Abstract
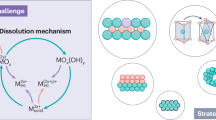
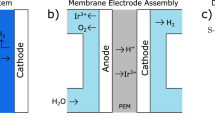
Membrane-electrode assembly (MEA)-based CO2 electrolysis shows great potential for industrial-scale chemical production, but long-term stability remains a key challenge. The degradation mechanisms of catalysts and electrodes in MEAs are not yet fully understood. Here a customized operando synchrotron X-ray characterization platform was established to track the time- and space-resolved evolution of ions and water movement, crystal structure and catalyst variations in MEAs. Using Au and Ag model catalysts, we show that the crystalline phase catalyst stability and catalyst–substrate adhesion are critical to MEA durability. Small- and wide-angle X-ray scattering analysis reveals that Au catalysts, with their robust crystal structure and stable catalyst–substrate adhesion, maintain stability under accelerated stress tests, whereas Ag catalysts degrade due to particle agglomeration, an undesirable dissolution–recrystallization process and detachment. This study demonstrates the advanced capabilities of operando X-ray techniques in elucidating catalyst and electrode degradation in CO2 electrolysers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information. Raw X-ray data generated at the European Synchrotron Radiation Facility (ESRF) large-scale facility are available at https://doi.esrf.fr/10.15151/ESRF-ES-1100173918 from 2026. Alternatively, these data are available from the corresponding author upon request. Source data are provided with this paper.
References
Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
Davis, S. J. et al. Net-zero emissions energy systems. Science 360, eaas9793 (2018).
Masel, R. I. et al. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 16, 118–128 (2021).
Ozden, A. et al. Carbon-efficient carbon dioxide electrolysers. Nat. Sustain. 5, 563–573 (2022).
Wakerley, D. et al. Gas diffusion electrodes, reactor designs and key metrics of low-temperature CO2 electrolysers. Nat. Energy 7, 130–143 (2022).
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Rabiee, H. et al. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: a review. Energy Environ. Sci. 14, 1959–2008 (2021).
Ge, L. et al. Electrochemical CO2 reduction in membrane-electrode assemblies. Chem 8, 663–692 (2022).
de Sousa, L., Benes, N. E. & Mul, G. Evaluating the effects of membranes, cell designs, and flow configurations on the performance of Cu-GDEs in converting CO2 to CO. ACS EST Eng. 2, 2034–2042 (2022).
Endrődi, B. et al. High carbonate ion conductance of a robust PiperION membrane allows industrial current density and conversion in a zero-gap carbon dioxide electrolyzer cell. Energy Environ. Sci. 13, 4098–4105 (2020).
Liu, Z., Yang, H., Kutz, R. & Masel, R. I. CO2 electrolysis to CO and O2 at high selectivity, stability and efficiency using sustainion membranes. J. Electrochem. Soc. 165, J3371 (2018).
Li, J. et al. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. Nat. Catal. 2, 1124–1131 (2019).
Wu, M. et al. Sequential *CO management via controlling in situ reconstruction for efficient industrial-current-density CO2-to-C2+ electroreduction. Proc. Natl Acad. Sci. USA 120, e2302851120 (2023).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Möller, T. et al. The product selectivity zones in gas diffusion electrodes during the electrocatalytic reduction of CO2. Energy Environ. Sci. 14, 5995–6006 (2021).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
Nwabara, U. O. et al. Towards accelerated durability testing protocols for CO2 electrolysis. J. Mater. Chem. A 8, 22557–22571 (2020).
Popović, S. et al. Stability and degradation mechanisms of copper-based catalysts for electrochemical CO2 reduction. Angew. Chem. Int. Ed. 59, 14736–14746 (2020).
Wu, Y. et al. Mitigating electrolyte flooding for electrochemical CO2 reduction via infiltration of hydrophobic particles in a gas diffusion layer. ACS Energy Lett. 7, 2884–2892 (2022).
Yang, K., Kas, R., Smith, W. A. & Burdyny, T. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2 reduction. ACS Energy Lett. 6, 33–40 (2021).
Cofell, E. R., Nwabara, U. O., Bhargava, S. S., Henckel, D. E. & Kenis, P. J. A. Investigation of electrolyte-dependent carbonate formation on gas diffusion electrodes for CO2 electrolysis. ACS Appl. Mater. Interfaces 13, 15132–15142 (2021).
Vass, Á., Kormányos, A., Kószó, Z., Endrődi, B. & Janáky, C. Anode catalysts in CO2 electrolysis: challenges and untapped opportunities. ACS Catal. 12, 1037–1051 (2022).
Liu, M. et al. The capping agent is the key: structural alterations of Ag NPs during CO2 electrolysis probed in a zero-gap gas-flow configuration. J. Catal. 404, 371–382 (2021).
Garg, S. et al. How alkali cations affect salt precipitation and CO2 electrolysis performance in membrane electrode assembly electrolyzers. Energy Environ. Sci. 16, 1631–1643 (2023).
Xu, Q. et al. Identifying and alleviating the durability challenges in membrane-electrode-assembly devices for high-rate CO electrolysis. Nat. Catal. 6, 1042–1051 (2023).
Moss, A. et al. In operando investigations of oscillatory water and carbonate effects in MEA-based CO2 electrolysis devices. Joule 7, 350–365 (2022).
Martens, I., Chattot, R. & Drnec, J. Decoupling catalyst aggregation, ripening, and coalescence processes inside operating fuel cells. J. Power Sources 521, 230851 (2022).
Dorofeev, G. A., Streletskii, A. N., Povstugar, I. V., Protasov, A. V. & Elsukov, E. P. Determination of nanoparticle sizes by X-ray diffraction. Colloid J. 74, 675–685 (2012).
Martens, I. et al. X-ray transparent proton-exchange membrane fuel cell design for in situ wide and small angle scattering tomography. J. Power Sources 437, 226906 (2019).
Aßmann, P., Gago, A. S., Gazdzicki, P., Friedrich, K. A. & Wark, M. Toward developing accelerated stress tests for proton exchange membrane electrolyzers. Curr. Opin. Electrochem. 21, 225–233 (2020).
Li, D. et al. Durability of anion exchange membrane water electrolyzers. Energy Environ. Sci. 14, 3393–3419 (2021).
Xu, Y. et al. Self-cleaning CO2 reduction systems: unsteady electrochemical forcing enables stability. ACS Energy Lett. 6, 809–815 (2021).
Disch, J., Bohn, L., Metzler, L. & Vierrath, S. Strategies for the mitigation of salt precipitation in zero-gap CO2 electrolyzers producing CO. J. Mater. Chem. A 11, 7344–7357 (2023).
Joensen, B. Ó. et al. Unveiling transport mechanisms of cesium and water in operando zero-gap CO2 electrolyzers. Joule 8, 1754–1771 (2024).
Ma, M., Zheng, Z., Yan, W., Hu, C. & Seger, B. Rigorous evaluation of liquid products in high-rate CO2/CO electrolysis. ACS Energy Lett. 7, 2595–2601 (2022).
Xu, Q. et al. Enriching surface-accessible CO2 in the zero-gap anion-exchange-membrane-based CO2 electrolyzer. Angew. Chem. Int. Ed. 62, e202214383 (2022).
Zeradjanin, A. R., Narangoda, P., Spanos, I., Masa, J. & Schlögl, R. How to minimise destabilising effect of gas bubbles on water splitting electrocatalysts? Curr. Opin. Electrochem. 30, 100797 (2021).
Graedel, T. E. Corrosion mechanisms for silver exposed to the atmosphere. J. Electrochem. Soc. 139, 1963 (1992).
Sachan, R. et al. Oxidation-resistant silver nanostructures for ultrastable plasmonic applications. Adv. Mater. 25, 2045–2050 (2013).
Lu, X. et al. In situ observation of the pH gradient near the gas diffusion electrode of CO2 reduction in alkaline electrolyte. J. Am. Chem. Soc. 142, 15438–15444 (2020).
Back, S., Yeom, M. S. & Jung, Y. Active sites of Au and Ag nanoparticle catalysts for CO2 electroreduction to CO. ACS Catal. 5, 5089–5096 (2015).
Clark, E. L. et al. Influence of atomic surface structure on the activity of Ag for the electrochemical reduction of CO2 to CO. ACS Catal. 9, 4006–4014 (2019).
Chen, X. et al. Simultaneous SAXS/WAXS/UV–vis study of the nucleation and growth of nanoparticles: a test of classical nucleation theory. Langmuir 31, 11678–11691 (2015).
Kuhl, K. P. et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 136, 14107–14113 (2014).
Cofell, E. R. et al. Potential cycling of silver cathodes in an alkaline CO2 flow electrolyzer for accelerated stress testing and carbonate inhibition. ACS Appl. Energy Mater. 5, 12013–12021 (2022).
Moss, A. et al. Versatile high energy X-ray transparent electrolysis cell for operando measurements. J. Power Sources 562, 232754 (2022).
Ashiotis, G. et al. The fast azimuthal integration Python library: pyFAI. J. Appl. Crystallogr. 48, 510–519 (2015).
Kieffer, J. & Karkoulis, D. PyFAI, a versatile library for azimuthal regrouping. J. Phys. Conf. Ser. 425, 202012 (2013).
Jinschek, J. R. & Helveg, S. Image resolution and sensitivity in an environmental transmission electron microscope. Micron 43, 1156–1168 (2012).
Acknowledgements
The research leading to these results has received funding from the ECOEthylene project from Innovation Fund Denmark (grant no. 8057-00018B), Danish National Research Foundation (grant no. DNRF146), Pioneer Center for Accelerating P2X Materials Discovery (CAPeX), DNRF (grant no. P3) and the Villum Center for the Science of Sustainable Fuels and Chemicals (grant no. 9455). We thank the European Synchroton Radiation Facility (ESRF) for providing the high-energy X-ray beam and ID 31 beamline staff for experimental support. We also thank S. Ullmann (Topsoe A/S) for his support on the transmission electron microscope, and Topsoe A/S is acknowledged for access to its TEM facility.
Author information
Authors and Affiliations
Contributions
Q.X. and J.A.Z.Z. organized this project, with B.S. and J.D. giving guidance. Q.X. wrote the manuscript and joined all revisions. Q.X., J.A.Z.Z., B.Ó.J. and L.T. performed the synchrotron beamline experiments, with A.S. and M.M. providing on-site support. A.B.M., S.G., S.Z. and I.C. participated in the whole discussion process. L.M.K. performed the electron microscope experiment, with S. Helveg giving guidance. L.M. and S. Huynh participated in GDE preparation and validation. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.A.Z.Z., L.T., L.M., S. Huynh and S.Z. have financial interests in Twelve, a company commercializing carbon dioxide electrolysis technology. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Andrew Beale and Tao Yao for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16 and Notes 1–9.
Source data
Source Data Fig. 1
Raw source data.
Source Data Fig. 2
Raw source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Raw source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Q., Zamora Zeledón, J.A., Joensen, B.Ó. et al. Operando X-ray characterization platform to unravel catalyst degradation under accelerated stress testing in CO2 electrolysis. Nat. Nanotechnol. 20, 889–896 (2025). https://doi.org/10.1038/s41565-025-01916-1
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-01916-1