Abstract
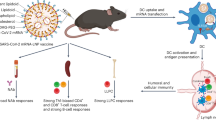
Lipid nanoparticles (LNPs) represent the leading delivery platform for mRNA vaccines with advantageous biocompatibility, scalability, adjuvant activity and often an acceptable safety profile. Here we investigate the physicochemical characteristics and adjuvanticity of four-component LNPs. Previous vaccine studies have demonstrated that altering the ionizable lipid influences the adjuvanticity of an LNP; however, the impact of the polyethylene glycol lipid and phospholipid has received less attention. Our mRNA–LNP vaccine formulations utilized different phospholipids and varying ratios of polyethylene glycol lipid, whereas the ionizable lipid and cholesterol remained approximately constant. We demonstrate that such modifications impact the magnitude and quality of the vaccine-elicited immune responses. We also dissect the underlying mechanisms and show that the biodistribution and cellular uptake of LNPs correlate with the magnitude and quality of the immune responses. These findings support the rational design of novel LNPs to tailor immune responses (cellular or humoral focused) based on the vaccine application.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
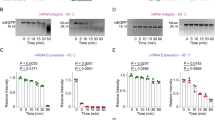
Source data are provided with this paper. The primary datasets for Supplementary Figs. 1–18, along with detailed results of the statistical analyses, are provided in Supplementary Data 1.
References
Hogan, M. J. & Pardi, N. mRNA vaccines in the COVID-19 pandemic and beyond. Annu. Rev. Med. 73, 17–39 (2022).
Patel, R., Kaki, M., Potluri, V. S., Kahar, P. & Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 18, 2002083 (2022).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Author(s) et al. COMIRNATY—COVID-19 Vaccine, mRNA Injection, Suspension [Package Insert] Report No. xxxx (Pfizer Laboratories Div Pfizer Inc., 2023).
Author(s) et al. SPIKEVAX—COVID-19 Vaccine, mRNA Injection, Suspension [Package Insert] Report No. xxxx (Moderna US, Inc., 2023).
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Karikó, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Pardi, N. et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 217, 345–351 (2015).
Alameh, M.-G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892.e7 (2021).
Tahtinen, S. et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 23, 532–542 (2022).
Cullis, P. R. & Hope, M. J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475 (2017).
Hald Albertsen, C. et al. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 188, 114416 (2022).
Dilliard, S. A. & Siegwart, D. J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 8, 282–300 (2023).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
LoPresti, S. T., Arral, M. L., Chaudhary, N. & Whitehead, K. A. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J. Control. Release 345, 819–831 (2022).
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).
Han, X. et al. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat. Nanotechnol. 18, 1105–1114 (2023).
Lokugamage, M. P. et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 5, 1059–1068 (2021).
Chander, N., Basha, G., Cheng, M. H. Y., Witzigmann, D. & Cullis, P. R. Lipid nanoparticle mRNA systems containing high levels of sphingomyelin engender higher protein expression in hepatic and extra-hepatic tissues. Mol. Ther. Methods Clin. Dev. 30, 235–245 (2023).
Tang, X. et al. Simultaneous dendritic cells targeting and effective endosomal escape enhance sialic acid-modified mRNA vaccine efficacy and reduce side effects. J. Control. Release 364, 529–545 (2023).
Kim, J. et al. Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through inhalation. ACS Nano 16, 14792–14806 (2022).
Zhang, R. et al. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater. Sci. 9, 1449–1463 (2021).
Álvarez-Benedicto, E. et al. Optimization of phospholipid chemistry for improved lipid nanoparticle (LNP) delivery of messenger RNA (mRNA). Biomater. Sci. 10, 549–559 (2022).
Lam, K. et al. Unsaturated, trialkyl ionizable lipids are versatile lipid-nanoparticle components for therapeutic and vaccine applications. Adv. Mater. 35, 2209624 (2023).
Puthanakit, T. et al. Phase II prefusion non-stabilised Covid-19 mRNA vaccine randomised study. Sci. Rep. 14, 2373 (2024).
Hassett, K. J. et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J. Control. Release 335, 237–246 (2021).
Kirchdoerfer, R. N. et al. Pre-fusion structure of a human coronavirus spike protein. Nature 531, 118–121 (2016).
Leung, A. K. K., Tam, Y. Y. C., Chen, S., Hafez, I. M. & Cullis, P. R. Microfluidic mixing: a general method for encapsulating macromolecules in lipid nanoparticle systems. J. Phys. Chem. B 119, 8698–8706 (2015).
Cheng, M. H. Y. et al. Induction of bleb structures in lipid nanoparticle formulations of mRNA leads to improved transfection potency. Adv. Mater. 35, e2303370 (2023).
Mendonça, M. C. P., Kont, A., Kowalski, P. S. & O’Driscoll, C. M. Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids. Drug Discov. Today 28, 103505 (2023).
Li, J. et al. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 10, 81–98 (2015).
Sallusto, F., Lanzavecchia, A., Araki, K. & Ahmed, R. From vaccines to memory and back. Immunity 33, 451–463 (2010).
Laczkó, D. et al. A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity 53, 724–732.e7 (2020).
Verma, C. et al. Cancer vaccines in the immunotherapy era: promise and potential. Vaccines 11, 1783 (2023).
Tallón de Lara, P., Castañón, H., Sterpi, M. & van den Broek, M. Antimetastatic defense by CD8+ T cells. Trends Cancer 8, 145–157 (2022).
Johansen, P. et al. Antigen kinetics determines immune reactivity. Proc. Natl Acad. Sci. USA 105, 5189–5194 (2008).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010).
Kulkarni, J. A., Cullis, P. R. & van der Meel, R. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 28, 146–157 (2018).
Bettini, E. et al. Distinct components of nucleoside-modified messenger RNA vaccines cooperate to instruct efficient germinal center responses. Preprint at bioRxiv https://doi.org/10.1101/2024.05.17.594726 (2024).
Yang, L. et al. Recent advances in lipid nanoparticles for delivery of mRNA. Pharmaceutics 14, 2682 (2022).
Remaut, K., Lucas, B., Braeckmans, K., Demeester, J. & De Smedt, S. C. Pegylation of liposomes favours the endosomal degradation of the delivered phosphodiester oligonucleotides. J. Control. Release 117, 256–266 (2007).
Tanaka, H. et al. Improvement of mRNA delivery efficiency to a T cell line by modulating PEG-lipid content and phospholipid components of lipid nanoparticles. Pharmaceutics 13, 2097 (2021).
Huang, Y., Jia, A., Wang, Y. & Liu, G. CD8+ T cell exhaustion in anti-tumour immunity: the new insights for cancer immunotherapy. Immunology 168, 30–48 (2023).
Zhu, Y. et al. Screening for lipid nanoparticles that modulate the immune activity of helper T cells towards enhanced antitumour activity. Nat. Biomed. Eng. 8, 544–560 (2024).
Bevers, S. et al. mRNA-LNP vaccines tuned for systemic immunization induce strong antitumor immunity by engaging splenic immune cells. Mol. Ther. 30, 3078–3094 (2022).
Luozhong, S. et al. Phosphatidylserine lipid nanoparticles promote systemic RNA delivery to secondary lymphoid organs. Nano Lett. 22, 8304–8311 (2022).
Gomi, M. et al. Delivering mRNA to secondary lymphoid tissues by phosphatidylserine-loaded lipid nanoparticles. Adv. Healthc. Mater. 12, e2202528 (2023).
Ben-Sasson, S. Z. et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J. Exp. Med. 210, 491–502 (2013).
Van Den Eeckhout, B. et al. Specific targeting of IL-1β activity to CD8+ T cells allows for safe use as a vaccine adjuvant. npj Vaccines 5, 64 (2020).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Baiersdörfer, M. et al. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35 (2019).
Vadovics, M., Muramatsu, H., Sárközy, A. & Pardi, N. Production and evaluation of nucleoside-modified mRNA vaccines for infectious diseases. Methods Mol. Biol. 2786, 167–181 (2024).
Freyn, A. W. et al. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol. Ther. 28, 1569–1584 (2020).
Heyes, J., Hall, K., Tailor, V., Lenz, R. & MacLachlan, I. Synthesis and characterization of novel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery. J. Control. Release 112, 280–290 (2006).
Ábrahám, E. et al. Expression and purification of the receptor-binding domain of SARS-CoV-2 spike protein in mammalian cells for immunological assays. FEBS Open Bio 14, 380–389 (2024).
Margine, I., Palese, P. & Krammer, F. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J. Vis. Exp. 81, e51112 (2013).
Stevens, J. et al. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science 303, 1866–1870 (2004).
Krammer, F. et al. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS ONE 7, e43603 (2012).
Parhiz, H. et al. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Control. Release 291, 106–115 (2018).
Acknowledgements
We thank the Cell & Developmental Biology Microscopy Core at the University of Pennsylvania. Additionally, the flow cytometry data were generated in the Penn Cytomics and Cell Sorting Shared Resource Laboratory at the University of Pennsylvania and is partially supported by a Abramson Cancer Center NCI Grant (P30 016520). The research identifier number is RRid:SCR_022376. The Pardi laboratory was supported by the National Institute of Allergy and Infectious Diseases (NIAID; R01AI146101, R01AI153064, P01AI158571 and U19AI181968). Z.L. was supported by the National Laboratory for Biotechnology (2022-2.1.1-NL-2022-00008) and the Hungarian Academy of Sciences (Lendület Program Grant (LP2017-7/2017)). K.A.L. and P.B. were supported by R01AI 152236. C.G.R. was supported by the NIH-NCI National Cancer Institute; R01CA283736 (C.G.R. and N.P.), the Parker Institute for Cancer Immunotherapy, 20221408 (C.G.R. and N.P.), and the University of California, Los Angeles, Immunology Advisory Committee (IAC) Award (C.G.R.). We also thank Y. Du, H. Sun and E. L. Prak of the Human Immunology Core (HIC) at the Perelman School of Medicine at the University of Pennsylvania for assistance with the Luminex assays, and M. Eldabbas, E. Maddox, T. Sinha and J. Shu for the preparation of deidentified primary human monocytes. The HIC is supported in part by NIH P30 AI045008 and P30 CA016520. HIC RRID: SCR_022380. We also thank M. Katona at the University of Pittsburgh for guidance in confocal imaging quantification methods; L. Palmer, H. Mahmoud, A. Martin, A. Liu and K. McClintock for their contributions and support throughout this project; and the Proteomics Research Group of the HUN-REN BRC Core Facility for performing the liquid chromatography/mass spectrometry analysis and validation of recombinant proteins.
Author information
Authors and Affiliations
Contributions
N.P. and J.H. conceived the study. N.P. designed the vaccine antigens. H.M. produced the mRNA vaccine antigens. P.S. encapsulated the mRNAs into LNPs and O.D. performed the immunization studies of PEG lipid titration. M.V. designed, performed and analysed the total IgG ELISA, IVIS and Luminex. K.A.L. and P.B. designed, performed and analysed the FRNT assay. B.T.G., M.V. and D.A. designed, performed and analysed the B cell studies. M.V., E.F.D., K.R., H.R.L. and C.G.R. designed, performed and analysed the T cell studies. M.V., W.Z., K.L., V.V.S., V.R.M., E.Á., G.C., A.H.B., K.N., T.M.L., T.L. and C.G.R. performed, designed and analysed the biodistribution studies. E.F.D., J.X., X.H. and M.J.M. designed, performed and analysed the in vitro LNP uptake studies. E.F.D. performed and analysed the IgG subtypes, IgA ELISA and innate cell infiltration to the injection site studies. E.F.D. and W.Z. designed, performed and analysed the endosomal escape assay. W.Z. performed and analysed the LNP protein corona assay. N.D.L., D.C., E.B. and M.L. designed, performed and analysed the in vivo LNP uptake studies. E.Á. and Z.L. produced the recombinant proteins. A.S. and T.M. helped with the mouse blood collection. M.V., K.L., E.F.D. and N.P. wrote the paper with help from co-authors.
Corresponding author
Ethics declarations
Competing interests
N.P. served on the mRNA strategic advisory board of Sanofi Pasteur in 2022 and Pfizer in 2023–2024. N.P. is also a member of the Scientific Advisory Board of AldexChem and BioNet, and has consulted for Vaccine Company Inc., Optimeos and Pasture Bio. K.L., J.H., O.D., W.Z. and P.S. are employees of Genevant Sciences Corp. and own shares or options of Genevant’s parent company. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Roy van der Meel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18, Tables 1–8, Methods and references.
Source data
Supplementary Data 1
Primary datasets for Supplementary Figs. 1–18, along with detailed results of statistical analyses.
Source Data Fig. 1
Primary datasets for Fig. 1, along with detailed results of statistical analyses.
Source Data Fig. 2
Primary datasets for Fig. 2, along with detailed results of statistical analyses.
Source Data Fig. 3
Primary datasets for Fig. 3, along with detailed results of statistical analyses.
Source Data Fig. 4
Primary datasets for Fig. 4, along with detailed results of statistical analyses.
Source Data Fig. 5
Primary datasets for Fig. 5, along with detailed results of statistical analyses.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vadovics, M., Zhao, W., Daley, E.F. et al. Tailoring the adjuvanticity of lipid nanoparticles by PEG lipid ratio and phospholipid modifications. Nat. Nanotechnol. 20, 1312–1322 (2025). https://doi.org/10.1038/s41565-025-01958-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-01958-5
This article is cited by
-
Optimal murine CD4+ T cell priming by mRNA-lipid nanoparticle vaccines requires endogenous antigen processing
Nature Communications (2026)
-
Lipid nanoparticle–based mRNA platforms for mucosal HIV vaccines: formulation advances, immune mechanisms, and translational pathways
Archives of Microbiology (2026)
-
mRNA vaccines: immunogenicity and quality characteristics
Journal of Nanobiotechnology (2025)