Abstract
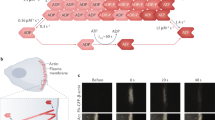
Self-organized criticality can occur in earthquakes, avalanches and biological processes, and is characterized by intermittent, scale-free energy dissipation. In living cells, the actin cytoskeleton undergoes dynamic structural reorganization, particularly during migration and division, where molecular motors generate mechanical stresses that drive large dissipative events. However, the mechanisms governing these critical transitions remain unclear. Here we show that cytoskeletal criticality emerges from the interplay between F-actin organization and active stress generation. Our study focuses on a minimal actomyosin system in vitro, which is composed of F-actin filaments, myosin II motors and nucleation-promoting factors. By systematically varying the actin connectivity and nematic order, we demonstrate that ordered and sparsely connected networks exhibit exponential stress dissipation, whereas disordered and highly connected networks show heavy-tailed distributions of energy release and the 1/f noise characteristic of self-organized criticality. Increased disorder leads to stress localization, shifting force propagation into stiffer mechanical modes, reminiscent of Anderson localization in condensed-matter systems. Furthermore, we show that network architecture directly regulates the myosin II filament size, establishing a chemical–mechanical feedback loop that modulates criticality. Our findings provide insights into the collective cytoskeletal dynamics, energy localization and cellular self-organization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. Raw data supporting the findings of this paper are available from the corresponding author upon reasonable request.
Code availability
Codes supporting the findings of this paper are available from the corresponding author upon reasonable request.
References
Bak, P. & Tang, C. Earthquakes as a self‐organized critical phenomenon. J. Geophys. Res.: Solid Earth 94, 15635–15637 (1989).
Wijngaarden, R. J., Welling, M. S., Aegerter, C. M. & Menghini, M. Avalanches and self-organized criticality in superconductors. Eur. Phys. J. B 50, 117–122 (2006).
Negoro, H., Kitamoto, S., Takeuchi, M. & Mineshige, S. Statistics of X-ray fluctuations from Cygnus X-1: reservoirs in the disk? Astrophys. J. 452, L49 (1995).
Bedard, C., Kroeger, H. & Destexhe, A. Does the 1/f frequency scaling of brain signals reflect self-organized critical states? Phys. Rev. Lett. 97, 118102 (2006).
Chialvo, D. R. Emergent complex neural dynamics. Nat. Phys. 6, 744–750 (2010).
Sneppen, K., Bak, P., Flyvbjerg, H. & Jensen, M. H. Evolution as a self-organized critical phenomenon. Proc. Natl Acad. Sci. USA 92, 5209–5213 (1995).
Utsu, T. A statistical study on the occurrence of aftershocks. Geophys. Mag. 30, 521–605 (1961).
Ōmori, F. On the After-Shocks of Earthquakes (The Univ. of Tokyo, 1895).
Bak, P., Christensen, K., Danon, L. & Scanlon, T. Unified scaling law for earthquakes. Phys. Rev. Lett. 88, 178501 (2002).
Gutenberg, B. & Richter, C. F. Frequency of earthquakes in California. Bull. Seismol. Soc. Am. 34, 185–188 (1944).
Bak, P. & Chen, K. Self-organized criticality. Sci. Am. 264, 46–53 (1991).
Bak, P. & Paczuski, M. The dynamics of fractals. Fractals 3, 415–429 (1995).
Bak, P., Tang, C. & Wiesenfeld, K. Self-organized criticality: an explanation of the 1/f noise. Phys. Rev. Lett. 59, 381 (1987).
Bak, P. & Creutz, M. in Fractals in Science 27–48 (Springer, 1994).
Davidsen, J. & Paczuski, M. 1/fα noise from correlations between avalanches in self-organized criticality. Phys. Rev. E 66, 050101 (2002).
Beggs, J. M. & Plenz, D. Neuronal avalanches in neocortical circuits. J. Neurosci. 23, 11167–11177 (2003).
Kobayashi, M. & Musha, T. 1/f fluctuation of heartbeat period. IEEE Trans. Biomed. Eng. 456–457 (1982).
Akselrod, S. et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213, 220–222 (1981).
Hooge, F. & Hoppenbrouwers, A. Amplitude distribution of 1/f noise. Physica 42, 331–339 (1969).
Voss, R. F. & Clarke, J. Flicker (1f) noise: equilibrium temperature and resistance fluctuations. Phys. Rev. B 13, 556 (1976).
Alencar, A. M. et al. Non-equilibrium cytoquake dynamics in cytoskeletal remodeling and stabilization. Soft Matter 12, 8506–8511 (2016).
Shi, Y., Porter, C. L., Crocker, J. C. & Reich, D. H. Dissecting fat-tailed fluctuations in the cytoskeleton with active micropost arrays. Proc. Natl Acad. Sci. USA 116, 13839–13846 (2019).
Shi, Y. et al. Pervasive cytoquakes in the actomyosin cortex across cell types and substrate stiffness. Integr. Biol. 13, 246–257 (2021).
Shi, Y., Sivarajan, S., Crocker, J. C. & Reich, D. H. Measuring cytoskeletal mechanical fluctuations and rheology with active micropost arrays. Curr. Protoc. 2, e433 (2022).
Sivarajan, S. et al. Lévy-distributed fluctuations in the living cell cortex. Phys. Rev. Res. 6, 043265 (2024).
Pollard, T. D. Mechanics of cytokinesis in eukaryotes. Curr. Opin. Cell Biol. 22, 50–56 (2010).
Blanchoin, L., Boujemaa-Paterski, R., Sykes, C. & Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 94, 235–263 (2014).
Murrell, M., Oakes, P. W., Lenz, M. & Gardel, M. L. Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 16, 486–498 (2015).
Smith, D. et al. Molecular motor-induced instabilities and cross linkers determine biopolymer organization. Biophys. J. 93, 4445–4452 (2007).
Murrell, M. P. & Gardel, M. L. F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. Proc. Natl Acad. Sci. USA 109, 20820–20825 (2012).
Silva, M. S. et al. Active multistage coarsening of actin networks driven by myosin motors. Proc. Natl Acad. Sci. USA 108, 9408–9413 (2011).
Vogel, S. K., Petrasek, Z., Heinemann, F. & Schwille, P. Myosin motors fragment and compact membrane-bound actin filaments. eLife 2, e00116 (2013).
Haviv, L., Gillo, D., Backouche, F. & Bernheim-Groswasser, A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J. Mol. Biol. 375, 325–330 (2008).
Li, F. & Higgs, H. N. The mouse formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335–1340 (2003).
Cory, G. O., Cramer, R., Blanchoin, L. & Ridley, A. J. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol. cell 11, 1229–1239 (2003).
Cao, L. et al. SPIN90 associates with mDia1 and the Arp2/3 complex to regulate cortical actin organization. Nat. Cell Biol. 22, 803–814 (2020).
Bovellan, M. et al. Cellular control of cortical actin nucleation. Curr. Biol. 24, 1628–1635 (2014).
Carvalho, K. et al. Actin polymerization or myosin contraction: two ways to build up cortical tension for symmetry breaking. Philos. Trans. R. Soc. B 368, 20130005 (2013).
Ideses, Y., Bernheim, A., Sonn, A. & Roichman, Y. Myosin II does it all: assembly, remodeling, and disassembly of actin networks are governed by myosin II activity. Biophys. J. 106, 568a–568a (2014).
Lau, A. W., Hoffman, B. D., Davies, A., Crocker, J. C. & Lubensky, T. C. Microrheology, stress fluctuations, and active behavior of living cells. Phys. Rev. Lett. 91, 198101 (2003).
Mizuno, D., Tardin, C., Schmidt, C. F. & Mackintosh, F. C. Nonequilibrium mechanics of active cytoskeletal networks. Science 315, 370–373 (2007).
Toyota, T., Head, D. A., Schmidt, C. F. & Mizuno, D. Non-Gaussian athermal fluctuations in active gels. Soft Matter 7, 3234–3239 (2011).
Ennomani, H. et al. Architecture and connectivity govern actin network contractility. Curr. Biol. 26, 616–626 (2016).
Liman, J. et al. The role of the Arp2/3 complex in shaping the dynamics and structures of branched actomyosin networks. Proc. Natl Acad. Sci. USA 117, 10825–10831 (2020).
Floyd, C., Levine, H., Jarzynski, C. & Papoian, G. A. Understanding cytoskeletal avalanches using mechanical stability analysis. Proc. Natl Acad. Sci. USA 118, e2110239118 (2021).
Li, C., Liman, J., Eliaz, Y. & Cheung, M. S. Forecasting avalanches in branched actomyosin networks with network science and machine learning. J. Phys. Chem. B 125, 11591–11605 (2021).
Floyd, C., Papoian, G. A. & Jarzynski, C. Quantifying dissipation in actomyosin networks. Interface Focus 9, 20180078 (2019).
Swartz, D. W. & Camley, B. A. Active gels, heavy tails, and the cytoskeleton. Soft Matter 17, 9876–9892 (2021).
Bernheim-Groswasser, A., Wiesner, S., Golsteyn, R. M., Carlier, M.-F. & Sykes, C. The dynamics of actin-based motility depend on surface parameters. Nature 417, 308–311 (2002).
Backouche, F., Haviv, L., Groswasser, D. & Bernheim-Groswasser, A. Active gels: dynamics of patterning and self-organization. Phys. Biol. 3, 264 (2006).
Bausch, A. & Kroy, K. A bottom-up approach to cell mechanics. Nat. Phys. 2, 231–238 (2006).
Claessens, M. M., Bathe, M., Frey, E. & Bausch, A. R. Actin-binding proteins sensitively mediate F-actin bundle stiffness. Nat. Mater. 5, 748–753 (2006).
Koenderink, G. H. et al. An active biopolymer network controlled by molecular motors. Proc. Natl Acad. Sci. USA 106, 15192–15197 (2009).
Bendix, P. M. et al. A quantitative analysis of contractility in active cytoskeletal protein networks. Biophys. J. 94, 3126–3136 (2008).
Lee, G. et al. Myosin-driven actin-microtubule networks exhibit self-organized contractile dynamics. Sci. Adv. 7, eabe4334 (2021).
Ricketts, S. N., Ross, J. L. & Robertson-Anderson, R. M. Co-entangled actin-microtubule composites exhibit tunable stiffness and power-law stress relaxation. Biophys. J. 115, 1055–1067 (2018).
Köster, D. V. et al. Actomyosin dynamics drive local membrane component organization in an in vitro active composite layer. Proc. Natl Acad. Sci. USA 113, E1645–E1654 (2016).
Murrell, M., Thoresen, T. & Gardel, M. Chapter Fifteen—reconstitution of contractile actomyosin arrays. Methods Enzymol. 540, 265–282 (2014).
Muresan, C. G. et al. F-actin architecture determines constraints on myosin thick filament motion. Nat. Commun. 13, 7008 (2022).
Linsmeier, I. et al. Disordered actomyosin networks are sufficient to produce cooperative and telescopic contractility. Nat. Commun. 7, 12615 (2016).
Stricker, J., Falzone, T. & Gardel, M. L. Mechanics of the F-actin cytoskeleton. J. Biomech. 43, 9–14 (2010).
Thoresen, T., Lenz, M. & Gardel, M. L. Reconstitution of contractile actomyosin bundles. Biophys. J. 100, 2698–2705 (2011).
Weirich, K. L. et al. Liquid behavior of cross-linked actin bundles. Proc. Natl Acad. Sci. USA 114, 2131–2136 (2017).
Stam, S. et al. Filament rigidity and connectivity tune the deformation modes of active biopolymer networks. Proc. Natl Acad. Sci. USA 114, E10037–E10045 (2017).
Starr, F. W., Douglas, J. F. & Sastry, S. The relationship of dynamical heterogeneity to the Adam-Gibbs and random first-order transition theories of glass formation. J. Chem. Phys. 138, 12A541 (2013).
Garrahan, J. P. Dynamic heterogeneity comes to life. Proc. Natl Acad. Sci. USA 108, 4701–4702 (2011).
Berthier, L. Dynamic heterogeneity in amorphous materials. Preprint at https://arxiv.org/abs/1106.1739 (2011).
Störzer, M., Gross, P., Aegerter, C. M. & Maret, G. Observation of the critical regime near Anderson localization of light. Phys. Rev. Lett. 96, 063904 (2006).
Anderson, P. W. Absence of diffusion in certain random lattices. Phys. Rev. 109, 1492 (1958).
Segev, M., Silberberg, Y. & Christodoulides, D. N. Anderson localization of light. Nat. Photon. 7, 197–204 (2013).
Wiersma, D. S. Disordered photonics. Nat. Photon. 7, 188–196 (2013).
Sun, Z. G. et al. Cofilin-mediated actin filament network flexibility facilitates 2D to 3D actomyosin shape change. Eur. J. Cell Biol. 103, 151379 (2024).
Van Hove, L. Correlations in space and time and Born approximation scattering in systems of interacting particles. Phys. Rev. 95, 249 (1954).
Hohenberg, P. C. & Halperin, B. I. Theory of dynamic critical phenomena. Rev. Mod. Phys. 49, 435 (1977).
Fisher, M. E. Renormalization group theory: its basis and formulation in statistical physics. Rev. Mod. Phys. 70, 653 (1998).
Bouchaud, J.-P. & Georges, A. Anomalous diffusion in disordered media: statistical mechanisms, models and physical applications. Phys. Rep. 195, 127–293 (1990).
Finer, J. T., Simmons, R. M. & Spudich, J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–119 (1994).
Molloy, J., Burns, J., Kendrick-Jones, J., Tregear, R. & White, D. Movement and force produced by a single myosin head. Nature 378, 209–212 (1995).
Kwak, S. G. & Kim, J. H. Central limit theorem: the cornerstone of modern statistics. Korean J. Anesthesiol. 70, 144–156 (2017).
Kumar, N., Singh, S. & Yadav, A. C. Linking space-time correlations for a class of self-organized critical systems. Phys. Rev. E 104, 064132 (2021).
Feder, H. J. S. & Feder, J. Self-organized criticality in a stick-slip process. Phys. Rev. Lett. 66, 2669 (1991).
Shi, Y. Dissecting Cytoskeletal Dynamics with Active Micropost Arrays. PhD thesis, Johns Hopkins Univ. (2020).
Chen, J., Chen, H., Cai, X., Weng, P. & Nie, H. Parameter estimation of stable distribution based on zero-order statistics. AIP Conf. Proc. 1864, 020171 (2017).
Samorodnitsky, G., Taqqu, M. S. & Linde, R. Stable non-Gaussian random processes: stochastic models with infinite variance. Bull. Lond. Math. Soc. 28, 554–555 (1996).
Zolotarev, V. M. One-Dimensional Stable Distributions (American Mathematical Soc., 1986).
Metzler, R. & Klafter, J. The random walk’s guide to anomalous diffusion: a fractional dynamics approach. Phys. Rep. 339, 1–77 (2000).
Murrell, M. & Gardel, M. L. Actomyosin sliding is attenuated in contractile biomimetic cortices. Mol. Biol. Cell 25, 1845–1853 (2014).
MacKintosh, F. C. & Levine, A. J. Nonequilibrium mechanics and dynamics of motor-activated gels. Phys. Rev. Lett. 100, 018104 (2008).
Reis, P. M., Ingale, R. A. & Shattuck, M. D. Caging dynamics in a granular fluid. Phys. Rev. Lett. 98, 188301 (2007).
Weeks, E. R., Crocker, J. C., Levitt, A. C., Schofield, A. & Weitz, D. A. Three-dimensional direct imaging of structural relaxation near the colloidal glass transition. Science 287, 627–631 (2000).
Guo, M. et al. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell 158, 822–832 (2014).
Gal, N., Lechtman-Goldstein, D. & Weihs, D. Particle tracking in living cells: a review of the mean square displacement method and beyond. Rheol. Acta 52, 425–443 (2013).
Weirich, K. L., Stam, S., Munro, E. & Gardel, M. L. Actin bundle architecture and mechanics regulate myosin II force generation. Biophys. J. 120, 1957–1970 (2021).
Kaminer, B. & Bell, A. L. Myosin filamentogenesis: effects of pH and ionic concentration. J. Mol. Biol. 20, 391–401 (1966).
Popov, K., Komianos, J. & Papoian, G. A. MEDYAN: mechanochemical simulations of contraction and polarity alignment in actomyosin networks. PLoS Comput. Biol. 12, e1004877 (2016).
Sheiko, S. S. et al. Adsorption-induced scission of carbon–carbon bonds. Nature 440, 191–194 (2006).
Lutz, J.-F., Lehn, J.-M., Meijer, E. & Matyjaszewski, K. From precision polymers to complex materials and systems. Nat. Rev. Mater. 1, 16024 (2016).
Li, Z. et al. Bottlebrush polymers: from controlled synthesis, self-assembly, properties to applications. Prog. Polym. Sci. 116, 101387 (2021).
Cardamone, L., Laio, A., Torre, V., Shahapure, R. & DeSimone, A. Cytoskeletal actin networks in motile cells are critically self-organized systems synchronized by mechanical interactions. Proc. Natl Acad. Sci. USA 108, 13978–13983 (2011).
Deriu, M. A. et al. Biomechanics of actin filaments: a computational multi-level study. J. Biomech. 44, 630–636 (2011).
Murphy, N., Wortis, R. & Atkinson, W. Generalized inverse participation ratio as a possible measure of localization for interacting systems. Phys. Rev. B 83, 184206 (2011).
Rizzo, T. & Tarzia, M. Localized phase of the Anderson model on the Bethe lattice. Phys. Rev. B 110, 184210 (2024).
Bueno, C., Liman, J., Schafer, N. P., Cheung, M. S. & Wolynes, P. G. A generalized Flory-Stockmayer kinetic theory of connectivity percolation and rigidity percolation of cytoskeletal networks. PLoS Comput. Biol. 18, e1010105 (2022).
Pollard, T. D. & Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003).
Mullins, R. D., Heuser, J. A. & Pollard, T. D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad. Sci. USA 95, 6181–6186 (1998).
Dalton, F. & Corcoran, D. Self-organized criticality in a sheared granular stick-slip system. Phys. Rev. E 63, 061312 (2001).
Rodríguez-Cruz, C. et al. Experimental observations of fractal landscape dynamics in a dense emulsion. Soft Matter 19, 6805–6813 (2023).
Alvarado, J., Sheinman, M., Sharma, A., MacKintosh, F. C. & Koenderink, G. H. Molecular motors robustly drive active gels to a critically connected state. Nat. Phys. 9, 591–597 (2013).
Tsuda, Y., Yasutake, H., Ishijima, A. & Yanagida, T. Torsional rigidity of single actin filaments and actin–actin bond breaking force under torsion measured directly by in vitro micromanipulation. Proc. Natl Acad. Sci. USA 93, 12937–12942 (1996).
Basko, D. M., Aleiner, I. L. & Altshuler, B. L. Metal–insulator transition in a weakly interacting many-electron system with localized single-particle states. Ann. Phys. 321, 1126–1205 (2006).
Chan, C., Beltzner, C. C. & Pollard, T. D. Cofilin dissociates Arp2/3 complex and branches from actin filaments. Curr. Biol. 19, 537–545 (2009).
Pandit, N. G. et al. Force and phosphate release from Arp2/3 complex promote dissociation of actin filament branches. Proc. Natl Acad. Sci. USA 117, 13519–13528 (2020).
Jung, W., Murrell, M. P. & Kim, T. F-actin fragmentation induces distinct mechanisms of stress relaxation in the actin cytoskeleton. Biophys. J. 110, 354a (2016).
Andrianantoandro, E. & Pollard, T. D. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24, 13–23 (2006).
Wioland, H. et al. ADF/cofilin accelerates actin dynamics by severing filaments and promoting their depolymerization at both ends. Curr. Biol. 27, 1956–1967. e1957 (2017).
Lappalainen, P. & Drubin, D. G. Cofilin promotes rapid actin filament turnover in vivo. Nature 388, 78–82 (1997).
Zhang, X.-F. et al. Regulation of axon growth by myosin II–dependent mechanocatalysis of cofilin activity. J. Cell Biol. 218, 2329–2349 (2019).
Jung, W. et al. Dynamic motions of molecular motors in the actin cytoskeleton. Cytoskeleton 76, 517–531 (2019).
Bertling, E. et al. Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol. Biol. cell 15, 2324–2334 (2004).
Kotila, T. et al. Structural basis of actin monomer re-charging by cyclase-associated protein. Nat. Commun. 9, 1892 (2018).
Chaudhry, F. et al. Srv2/cyclase-associated protein forms hexameric shurikens that directly catalyze actin filament severing by cofilin. Mol. Biol. Cell 24, 31–41 (2013).
Alimov, N., Hoeprich, G. J., Padrick, S. B. & Goode, B. L. Cyclase-associated protein interacts with actin filament barbed ends to promote depolymerization and formin displacement. J. Biol. Chem. 299, 105367 (2023).
Guo, S., Hoeprich, G. J., Magliozzi, J. O., Gelles, J. & Goode, B. L. Dynamic remodeling of actin networks by cyclase-associated protein and CAP-Abp1 complexes. Curr. Biol. 33, 4484–4495.e4485 (2023).
Yu, Q., Li, J., Murrell, M. P. & Kim, T. Balance between force generation and relaxation leads to pulsed contraction of actomyosin networks. Biophys. J. 115, 2003–2013 (2018).
Vidiella, B. et al. Engineering self-organized criticality in living cells. Nat. Commun. 12, 4415 (2021).
Walleczek J. Self-Organized Biological Dynamics and Nonlinear Control: Toward Understanding Complexity, Chaos and Emergent Function in Living Systems (Cambridge Univ. Press, 2006).
Kim, T., Hwang, W., Lee, H. & Kamm, R. D. Computational analysis of viscoelastic properties of crosslinked actin networks. PLoS Comput. Biol. 5, e1000439 (2009).
Luan, Y., Lieleg, O., Wagner, B. & Bausch, A. R. Micro- and macrorheological properties of isotropically cross-linked actin networks. Biophys. J. 94, 688–693 (2008).
Lieleg, O., Claessens, M. M. & Bausch, A. R. Structure and dynamics of cross-linked actin networks. Soft Matter 6, 218–225 (2010).
Sun, Z. G. & Murrell, M. Cofilin-mediated filament softening and crosslinking counterbalance to enhance actin network flexibility. Phys. Rev. Lett. 133, 218402 (2024).
Shin, J. H., Gardel, M., Mahadevan, L., Matsudaira, P. & Weitz, D. Relating microstructure to rheology of a bundled and cross-linked F-actin network in vitro. Proc. Natl Acad. Sci. USA 101, 9636–9641 (2004).
Gardel, M. L. et al. Elastic behavior of cross-linked and bundled actin networks. Science 304, 1301–1305 (2004).
Gardel, M. L. et al. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc. Natl Acad. Sci. USA 103, 1762–1767 (2006).
Gardel, M. et al. Scaling of F-actin network rheology to probe single filament elasticity and dynamics. Phys. Rev. Lett. 93, 188102 (2004).
Verkhovsky, A. B. & Borisy, G. G. Non-sarcomeric mode of myosin II organization in the fibroblast lamellum. J. Cell Biol. 123, 637–652 (1993).
Miura, K. Bleach correction ImageJ plugin for compensating the photobleaching of time-lapse sequences. F1000Res. 9, 1494 (2020).
Acknowledgements
We are grateful for insightful discussions with C. O’Hern, M. Shattuck and J. (J.) Treado. We acknowledge the initial contribution related to this project by C. Muresan, A. Pasha Tabatabai and L. Lanier. This work was supported by funding through ARO MURI W911NF-14-1-0403, National Institutes of Health (NIH) R01 1R01GM126256, NIH U54 CA209992 and Human Frontiers Science Program (HFSP) grant no. RGY0073/2018 to M.M. We acknowledge the University of Maryland supercomputing resources (https://hpcc.umd.edu) made available for conducting the computational research reported in this paper.
Author information
Authors and Affiliations
Contributions
Z.G.S. and M.M. designed and conceived the work and drafted the paper. Z.G.S., M.M., C.F. and N.Z. edited the paper. Z.G.S. performed the experiments. Z.G.S. analysed the experimental data. N.Z. and P.K. performed the simulations. Z.G.S., N.Z. and P.K. analysed the simulation data. M.M. and G.P. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Yu Shi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14, description of the simulation model and captions for Supplementary Videos.
Supplementary Video 1
0.74-nM Arp2/3-nucleated F-actin deformed by MTFs.
Supplementary Video 2
0.74-nM Arp2/3-nucleated F-actin deformed by MTF composite.
Supplementary Video 3
MEDYAN simulation of Rarp = 0.002 F-actin deformed by MTFs.
Supplementary Video 4
MEDYAN simulation of Rarp = 0.02 F-actin deformed by MTFs.
Supplementary Video 5
MEDYAN simulation of Rarp = 0.004 F-actin filament large bending by MTFs.
Supplementary Video 6
MEDYAN simulation of Rarp = 0.02 F-actin filament large bending by MTFs.
Supplementary Video 7
MEDYAN simulation of Rarp = 0.02 F-actin filament large displacements and energy release over time.
Source data
Source Data Figs. 1–5
Source data for Figs. 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Z.G., Zimmerberg, N., Kelly, P. et al. Feedback between F-actin organization and active stress governs criticality and energy localization in the cell cytoskeleton. Nat. Phys. 21, 1290–1302 (2025). https://doi.org/10.1038/s41567-025-02919-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41567-025-02919-4
This article is cited by
-
From actin to action
Nature Physics (2025)