Abstract
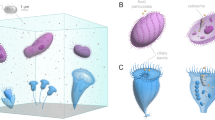
Many single-celled organisms exhibit both solitary and colonial existence. An important step towards multicellularity, which is associated with benefits such as enhanced nutrient uptake, was the formation of colonies of unicellular organisms. However, the initial drivers that favoured individual cells aggregating into more complex colonies are less clear. Here we show that hydrodynamic coupling between proximate neighbours results in faster feeding flows for neighbouring ciliates, such that individuals within a dynamic colony have stronger average feeding flows than solitary individuals. Flows generated by individuals acting together reach higher velocities, thus allowing access to a wider range of prey resources than individuals acting on their own. Moreover, we find that accrued feeding benefits are typically asymmetric: whereas all individuals benefit from acting together, those with slower solitary currents gain more from partnering than those with faster currents. We find that colonial organization in simple unicellular organisms is beneficial for all its members. This provides fundamental insights into the selective forces favouring the early evolution of multicellular organization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data supporting the findings of this paper are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
Code developed as part of this work will be available from the corresponding authors upon reasonable request.
References
Fenchel, T. Suspension feeding in ciliated Protozoa: structure and function of feeding organelles. Arch. Protistenkd. 123, 239–260 (1980).
Goldstein, R. E. Green algae as model organisms for biological fluid dynamics. Annu. Rev. Fluid Mech. 47, 343–375 (2015).
Jorgensen, C. B. Comparative physiology of suspension feeding. Annu. Rev. Physiol. 37, 57–79 (1975).
Riisgard, H. U. & Larsen, P. S. Particle capture mechanisms in suspension-feeding invertebrates. Mar. Ecol. Prog. Ser. 418, 255–293 (2010).
Gilpin, W., Bull, M. S. & Prakash, M. The multiscale physics of cilia and flagella. Nat. Rev. Phys. 2, 74–88 (2020).
King, N. The unicellular ancestry of animal development. Dev. Cell 7, 313–325 (2004).
Bonner, J. T. The origins of multicellularity. Integr. Biol. 1, 27–36 (1998).
van Gestel, J. & Tarnita, C. E. On the origin of biological construction, with a focus on multicellularity. Proc. Natl Acad. Sci. USA 114, 11018–11026 (2017).
Grosberg, R. K. & Strathmann, R. R. The evolution of multicellularity: a minor major transition? Annu. Rev. Ecol. Evol. Syst. 38, 621–654 (2007).
Short, M. B. et al. Flows driven by flagella of multicellular organisms enhance long-range molecular transport. Proc. Natl Acad. Sci. USA 103, 8315–8319 (2006).
Roper, M., Dayel, M. J., Pepper, R. E. & Koehl, M. A. Cooperatively generated stresslet flows supply fresh fluid to multicellular choanoflagellate colonies. Phys. Rev. Lett. 110, 228104 (2013).
Solari, C. A., Ganguly, S., Kessler, J. O., Michod, R. E. & Goldstein, R. E. Multicellularity and the functional interdependence of motility and molecular transport. Proc. Natl Acad. Sci. USA 103, 1353–1358 (2006).
Koufopanou, V. & Bell, G. Soma and germ: an experimental approach using Volvox. Proc. R. Soc. B: Biol. Sci. 254, 107–113 (1993).
Tartar, V. The Biology of Stentor (Pergamon, 1961).
Slabodnick, M. M. et al. The kinase regulator Mob1 acts as a patterning protein for Stentor morphogenesis. PLoS Biol. 12, e1001861 (2014).
Wan, K. Y. et al. Reorganization of complex ciliary flows around regenerating Stentor coeruleus. Philos. Trans. R. Soc. B 375, 20190167 (2020).
Kowalczyk, W., Zima, B. E. & Delgado, A. A biological seeding particle approach for μ-PIV measurements of a fluid flow provoked by microorganisms. Exp. Fluids 43, 147–150 (2007).
Ukita, H. & Idaka, M. Visualization and analysis of a micro-flow generated by an optical rotator. Opt. Rev. 7, 448–450 (2000).
Kanso, E. A., Lopes, R. M., Strickler, J. R., Dabiri, J. O. & Costello, J. H. Teamwork in the viscous oceanic microscale. Proc. Natl Acad. Sci. USA 118, e2018193118 (2021).
Pepper, R. E., Roper, M., Ryu, S., Matsudaira, P. & Stone, H. A. Nearby boundaries create eddies near microscopic filter feeders. J. R. Soc. Interface 7, 851–862 (2010).
Fenchel, T. in Nitrogen Cycling in Coastal Marine Environments Ch. 3 (eds Blackburn, T. H. & Sørensen, J.) 59–65 (Wiley, 1988).
Liron, N. & Mochon, S. Stokes flow for a stokes-let between 2 parallel flat plates. J. Eng. Math. 10, 287–303 (1976).
Blake, J. R. A note on the image system for a stokeslet in a no-slip boundary. Math. Proc. Camb. Philos. Soc. 70, 303–310 (1971).
Blake, J. R. & Otto, S. R. Ciliary propulsion, chaotic filtration and a ‘blinking’ stokeslet. J. Eng. Math. 30, 151–168 (1996).
Cortez, R. The method of regularized stokeslets. SIAM J. Sci. Comput. 23, 1204–1225 (2006).
Pepper, R. E. et al. A new angle on microscopic suspension feeders near boundaries. Biophys. J. 105, 1796–1804 (2013).
Jones, A. R., Jahn, T. L. & Fonseca, J. R. Contraction of protoplasm. III. Cinematographic analysis of the contraction of some heterotrichs. J. Cell. Physiol. 75, 1–7 (1970).
Wood, D. C. Electrophysiological studies of the protozoan, Stentor coeruleus. J. Neurobiol. 1, 363–377 (1970).
Fenchel, T. Filter-feeding in colonial protists. Protist 170, 283–286 (2019).
Dexter, J. P., Prabakaran, S. & Gunawardena, J. A complex hierarchy of avoidance behaviors in a single-cell Eukaryote. Curr. Biol. 29, 4323–4329.e2 (2019).
Christensen-Dalsgaard, K. K. & Fenchel, T. Increased filtration efficiency of attached compared to free-swimming flagellates. Aquat. Microb. Ecol. 33, 77–86 (2003).
Solari, C. A. et al. Flagellar phenotypic plasticity in volvocalean algae correlates with Peclet number. J. R. Soc. Interface 8, 1409–1417 (2011).
Kirkegaard, J. B. & Goldstein, R. E. Filter-feeding, near-field flows, and the morphologies of colonial choanoflagellates. Phys. Rev. E 94, 052401 (2016).
Goodenough, U. W. Motile detergent-extracted cells of Tetrahymena and Chlamydomonas. J. Cell Biol. 96, 1610–1621 (1983).
Pepper, R. E. et al. The effect of external flow on the feeding currents of sessile microorganisms. J. R. Soc. Interface 18, 20200953 (2021).
Rode, M., Meucci, G., Seegert, K., Kiørboe, T. & Andersen, A. Effects of surface proximity and force orientation on the feeding flows of microorganisms on solid surfaces. Phys. Rev. Fluids 5, 123104 (2020).
Slabodnick, M. M. et al. The macronuclear genome of Stentor coeruleus reveals tiny introns in a giant cell. Curr. Biol. 27, 569–575 (2017).
De Terra, N. Culture of Stentor coeruleus on Colpidium campylum. J. Protozool. 13, 491–492 (1966).
Gilpin, W., Prakash, V. N. & Prakash, M. Flowtrace: simple visualization of coherent structures in biological fluid flows. J. Exp. Biol. 220, 3411–3418 (2017).
Gimbutas, Z., Greengard, L. & Veerapaneni, S. Simple and efficient representations for the fundamental solutions of Stokes flow in a half-space. J. Fluid Mech. 776, R1 (2015).
Ainley, J., Durkin, S., Embid, R., Boindala, P. & Cortez, R. The method of images for regularized stokeslets. J. Comput. Phys. 227, 4600–4616 (2008).
Acknowledgements
S.S. was supported by Whitman Early Career Award from MBL and startup funds from Emory University. Funding support came from NIH NIGMS (Grant No. R35GM143050 to S.S.), NIH NIGMS (Grant No. R35GM130327 to W.M.), NIH (Grant No. R01HL153622 to E.K.), NSF (Grant Nos. IOS-2034043 and CBET-2100209 to E.K.), NSF (Grant Nos. CBET-2100705 and IOS-2114171 to J.H.C.), NSF (Grant Nos. CBET-2100156 and IOS-21141691 to S.P.C.), ONR (Grant No. N00014-22-1-2655 to E.K.) and ONR (Grant No. N00014-23-1-2754 to J.H.C.). We thank M. Slabodnick for invaluable advice on Stentor culturing and J. Allen for help with microscopy. S.S. is grateful to J. Lippincott-Schwartz and R. Phillips for their enthusiastic support for this project. S.S. thanks A. Knoof, for invaluable help in collecting stentors from ponds across the Northeast.
Author information
Authors and Affiliations
Contributions
S.S. and J.H.C. designed the experiments. H.G. and E.K. designed the mathematical model. S.S. conducted the experiments. H.G. performed the simulations. S.S., S.P.C. and W.M. analysed the experimental data. H.G. and E.K. analysed the model results. S.S., J.H.C., H.G. and E.K. put together all the results and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Experimental setup for visualizing empirical flowfields.
Schematic representation of the imaging chamber formed by attaching two coverslips on either side of a polydimethylsiloxane (PDMS) ring 5 mm in diameter and 700 µm high. The microscope is positioned below the PDMS chamber. Stentors (blue schematic) usually anchored themselves on the lower glass coverslip attached to the PDMS chamber.
Extended Data Fig. 3 Methodology for measurement of feeding currents.
Since only the orthogonal portion of the flowfields in the region between the two vortices reaches the oral opening and can be productively filtered for prey, only the velocity component normal to the oral opening, Ufeeding (thick black arrow), is taken as a measure of the feeding current. Due to mixing between tracer and cilia movements, feeding current velocities were measured 0.25 mm from the oral opening to prevent contamination of the PIV analysis due to its inability to separate tracer movements from ciliary beating. Feeding current (Ufeeding) for each individual in a pair or alone was determined by calculating the average velocity across a line the size of the oral opening of each individual. Thin black arrows denote the flow streamlines. Thin double-headed arrow denotes separation between the two Stentors. Thick arrow denotes the flow directed towards the oral opening of each Stentor.
Extended Data Fig. 4 Feeding current velocities of solitary individuals.
Distribution of velocities of the feeding current generated by solitary Stentor individuals (n = 17) measured at 0.25 mm from the ciliary band.
Extended Data Fig. 5 Ciliary wave velocity remains unchanged with proximity.
Kymographs showing the frequency of the metachronal waves for the two individuals in the pair shown in Fig. 2a,b. The frequency of the metachronal wave for the left and the right S. coeruleus in the pair remains unchanged as the distance, Δ, between the two individuals changes from (a) Δ = 52 µm (left: 16.7 Hz, right: 15.4) to (b) Δ = 246 µm (left: 16.6 Hz, right: 15.2 Hz).
Extended Data Fig. 6 Streamlines generated by the model Stentor.
Flow fields of a single Stentor modeled as a regularized force (black arrowhead) bounded by one wall (θ = 0.25π), viewing from above (left panel) and from side (right panel). Blue lines and arrows denote the streamlines and the direction of the flow.
Extended Data Fig. 7 Streamlines generated by a pair of model Stentor individuals.
(a) Fluid flows generated by a Stentor pair, modeled as a pair of regularized Stokeslets of strengths F1 and F2 respectively near a wall with force ratio F1/F2 = 1, inter-Stentor separation distance Δ = 2 (left) and Δ = 0.5 (right) (b) Force ratio F1/F2 = 2 and inter-Stentor separation distances Δ = 2 (left), and Δ = 0.5 (right). Top row: flow streamlines. Bottom row: downward velocity component of the flow velocity determined along the horizontal line z = 1.25H.
Extended Data Fig. 8 Feeding flow rate and the benefit for individual Stentor in a pair.
(a-c) Feeding flow rate of Stentor 1 U(1)feeding in a Stentor pair whose swaying angles ϕ1,2 (a), inclination angles θ1,2 (b), and the separation distances ∆ (c) are subject to Gaussian noises. The horizontal dashed lines represent the feeding flow rate of solitary Stentor. The gray vertical lines separate the cases of having a stronger neighbor (left) and weaker neighbor (right), same as in (d–f). (d-f) The benefit of “being together” for Stentor 1 in a Stentor pair whose swaying angles (d), inclination angles (e), and the separation distances (f) are subject to Gaussian noises. The mean (lines) and the standard deviations (error bars) are obtained from 100 Monte Carlo simulations. F1 and F2 are the individual strengths of Stentors 1 and 2.
Supplementary information
Supplementary Video 2
Feeding flow of an individual Stentor visualized using milk particles as tracers. The time-lapse video was generated using dark-field microscopy. Also see Fig. 1d.
Supplementary Video 4
PIV analysis of the flow field of an individual Stentor showing the direction (denoted by the arrows) and the magnitude of the feeding flow. Arrows are pseudo-coloured to denote the magnitude of the feeding flow velocity. The scale is from 0 (black arrows) to 100 µm s−1 (white arrows). Also see Fig. 1e.
Supplementary Video 6
PIV analysis of the flow field generated by a pair of Stentor individuals showing the direction (denoted by the arrows) and magnitude of the feeding flow. Arrows are pseudo-coloured to denote the magnitude of the feeding flow velocity. The scale is from 0 (black arrows) to 300 µm s−1 (white arrows). Also see Fig. 2b.
Source data
Source Data Fig. 2
Data shown in Fig. 2c,d.
Source Data Fig. 3
Data shown in Fig. 3c–i.
Source Data Fig. 4
Data shown in Fig. 4b,c including the insets.
Source Data Fig. 5
Data shown in Fig. 5a,b.
Source Data Extended Data Fig. 4
Data shown in Extended Data Fig. 4
Source Data Extended Data Fig. 7
Data shown in Extended Data Fig. 7a,b.
Source Data Extended Data Fig. 8
Data shown in Extended Data Fig. 8a–f.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shekhar, S., Guo, H., Colin, S.P. et al. Cooperative hydrodynamics accompany multicellular-like colonial organization in the unicellular ciliate Stentor. Nat. Phys. 21, 624–631 (2025). https://doi.org/10.1038/s41567-025-02787-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41567-025-02787-y
This article is cited by
-
Good feeders make good neighbours
Nature Physics (2025)