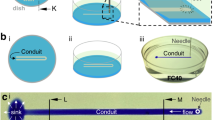
Abstract
Filamentous fungi play crucial roles in global carbon and nutrient cycling, soil carbon sequestration, agricultural soil management, contaminant fate and transport, biofouling of engineered materials and human health. Although these processes typically involve multiple fluid phases in porous media, the mechanisms by which fungi regulate fluid flow remain poorly understood, limiting our ability to predict and harness fungus-mediated processes. The complexity and opacity of porous media further obscure our understanding of how fungi influence fluid flow and distribution. Here we explore the impact of filamentous fungi on multiphase flow and fluid redistribution using a dual-porosity microfluidic chip, featuring a flow channel embedded within tight porous media. Our pore-scale visualizations show that filamentous fungi can actively induce multiphase flow and mobilize trapped fluid phases in porous media through localized clogging and hyphal-induced pore invasion, enhancing the oil–water interfacial area and redistribution of fluid phases. This study reveals the mechanisms by which filamentous fungi modulate fluid flow and distribution, offering insights into harnessing fungal processes to enhance applications such as bioremediation and carbon sequestration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information.
References
Tedersoo, L. et al. Global diversity and geography of soil fungi. Science 346, 6213 (2014).
Grossart, H. P. et al. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 17, 339–354 (2019).
Limon, J. J., Skalski, J. H. & Underhill, D. M. Commensal fungi in health and disease. Cell Host Microbe 22, 156–165 (2017).
Niu, L. et al. Ignored fungal community in activated sludge wastewater treatment plants: diversity and altitudinal characteristics. Environ. Sci. Pollut. Res. 24, 4185–4193 (2017).
Coleine, C., Stajich, J. E. & Selbmann, L. Fungi are key players in extreme ecosystems. Trends Ecol. Evol. 37, 517–528 (2022).
Butinar, L., Frisvad, J. C. & Gunde-Cimerman, N. Hypersaline waters—a potential source of foodborne toxigenic aspergilli and penicillia. FEMS Microbiol. Ecol. 77, 186–199 (2011).
Nuppunen-Puputti, M. et al. Rock surface fungi in deep continental biosphere-exploration of microbial community formation with subsurface in situ biofilm trap. Microorganisms 9, 64 (2020).
Zhdanova, N. N., Tugay, T., Dighton, J., Zheltonozhsky, V. & Mcdermott, P. Ionizing radiation attracts soil fungi. Mycol. Res. 108, 1089–1096 (2004).
Vaksmaa, A. et al. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 10, 1–21 (2023).
Lankiewicz, T. S. et al. Lignin deconstruction by anaerobic fungi. Nat. Microbiol. 8, 596–610 (2023).
Arntzen, M. Ø. et al. Quantitative comparison of the biomass-degrading enzyme repertoires of five filamentous fungi. Sci. Rep. 10, 20267 (2020).
Bar-On, Y. M., Phillips, R. & Milo, R. The biomass distribution on Earth. Proc. Natl Acad. Sci. USA 115, 6506–6511 (2018).
Baxi, S. N. et al. Exposure and health effects of fungi on humans. J. Allergy Clin. Immunol. Pract. 4, 396–404 (2016).
Banerjee, S. & van der Heijden, M. G. A. Soil microbiomes and one health. Nat. Rev. Microbiol. 21, 6–20 (2023).
Li, Q., Liu, J. & Gadd, G. M. Fungal bioremediation of soil co-contaminated with petroleum hydrocarbons and toxic metals. Appl. Microbiol. Biotechnol. 104, 8999–9008 (2020).
Zhang, J. H., Xue, Q. H., Gao, H., Ma, X. & Wang, P. Degradation of crude oil by fungal enzyme preparations from Aspergillus spp. for potential use in enhanced oil recovery. J. Chem. Technol. Biotechnol. 91, 865–875 (2016).
Qian, X., Fang, C., Huang, M. & Achal, V. Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil. J. Clean. Prod. 164, 198–208 (2017).
Liu, F., Shah, D. S. & Gadd, G. M. Role of protein in fungal biomineralization of copper carbonate nanoparticles. Curr. Biol. 31, 358–368.e3 (2021).
Dusengemungu, L., Kasali, G., Gwanama, C. & Mubemba, B. Overview of fungal bioleaching of metals. Environ. Adv. 5, 100083 (2021).
Kang, X., Csetenyi, L. & Gadd, G. M. Colonization and bioweathering of monazite by Aspergillus niger: solubilization and precipitation of rare earth elements. Environ. Microbiol. 23, 3970–3986 (2021).
Yarzábal Rodríguez, L. A. et al. Exploring extremophilic fungi in soil mycobiome for sustainable agriculture amid global change. Nat. Commun. 15, 6951 (2024).
Duan, S. et al. Cross-kingdom nutrient exchange in the plant–arbuscular mycorrhizal fungus–bacterium continuum. Nat. Rev. Microbiol. https://doi.org/10.1038/s41579-024-01073-7 (2024).
Compant, S. et al. Harnessing the plant microbiome for sustainable crop production. Nat. Rev. Microbiol. https://doi.org/10.1038/s41579-024-01079-1 (2024).
Scheidweiler, D., Peter, H., Pramateftaki, P., Anna, Pde & Battin, T. J. Unraveling the biophysical underpinnings to the success of multispecies biofilms in porous environments. ISME J. 13, 1700–1710 (2019).
de Anna, P., Pahlavan, A. A., Yawata, Y., Stocker, R. & Juanes, R. Chemotaxis under flow disorder shapes microbial dispersion in porous media. Nat. Phys. 17, 68–73 (2021).
Rusconi, R., Guasto, J. S. & Stocker, R. Bacterial transport suppressed by fluid shear. Nat. Phys. 10, 212–217 (2014).
Alcolombri, U. et al. Sinking enhances the degradation of organic particles by marine bacteria. Nat. Geosci. 14, 775–780 (2021).
Kurz, D. L. et al. Competition between growth and shear stress drives intermittency in preferential flow paths in porous medium biofilms. Proc. Natl Acad. Sci. USA 119, e2122202119 (2022).
Secchi, E. et al. The structural role of bacterial eDNA in the formation of biofilm streamers. Proc. Natl Acad. Sci. USA 119, 1–10 (2022).
Gao, B., Wang, X. & Ford, R. M. Chemotaxis along local chemical gradients enhanced bacteria dispersion and PAH bioavailability in a heterogenous porous medium. Sci. Total Environ. 859, 160004 (2023).
Lee, S. H., Secchi, E. & Kang, P. K. Rapid formation of bioaggregates and morphology transition to biofilm streamers induced by pore-throat flows. Proc. Natl Acad. Sci. USA 120, 1–10 (2023).
Bhattacharjee, T. & Datta, S. S. Bacterial hopping and trapping in porous media. Nat. Commun. 10, 2075 (2019).
Stocker, R., Seymour, J. R., Samadani, A., Hunt, D. E. & Polz, M. F. Rapid chemotactic response enables marine bacteria to exploit ephemeral microscale nutrient patches. Proc. Natl Acad. Sci. USA 105, 4209–4214 (2008).
Stocker, R. Marine microbes see a sea of gradients. Science 338, 628–633 (2012).
Drescher, K., Shen, Y., Bassler, B. L. & Stone, H. A. Biofilm streamers cause catastrophic disruption of flow with consequences for environmental and medical systems. Proc. Natl Acad. Sci. USA 110, 4345–4350 (2013).
Rusconi, R., Lecuyer, S., Autrusson, N., Guglielmini, L. & Stone, H. A. Secondary flow as a mechanism for the formation of biofilm streamers. Biophys. J. 100, 1392–1399 (2011).
Yang, J. Q. et al. Evidence for biosurfactant-induced flow in corners and bacterial spreading in unsaturated porous media. Proc. Natl Acad. Sci. USA. 118, 38 (2021).
Islam, M. R., Tudryn, G., Bucinell, R., Schadler, L. & Picu, R. C. Morphology and mechanics of fungal mycelium. Sci. Rep. 7, 1–12 (2017).
Wösten, H. A. B. et al. How a fungus escapes the water to grow into the air. Curr. Biol. 9, 85–88 (1999).
Or, D., Smets, B. F., Wraith, J. M., Dechesne, A. & Friedman, S. P. Physical constraints affecting bacterial habitats and activity in unsaturated porous media—a review. Adv. Water Resour. 30, 1505–1527 (2007).
Bengtson, S. et al. Deep-biosphere consortium of fungi and prokaryotes in Eocene subseafloor basalts. Geobiology 12, 489–496 (2014).
Ivarsson, M. et al. A fungal-prokaryotic consortium at the basalt–zeolite interface in subseafloor igneous crust. PLoS ONE 10, 1–19 (2015).
Kohlmeier, S. et al. Taking the fungal highway: mobilization of pollutant-degrading bacteria by fungi. Environ. Sci. Technol. 39, 4640–4646 (2005).
Guhr, A., Borken, W., Spohn, M. & Matzner, E. Redistribution of soil water by a saprotrophic fungus enhances carbon mineralization. Proc. Natl Acad. Sci. USA 112, 14647–14651 (2015).
Brand, A. & Gow, N. A. Mechanisms of hypha orientation of fungi. Curr. Opin. Microbiol. 12, 350–357 (2009).
Ugalde, U. & Rodriguez-Urra, A. B. The Mycelium Blueprint: insights into the cues that shape the filamentous fungal colony. Appl. Microbiol. Biotechnol. 98, 8809–8819 (2014).
Lee, K. et al. Fungal quorum quenching: a paradigm shift for energy savings in membrane bioreactor (MBR) for wastewater treatment. Environ. Sci. Technol. 50, 10914–10922 (2016).
National Academies of Sciences, Engineering and Medicine. Characterization, Modeling, Monitoring, and Remediation of Fractured Rock (The National Academies Press, 2020); https://doi.org/10.17226/21742
El-Masri, H. Toxicological Profile for Naphthalene, 1-Methylnaphthalene, and 2-Methylnaphthalene (Agency for Toxic Substances and Disease Registry, 2005).
Feder, J., Flekkøy, E. G. & Hansen, A. Physics of Flow in Porous Media (Cambridge Univ. Press, 2022); https://doi.org/10.1017/9781009100717
Haines, W. B. Studies in the physical properties of soil. V. The hysteresis effect in capillary properties, and the modes of moisture distribution associated therewith. J. Agric. Sci. 20, 97–116 (1930).
Måløy, K. J., Furuberg, L., Feder, J. & Jøssang, T. Dynamics of slow drainage in porous media. Phys. Rev. Lett. 68, 2161–2164 (1992).
Moura, M., Måløy, K. J., Flekkøy, E. G. & Toussaint, R. Intermittent dynamics of slow drainage experiments in porous media: characterization under different boundary conditions. Front. Phys. 7, 217 (2020).
Morrow, N. R. Physics and thermodynamics of capillary action in porous media. Ind. Eng. Chem. 62, 32–56 (1970).
Moebius, F. & Or, D. Interfacial jumps and pressure bursts during fluid displacement in interacting irregular capillaries. J. Colloid Interface Sci. 377, 406–415 (2012).
Kurz, D. L., Secchi, E., Stocker, R. & Jimenez-Martinez, J. Morphogenesis of biofilms in porous media and control on hydrodynamics. Environ. Sci. Technol. 57, 5666–5677 (2023).
Zhao, B., MacMinn, C. W. & Juanes, R. Wettability control on multiphase flow in patterned microfluidics. Proc. Natl Acad. Sci. USA 113, 10251–10256 (2016).
Chui, J. Y. Y., Douarche, C., Auradou, H. & Juanes, R. Rheology of bacterial superfluids in viscous environments. Soft Matter 17, 7004–7013 (2021).
Moura, M., Fiorentino, E.-A., Måløy, K. J., Schäfer, G. & Toussaint, R. Impact of sample geometry on the measurement of pressure-saturation curves: experiments and simulations. Water Resour. Res. 51, 8900–8926 (2015).
Budek, A., Garstecki, P., Samborski, A. & Szymczak, P. Thin-finger growth and droplet pinch-off in miscible and immiscible displacements in a periodic network of microfluidic channels. Phys. Fluids 27, 112109 (2015).
Cremer, C. J. M. & Neuweiler, I. How dynamic boundary conditions induce solute trapping and quasi-stagnant zones in laboratory experiments comprising unsaturated heterogeneous porous media. Water Resour. Res. 55, 10765–10780 (2019).
Ryan, M. C., MacQuarrie, K. T. B., Harman, J. & McLellan, J. Field and modeling evidence for a “stagnant flow” zone in the upper meter of sandy phreatic aquifers. J. Hydrol. 233, 223–240 (2000).
National Research Council. In Situ Bioremediation: When Does it Work? (The National Academies Press, 1993); https://doi.org/10.17226/2131
Xu, M. et al. Responses of microbial community functional structures to pilot-scale uranium in situ bioremediation. ISME J. 4, 1060–1070 (2010).
Niu, J., Liu, Q., Lv, J. & Peng, B. Review on microbial enhanced oil recovery: mechanisms, modeling and field trials. J. Pet. Sci. Eng. 192, 107350 (2020).
She, H., Kong, D., Li, Y., Hu, Z. & Guo, H. Recent Advance of Microbial Enhanced Oil Recovery (MEOR) in China. Geofluids 2019, 1871392 (2019).
Rosenberg, M., Barki, M., Bar-Ness, R., Goldberg, S. & Doyle, R. J. Microbial Adhesion to Hydrocarbons (MATH). Biofouling 4, 121–128 (1991).
Iglauer, S., Pentland, C. H. & Busch, A. CO2 wettability of seal and reservoir rocks and the implications for carbon geo-sequestration. Water Resour. Res. 51, 729–774 (2015).
Drake, H. et al. Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat. Commun. 8, 55 (2017).
Sohlberg, E. et al. Revealing the unexplored fungal communities in deep groundwater of crystalline bedrock fracture zones in Olkiluoto, Finland. Front. Microbiol. 6, 573 (2015).
Suetrong, S. et al. Unravelling the hidden diversity of cave mycobiota in Thailand’s Satun Geopark. Sci. Rep. 13, 19162 (2023).
Poli, A. et al. Cultivable fungal diversity in two karstic caves in Italy: under-investigated habitats as source of putative novel taxa. Sci. Rep. 14, 4164 (2024).
Jiménez-Martínez, J. et al. Pore-scale mechanisms for the enhancement of mixing in unsaturated porous media and implications for chemical reactions. Geophys. Res. Lett. 42, 5316–5324 (2015).
Heyer, J., Galschenko, V. F. & Dunfield, P. F. Molecular phylogeny of type II methane-oxidizing bacteria isolated from various environments. Microbiology 148, 2831–2846 (2002).
Friend, J. & Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 4, 026502 (2010).
Lee, J. N., Park, C. & Whitesides, G. M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 75, 6544–6554 (2003).
Medici, G., West, L. J. & Banwart, S. A. Groundwater flow velocities in a fractured carbonate aquifer-type: implications for contaminant transport. J. Contam. Hydrol. 222, 1–16 (2019).
Cook, P. A Guide to Regional Groundwater Flow in Fractured Rock Aquifers (CSIRO Land and Water, 2003).
Hu, F. et al. An improved technology for monitoring groundwater flow velocity and direction in fractured rock system based on colloidal particles motion. Sci. Rep. 14, 7685 (2024).
Streltsova, T. D. Hydrodynamics of groundwater flow in a fractured formation. Water Resour. Res. 12, 405–414 (1976).
Acknowledgements
This work was supported by the MnDRIVE Advancing Industry, Conserving Our Environment, and a Seed Grant from the Biotechnology & Biomanufacturing Innovation Center at the University of Minnesota. S.H.L. acknowledges the Postdoctoral Fellowship Program Nurturing Next-generation Researchers granted (award number 2018R1A6A3A03012913, S.H.L.) by National Research Foundation of Korea (NRF). M.M. acknowledges the support of the Research Council of Norway through project numbers 262644 (PoreLab SFF, M.M.), 324555 (FlowConn YFF, M.M.) and 309073 (COLOSSAL INTPART, M.M.). The preparation of PDMS microfluidic devices was conducted in the Minnesota Nano Center, which is supported by the NSF through the National Nano Coordinated Infrastructure Network (NNCI) under award number ECCS-2025124.
Author information
Authors and Affiliations
Contributions
S.H.L. and P.K.K. designed research; S.H.L. performed experiments; S.H.L., S.S. and C.S. conducted microbiome analysis; S.H.L., M.M., S.S., C.S. and P.K.K. analysed data; and S.H.L. and P.K.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Ilenia Battiato and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Video 1
Clogging of the main channel by filamentous fungi drives displacement of NAPL from model fractured aquifer system. The top panel shows the overview of the dual-porosity chip, the middle panel displays the invasion area colour-coded with time of change and the bottom panel shows the pressure drop and the volume of displaced oil from the chip. The experiment was performed as described in the Methods in the chip with regularly arranged pillars. The colour coding by the time of change was performed by assigning the time value to the pixels of newly invaded area of binarized images.
Supplementary Video 2
Hydrophilic hypha induced pore invasion at the single pore level. Images were recorded after the syringe pump was stopped once the fungal colony attached and started to grow in the chip with regularly arranged pillars. The pore throat or distance between pillars was 10 µm.
Supplementary Video 3
Fungi-induced NAPL displacement replicate experiment 1. A replicate experiment with regularly arranged pillar porous media chips were conducted. The results indicate the fungi-induced NAPL displacement is reproducible. The inset shows an enlarged video of the region colonized by fungi indicated by white rectangle. The oil phase was stained with fluorescent tracer.
Supplementary Video 4
Fungi-induced NAPL displacement replicate experiment 2. A replicate experiment with regularly arranged pillar porous media chips were conducted. The results indicate the fungi-induced NAPL displacement is reproducible.
Supplementary Video 5
Filamentous fungi-enhanced oil displacement is reproduced in randomized porous media. The top panel shows the overview of the dual-porosity chip, and the bottom panel shows a zoomed-in video of area colonized by a fungus, as well as the pressure drop and the volume of displaced oil from the chip. The experiment was performed as described in the Methods in the chip with randomly arranged pillars.
Supplementary Video 6
Invasion of NAPL-filled pores with large throats by penetration of multiple hydrophilic hyphae. Images were recorded after the syringe pump was stopped once the fungal colony attached and started to grow in the chip with regularly arranged pillars. The pore throat or distance between pillars was 100 µm.
Supplementary Video 7
Alteration of oil blob shape by poking of hydrophilic hyphal tip. Images were recorded after the syringe pump was stopped once the fungal colony attached and started to grow in the main flow channel. The oil blob shown with white outline sits on the glass surface in water saturated conduit. The change in the oil blob’s shape upon contact with hyphae indicates their hydrophilic nature.
Supplementary Video 8
Invasion of NAPL-filled pores with small throats by penetration of a single hydrophilic hypha. Images were recorded after the syringe pump was stopped once the fungal colony attached and started to grow in the chip with regularly arranged pillars. The pore throat or distance between pillars was 10 µm.
Supplementary Video 9
Split of oil island or bridging of water via hyphal penetration. Images were recorded after the syringe pump was stopped once the fungal colony attached and started to grow in the chip with regularly arranged pillars. The pore throat or distance between pillars was 10 µm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S.H., Moura, M., Srivastava, S. et al. Filamentous fungi control multiphase flow and fluid distribution in porous media. Nat. Phys. 21, 1719–1727 (2025). https://doi.org/10.1038/s41567-025-03020-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41567-025-03020-6