises and challenges of adoptive T-cell therapies for solid tumours. Br. J. Cancer 124, 1759–1776 (2021).
Flugel, C. L. et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 20, 49–62 (2023).
Baker, D. J., Arany, Z., Baur, J. A., Epstein, J. A. & June, C. H. CAR T therapy beyond cancer: the evolution of a living drug. Nature 619, 707–715 (2023).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167 (2020).
Ikeda, H. et al. Immune evasion through mitochondrial transfer in the tumour microenvironment. Nature 638, 225–236 (2025).
Saha, T. et al. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat. Nanotechnol. 17, 98–106 (2022).
Elia, I. et al. Tumor cells dictate anti-tumor immune responses by altering pyruvate utilization and succinate signaling in CD8+ T cells. Cell Metab. 34, 1137–1150. e1136 (2022).
Kovács, S. A., Fekete, J. T. & Győrffy, B. Predictive biomarkers of immunotherapy response with pharmacological applications in solid tumors. Acta Pharmacol. Sin. 44, 1879–1889 (2023).
Goode, A. et al. Defective recognition of LC3B by mutant SQSTM1/p62 implicates impairment of autophagy as a pathogenic mechanism in ALS-FTLD. Autophagy 12, 1094–1104 (2016).
Johansen, T. & Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 7, 279–296 (2011).
Guo, X. et al. Mito-bomb: targeting mitochondria for cancer therapy. Adv. Mater. 33, e2007778 (2021).
Kumar, P., Nagarajan, A. & Uchil, P. D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018, 465–468 (2018).
Jia, F. et al. Stabilizing RNA nanovaccines with transformable hyaluronan dynamic hydrogel for durable cancer immunotherapy. Adv. Funct. Mater. 33, 2204636 (2023).
Yamashita, A. et al. The actions and metabolism of lysophosphatidylinositol, an endogenous agonist for GPR55. Prostaglandins Other Lipid Mediat. 107, 103–116 (2013).
Zhou, X.-L., Guo, X., Song, Y.-P., Zhu, C.-Y. & Zou, W. The LPI/GPR55 axis enhances human breast cancer cell migration via HBXIP and p-MLC signaling. Acta Pharmacol. Sin. 39, 459–471 (2018).
Raskov, H., Orhan, A., Christensen, J. P. & Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 124, 359–367 (2021).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52, 17–35 (2020).
Leone, P. et al. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. JNCI: J. Natl Cancer Inst. 105, 1172–1187 (2013).
Acknowledgements
This work was financially supported by the Fundamental Research Center Project (T2288102), the Research Grant (52361145850) of the National Natural Science Foundation of China and the Joint Project of Pinnacle Disciplinary Group, the Second Affiliated Hospital of Chongqing Medical. We thank the Core Facility of the Center of Biomedical Analysis, Tsinghua University, for assistance with confocal microscopy.
Author information
Authors and Affiliations
Contributions
H.W. conceived the project. H.W., X.P., G.N., M.T. and Z.W. analysed the data and wrote the manuscript. Z.W. and X.P. conducted all the experiments with help from M.T. and Z.F. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Jianliang Shen and Luca Simula for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
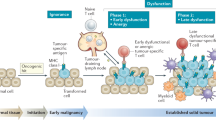
Extended Data Fig. 1 Schematic representation of mitoNID-mediated mitochondria degradation for enhanced CD8+ T cell therapy.
Treatment with mitoNIDs facilitates mitophagy by promoting the proximity between mitochondria and autophagy-related protein LC3, consequently stimulating mitochondrial degradation within tumor cells. This process elevates the level of the metabolite LPI, triggering the activation of the GPR55-ErK signaling pathway, thereby fostering the proliferation of CD8+ T cells. Furthermore, mitochondrial degradation leads to the upregulation of MHC-I expression and the downregulation of BCL-XL expression in tumor cells, consequently enhancing tumor cell recognition and sensitivity by CD8+ T cells. In vivo immunotherapy strategies, including adoptive T-cell therapy, CAR T-cell therapy, and mRNA cancer vaccines, were validated for the enhanced therapeutic outcomes through the co-administration of mitoNIDs.
Extended Data Fig. 2 MitoNIDs boost the killing ability of CD8+ T cells against tumor cells.
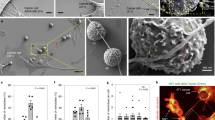
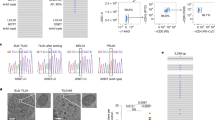
a, Schematic diagram illustrating the experimental process of mitoNIDs enhancing CD8+ T cell toxicity. b, Images of B16-OVA tumor cells after treatment with various E:T ratios of OT-1 CD8+ T cells with or without mitoNIDs. Scale bar, 50 μm. c, LDH release in the culture supernatant. d, The degree of apoptosis of B16-OVA tumor cells detected by flow cytometry. e, Cell viability of OT-1 CD8+ T cell-treated B16-OVA tumor cells. f, Immunoblots showing cleaved caspase-3 in B16-OVA tumor cells after treatment with mitoNIDs and OT-1 CD8+ T cells. g, Images of A549 tumor cells after treatment with EGFR-CAR-T cells with or without mitoNIDs. Scale bar, 50 μm. h, The degree of apoptosis of A549 tumor cells detected by flow cytometry. i, Cell viability of EGFR-CAR-T cell-treated A549 tumor cells. j, Schematic diagram illustrating the experimental process of mitoNIDs overcoming the tolerance of tumor cells to CD8+ T cells. k, Images of B16-OVA tumor cells after treatment with OT-1 CD8+ T cells with or without mitoNIDs. Scale bar, 50 μm. l, LDH release in the culture supernatant. m, The degree of apoptosis of B16-OVA cells detected by flow cytometry. n, Cell viability of B16-OVA tumor cells. o, Immunoblots showing cleaved caspase-3 in B16-OVA cells after treatment with mitoNIDs and OT-1 CD8+ T cells. p, Microscopic images of A549 tumor cells after treatment with EGFR-CAR-T cells with or without mitoNIDs. Scale bar, 50 μm. q, The degree of apoptosis of A549 tumor cells detected by flow cytometry. r, Cell viability of EGFR-CAR-T cell-treated A549 tumor cells. All data are shown as the mean ± s.d. (n = 3 biologically independent cell samples). Two-way ANOVA with Šídák’s multiple comparison was used to calculate statistical differences in c, d, e, h, i, l, m, n, q and r.
Supplementary information
Supplementary Information
Supplementary Figs. 1–57, Tables 1, experimental procedures, unprocessed western blots and references.
Supplementary Data
Statistical source data.
Source data
Source Data Fig. 1
Statistical source data and unprocessed western blots.
Source Data Fig. 1
Statistical source data and unprocessed western blots.
Source Data Fig. 2
Statistical source data and unprocessed western blots.
Source Data Fig. 2
Statistical source data and unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data and unprocessed western blots.
Source Data Fig. 6
Statistical source data and unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data and unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data and unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, X., Wang, Z., Tan, M. et al. Nanoinducer-mediated mitochondria-selective degradation enhances T cell immunotherapy against multiple cancers. Nat. Nanotechnol. 20, 947–958 (2025). https://doi.org/10.1038/s41565-025-01909-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-01909-0
This article is cited by
-
Recent advances in targeting protein degradation for tumor immunotherapy
Journal of Hematology & Oncology (2025)
-
Degrading cancer cell mitochondria to improve T cell-mediated killing
Nature Nanotechnology (2025)
-
Mitochondrial metabolic reprogramming in colorectal cancer: mechanisms of resistance and future clinical interventions
Cell Death Discovery (2025)
-
Research on the interactive mechanisms between mitochondrial variations and immune responses in gliomas based on integrated visualization analysis
Discover Oncology (2025)