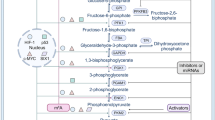
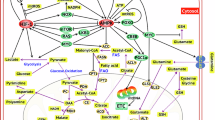
Abstract
Glucose metabolic enzymes and their metabolites not only provide energy and building blocks for synthesizing macromolecules but also possess non-canonical or moonlighting functions in response to extracellular and intracellular signalling. These moonlighting functions modulate various cellular activities, including gene expression, cell cycle progression, DNA repair, autophagy, senescence and apoptosis, cell proliferation, remodelling of the tumour microenvironment and immune responses. These functions integrate glucose metabolism with other essential cellular activities, driving cancer progression. Targeting these moonlighting functions could open new therapeutic avenues and lead to cancer-specific treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bian, X. et al. Regulation of gene expression by glycolytic and gluconeogenic enzymes. Trends Cell Biol. 32, 786–799 (2022).
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011).
Xu, D. et al. The evolving landscape of noncanonical functions of metabolic enzymes in cancer and other pathologies. Cell Metab. 33, 33–50 (2021).
Warburg, O., Wind, F. & Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 (1927).
Thompson, C. B. et al. A century of the Warburg effect. Nat. Metab. 5, 1840–1843 (2023).
Ashton, T. M., McKenna, W. G., Kunz-Schughart, L. A. & Higgins, G. S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 24, 2482–2490 (2018).
El-Botty, R. et al. Oxidative phosphorylation is a metabolic vulnerability of endocrine therapy and palbociclib resistant metastatic breast cancers. Nat. Commun. 14, 4221 (2023).
Menk, A. V. et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 22, 1509–1521 (2018).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 (2008). This study reveals the coordinated regulation of glycolysis and mitochondrial activity by the protein kinase activity of phosphoglycerate kinase 1 to drive the Warburg effect.
Li, X. et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol. Cell 61, 705–719 (2016).
Claps, G. et al. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 19, 749–762 (2022).
Miao, P., Sheng, S., Sun, X., Liu, J. & Huang, G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life 65, 904–910 (2013).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Lu, Z. & Hunter, T. Metabolic kinases moonlighting as protein kinases. Trends Biochem. Sci. 43, 301–310 (2018).
Dai, J. et al. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J. Exp. Med. 216, 2883–2899 (2019).
Wang, Y., Xia, Y. & Lu, Z. Metabolic features of cancer cells. Cancer Commun. 38, 65 (2018).
Tessarz, P. & Kouzarides, T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 15, 703–708 (2014).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019). This study reveals that lactylation occurs on histones, thereby regulating gene expression.
Yang, W. et al. EGFR-induced and PKCepsilon monoubiquitylation-dependent NF-kappaB activation upregulates PKM2 expression and promotes tumorigenesis. Mol. Cell 48, 771–784 (2012).
Yang, W. et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 14, 1295–1304 (2012). This study reveals that nuclear pyruvate kinase M2 activates MYC to promote glycolytic gene expression, demonstrating that the Warburg effect is regulated by a nuclear glycolytic enzyme upon receptor tyrosine kinase activation.
Lu, Z. & Hunter, T. Prolyl isomerase Pin1 in cancer. Cell Res. 24, 1033–1049 (2014).
Lv, L. et al. Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol. Cell 52, 340–352 (2013).
Wang, H. J. et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1alpha-mediated glucose metabolism. Proc. Natl Acad. Sci. USA 111, 279–284 (2014).
Yang, W. et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell 150, 685–696 (2012). This study reveals that pyruvate kinase M2 can function as a protein kinase and phosphorylate histone H3.
Yang, W. et al. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature 480, 118–122 (2011). This study reveals that receptor tyrosine kinase activation confers a nuclear function on pyruvate kinase M2, allowing it to regulate β-catenin and promote tumour cell proliferation.
Hosios, A. M., Fiske, B. P., Gui, D. Y. & Vander Heiden, M. G. Lack of evidence for PKM2 protein kinase activity. Mol. Cell 59, 850–857 (2015).
Yang, W. & Lu, Z. Nuclear PKM2 regulates the Warburg effect. Cell Cycle 12, 3154–3158 (2013).
Wang, X. et al. PKM2-induced the phosphorylation of histone H3 contributes to EGF-mediated PD-L1 transcription in HCC. Front. Pharmacol. 11, 577108 (2020).
Li, S. et al. Serine and SAM responsive complex SESAME regulates histone modification crosstalk by sensing cellular metabolism. Mol. Cell 60, 408–421 (2015).
Yu, Q. et al. Regulation of SESAME-mediated H3T11 phosphorylation by glycolytic enzymes and metabolites. PLoS ONE 12, e0175576 (2017).
Hu, P. et al. Nuclear-localized pyruvate kinases control phosphorylation of histone H3 on threonine 11. Nat. Plants 10, 1682–1697 (2024).
Zhang, Y. et al. Metabolic switch regulates lineage plasticity and induces synthetic lethality in triple-negative breast cancer. Cell Metab. 36, 193–208.e8 (2024).
Jing, Y. Y. et al. Epigenetic regulation of the Warburg effect by H2B monoubiquitination. Cell Death Differ. 27, 1660–1676 (2020).
Liu, Y. et al. Nuclear lactate dehydrogenase A senses ROS to produce alpha-hydroxybutyrate for HPV-induced cervical tumor growth. Nat. Commun. 9, 4429 (2018).
Kornberg, M. D. et al. GAPDH mediates nitrosylation of nuclear proteins. Nat. Cell Biol. 12, 1094–1100 (2010).
Yin, C. et al. ALDOB/KAT2A interactions epigenetically modulate TGF-beta expression and T cell functions in hepatocellular carcinogenesis. Hepatology 81, 77–93 (2023).
Shi, R. et al. Fructose-1,6-bisphosphatase 1 suppresses PPARalpha-mediated gene transcription and non-small-cell lung cancer progression. Am. J. Cancer Res. 13, 4742–4754 (2023).
Wang, Z. et al. Fructose-1,6-bisphosphatase 1 functions as a protein phosphatase to dephosphorylate histone H3 and suppresses PPARalpha-regulated gene transcription and tumour growth. Nat. Cell Biol. 24, 1655–1665 (2022). This study is the first to report a metabolic enzyme functioning as a protein phosphatase.
Li, B. et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 513, 251–255 (2014).
Moreno-Yruela, C. et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci. Adv. 8, eabi6696 (2022).
Torrini, C. et al. Lactate is an epigenetic metabolite that drives survival in model systems of glioblastoma. Mol. Cell 82, 3061–3076.e6 (2022).
Latham, T. et al. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 40, 4794–4803 (2012).
Takata, N. et al. Lactate-dependent transcriptional regulation controls mammalian eye morphogenesis. Nat. Commun. 14, 4129 (2023).
Heim, C. E. et al. Lactate production by Staphylococcus aureus biofilm inhibits HDAC11 to reprogramme the host immune response during persistent infection. Nat. Microbiol. 5, 1271–1284 (2020).
Zhu, R. et al. ACSS2 acts as a lactyl-CoA synthetase and couples KAT2A to function as a lactyltransferase for histone lactylation and tumor immune evasion. Cell Metab. 37, 361–376 (2024). This study is the first to identify a mammalian lactyl-coenzyme A synthetase.
Liu, R. et al. Nuclear GTPSCS functions as a lactyl-CoA synthetase to promote histone lactylation and gliomagenesis. Cell Metab. 37, 377–394 (2024).
Wang, S. et al. Lactate reprograms glioblastoma immunity through CBX3-regulated histone lactylation. J. Clin. Investig. 134, e176851 (2024).
Huang, Z. W. et al. STAT5 promotes PD-L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia. Signal. Transduct. Target. Ther. 8, 391 (2023).
Li, W. et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy 20, 114–130 (2024).
He, Y. et al. Numb/Parkin-directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation. Cell Rep. 42, 112033 (2023).
Li, X. M. et al. Histone lactylation inhibits RARgamma expression in macrophages to promote colorectal tumorigenesis through activation of TRAF6-IL-6-STAT3 signaling. Cell Rep. 43, 113688 (2024).
Noe, J. T. et al. Lactate supports a metabolic–epigenetic link in macrophage polarization. Sci. Adv. 7, eabi8602 (2021).
Ippolito, L. et al. Lactate rewires lipid metabolism and sustains a metabolic–epigenetic axis in prostate cancer. Cancer Res. 82, 1267–1282 (2022).
Gao, X., Wang, H., Yang, J. J., Liu, X. & Liu, Z. R. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell 45, 598–609 (2012).
Damasceno, L. E. A. et al. PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation. J. Exp. Med. 217, e20190613 (2020).
Angiari, S. et al. Pharmacological activation of pyruvate kinase M2 inhibits CD4(+) T cell pathogenicity and suppresses autoimmunity. Cell Metab. 31, 391–405.e8 (2020).
Zhao, X. et al. Pyruvate kinase M2 interacts with nuclear sterol regulatory element-binding protein 1a and thereby activates lipogenesis and cell proliferation in hepatocellular carcinoma. J. Biol. Chem. 293, 6623–6634 (2018).
Yu, P. et al. PKM2-c-Myc-survivin cascade regulates the cell proliferation, migration, and tamoxifen resistance in breast cancer. Front. Pharmacol. 11, 550469 (2020).
Luo, W. et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 (2011).
Zheng, F. et al. The HIF-1alpha antisense long non-coding RNA drives a positive feedback loop of HIF-1alpha mediated transactivation and glycolysis. Nat. Commun. 12, 1341 (2021).
Palsson-McDermott, E. M. et al. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab. 21, 347 (2015).
Yang, L. et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 5, 4436 (2014).
Hamabe, A. et al. Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial–mesenchymal transition. Proc. Natl Acad. Sci. USA 111, 15526–15531 (2014).
Morfouace, M. et al. Control of glioma cell death and differentiation by PKM2–Oct4 interaction. Cell Death Dis. 5, e1036 (2014).
Jin, X. et al. Pyruvate kinase M2 promotes the activation of dendritic cells by enhancing IL-12p35 expression. Cell Rep. 31, 107690 (2020).
Guo, D., Meng, Y., Jiang, X. & Lu, Z. Hexokinases in cancer and other pathologies. Cell Insight 2, 100077 (2023).
Guo, D. et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IκBα. Cell Metab. 34, 1312–1324.e6 (2022). This study reveals that the Warburg effect can directly regulate tumour cell immune evasion through the protein kinase activity of hexokinase 2.
He, H., Xiao, L., Wang, J., Guo, D. & Lu, Z. Aerobic glycolysis promotes tumor immune evasion and tumor cell stemness through the noncanonical function of hexokinase 2. Cancer Commun. 43, 387–390 (2023).
Thomas, G. E. et al. The metabolic enzyme hexokinase 2 localizes to the nucleus in AML and normal haematopoietic stem and progenitor cells to maintain stemness. Nat. Cell Biol. 24, 872–884 (2022).
Dasgupta, S. et al. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer. Nature 556, 249–254 (2018).
Meng, J., Chen, X. & Han, Z. PFKFB4 promotes lung adenocarcinoma progression via phosphorylating and activating transcriptional coactivator SRC-2. BMC Pulm. Med. 21, 60 (2021).
Xu, D. et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature 580, 530–535 (2020). This study reveals an integrated regulation of glucose metabolism and lipogenesis by the protein kinase activity of phosphoenolpyruvate carboxykinase 1.
Jiang, H., Zhu, L., Xu, D. & Lu, Z. A newly discovered role of metabolic enzyme PCK1 as a protein kinase to promote cancer lipogenesis. Cancer Commun. 40, 389–394 (2020).
Shao, F. et al. Association of phosphoenolpyruvate carboxykinase 1 protein kinase activity-dependent sterol regulatory element-binding protein 1 activation with prognosis of oesophageal carcinoma. Eur. J. Cancer 142, 123–131 (2020).
Liao, K. et al. A feedback circuitry between polycomb signaling and fructose-1,6-bisphosphatase enables hepatic and renal tumorigenesis. Cancer Res. 80, 675–688 (2020).
Huangyang, P. et al. Fructose-1,6-bisphosphatase 2 inhibits sarcoma progression by restraining mitochondrial biogenesis. Cell Metab. 31, 174–188.e7 (2020).
De Rosa, V. et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 16, 1174–1184 (2015).
Liu, Q. et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell 184, 5559–5576.e19 (2021).
Zheng, L., Roeder, R. G. & Luo, Y. S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 114, 255–266 (2003).
Gao, X. et al. NleB, a bacterial effector with glycosyltransferase activity, targets GAPDH function to inhibit NF-kappaB activation. Cell Host Microbe 13, 87–99 (2013).
Mondragon, L. et al. GAPDH overexpression in the T cell lineage promotes angioimmunoblastic T cell lymphoma through an NF-kappaB-dependent mechanism. Cancer Cell 36, 268–287.e10 (2019).
Lee, J. H. et al. EGFR-phosphorylated platelet isoform of phosphofructokinase 1 promotes PI3K activation. Mol. Cell 70, 197–210.e7 (2018). This study reveals that phosphofructokinase 1, a rate-limiting glycolytic enzyme, functions as a signalling molecule essential for receptor tyrosine kinase-mediated PI3K–AKT activation.
Lee, J. H. et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat. Commun. 8, 949 (2017).
Lee, J. H. et al. Phosphofructokinase 1 platelet isoform promotes beta-catenin transactivation for tumor development. Front. Oncol. 10, 211 (2020).
Enzo, E. et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 34, 1349–1370 (2015).
Gao, X. et al. Nuclear PFKP promotes CXCR4-dependent infiltration by T cell acute lymphoblastic leukemia. J. Clin. Invest. 131, e143119 (2021).
Song, J. et al. Aldolase A accelerates cancer progression by modulating mRNA translation and protein biosynthesis via noncanonical mechanisms. Adv. Sci. 10, e2302425 (2023).
Singh, R. & Green, M. R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science 259, 365–368 (1993).
Ding, R. et al. Lactate modulates RNA splicing to promote CTLA-4 expression in tumor-infiltrating regulatory T cells. Immunity 57, 528–540 (2024).
Sun, L. et al. Lactylation of METTL16 promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric cancer. Nat. Commun. 14, 6523 (2023).
Sizemore, S. T. et al. Pyruvate kinase M2 regulates homologous recombination-mediated DNA double-strand break repair. Cell Res. 28, 1090–1102 (2018).
Qu, J. et al. Phosphoglycerate mutase 1 regulates dNTP pool and promotes homologous recombination repair in cancer cells. J. Cell Biol. 216, 409–424 (2017).
Gustafsson, N. M. S. et al. Targeting PFKFB3 radiosensitizes cancer cells and suppresses homologous recombination. Nat. Commun. 9, 3872 (2018).
Shi, M. et al. GAPDH facilitates homologous recombination repair by stabilizing RAD51 in an HDAC1-dependent manner. EMBO Rep. 24, e56437 (2023).
Ci, S. et al. Src-mediated phosphorylation of GAPDH regulates its nuclear localization and cellular response to DNA damage. FASEB J. 34, 10443–10461 (2020).
Sobanski, T. et al. The fructose-bisphosphate, Aldolase A (ALDOA), facilitates DNA-PKcs and ATM kinase activity to regulate DNA double-strand break repair. Sci. Rep. 13, 15171 (2023).
Wu, S. et al. Pyruvate facilitates FACT-mediated gammaH2AX loading to chromatin and promotes the radiation resistance of glioblastoma. Adv. Sci. 9, e2104055 (2022).
Qian, X. et al. Phosphoglycerate kinase 1 phosphorylates Beclin1 to induce autophagy. Mol. Cell 65, 917–931 (2017).
Roberts, D. J., Tan-Sah, V. P., Ding, E. Y., Smith, J. M. & Miyamoto, S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol. Cell 53, 521–533 (2014).
Qian, X., Li, X. & Lu, Z. Protein kinase activity of the glycolytic enzyme PGK1 regulates autophagy to promote tumorigenesis. Autophagy 13, 1246–1247 (2017).
He, C. L. et al. Pyruvate kinase M2 activates mTORC1 by phosphorylating AKT1S1. Sci. Rep. 6, 21524 (2016).
Lee, M. N. et al. Glycolytic flux signals to mTOR through glyceraldehyde-3-phosphate dehydrogenase-mediated regulation of Rheb. Mol. Cell. Biol. 29, 3991–4001 (2009).
Chang, C. et al. AMPK-dependent phosphorylation of GAPDH triggers Sirt1 activation and is necessary for autophagy upon glucose starvation. Mol. Cell 60, 930–940 (2015).
Iqbal, I. K., Bajeli, S., Sahu, S., Bhat, S. A. & Kumar, A. Hydrogen sulfide-induced GAPDH sulfhydration disrupts the CCAR2–SIRT1 interaction to initiate autophagy. Autophagy 17, 3511–3529 (2021).
Huang, R. et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 57, 456–466 (2015).
Li, M. et al. Fructose-1,6-bisphosphatase 1 dephosphorylates and inhibits TERT for tumor suppression. Nat. Chem. Biol. 20, 1505–1513 (2024). This study reveals that fructose-1,6-bisphosphatase 1 functions as a protein phosphatase, negatively regulating TERT and telomeres to govern immortality and ageing.
Zhu, W. et al. Fructose-1,6-bisphosphatase 1 dephosphorylates IκBα and suppresses colorectal tumorigenesis. Cell Res. 33, 245–257 (2023).
Zhang, G., Tao, J., Lin, L., Qiu, W. & Lu, Z. Repurposing FBP1: dephosphorylating IκBα to suppress NFκB. Cell Res. 33, 419–420 (2023).
Yang, J. S. et al. GAPDH inhibits intracellular pathways during starvation for cellular energy homeostasis. Nature 561, 263–267 (2018).
Jacquin, M. A. et al. GAPDH binds to active Akt, leading to Bcl-xL increase and escape from caspase-independent cell death. Cell Death Differ. 20, 1043–1054 (2013).
Jang, M. et al. Glyceraldehyde-3-phosphate, a glycolytic intermediate, plays a key role in controlling cell fate via inhibition of caspase activity. Mol. Cell 28, 559–563 (2009).
Majewski, N. et al. Hexokinase–mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell 16, 819–830 (2004).
Qi, H. et al. Succinylation-dependent mitochondrial translocation of PKM2 promotes cell survival in response to nutritional stress. Cell Death Dis. 10, 170 (2019).
Liang, J. et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 27, 329–351 (2017).
Huang, C. et al. Lactate promotes resistance to glucose starvation via upregulation of Bcl-2 mediated by mTOR activation. Oncol. Rep. 33, 875–884 (2015).
Hsu, C. C. et al. Inositol serves as a natural inhibitor of mitochondrial fission by directly targeting AMPK. Mol. Cell 81, 3803–3819.e7 (2021).
Meng, Y. et al. Glycolytic enzyme PFKL governs lipolysis by promoting lipid droplet-mitochondria tethering to enhance beta-oxidation and tumor cell proliferation. Nat. Metab. 6, 1092–1107 (2024). This study reveals that phosphofructokinase, liver-type coordinately regulates glycolysis and lipolysis through its protein kinase activity.
Chen, J. et al. PFKP alleviates glucose starvation-induced metabolic stress in lung cancer cells via AMPK-ACC2 dependent fatty acid oxidation. Cell Discov. 8, 52 (2022).
Zhang, T. et al. G6PD maintains the VSMC synthetic phenotype and accelerates vascular neointimal hyperplasia by inhibiting the VDAC1-Bax-mediated mitochondrial apoptosis pathway. Cell Mol. Biol. Lett. 29, 47 (2024).
Zhong, B. et al. Glucose-6-phosphate dehydrogenase neutralizes stresses by supporting reductive glutamine metabolism and AMPK activation. Signal. Transduct. Target. Ther. 6, 46 (2021).
Li, X. et al. Nuclear PGK1 alleviates ADP-dependent inhibition of CDC7 to promote DNA replication. Mol. Cell 72, 650–660 (2018).
Jiang, Y. et al. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol. Cell 53, 75–87 (2014). This study reveals that pyruvate kinase M2 functions as a protein kinase and directly regulates mitosis progression.
Jiang, Y. et al. PKM2 phosphorylates MLC2 and regulates cytokinesis of tumour cells. Nat. Commun. 5, 5566 (2014).
Liu, W. et al. Lactate regulates cell cycle by remodelling the anaphase promoting complex. Nature 616, 790–797 (2023). This study reveals that lactate functions as an anaphase-promoting complex/cyclosome modulator to control mitotic exit.
Orozco, J. M. et al. Dihydroxyacetone phosphate signals glucose availability to mTORC1. Nat. Metab. 2, 893–901 (2020).
Liu, J. et al. Metabolic enzyme LDHA activates Rac1 GTPase as a noncanonical mechanism to promote cancer. Nat. Metab. 4, 1830–1846 (2022).
Qian, X. et al. PTEN suppresses glycolysis by dephosphorylating and inhibiting autophosphorylated PGK1. Mol. Cell 76, 516–527.e7 (2019).
Liu, F. et al. PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat. Cell Biol. 19, 1358–1370 (2017).
Yang, Y. C. et al. Cytosolic PKM2 stabilizes mutant EGFR protein expression through regulating HSP90–EGFR association. Oncogene 36, 4234 (2017).
Keller, K. E., Doctor, Z. M., Dwyer, Z. W. & Lee, Y. S. SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol. Cell 53, 700–709 (2014).
Cheng, T. Y. et al. Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene 37, 1730–1742 (2018).
Zhang, D. et al. Phosphoglycerate mutase 1 promotes cancer cell migration independent of its metabolic activity. Oncogene 36, 2900–2909 (2017).
Blaha, C. S. et al. A non-catalytic scaffolding activity of hexokinase 2 contributes to EMT and metastasis. Nat. Commun. 13, 899 (2022).
Li, M. et al. Aldolase B suppresses hepatocellular carcinogenesis by inhibiting G6PD and pentose phosphate pathways. Nat. Cancer 1, 735–747 (2020).
He, X. et al. Loss of hepatic aldolase B activates Akt and promotes hepatocellular carcinogenesis by destabilizing the Aldob/Akt/PP2A protein complex. PLoS Biol. 18, e3000803 (2020).
Gu, L. et al. Fructose-1,6-bisphosphatase is a nonenzymatic safety valve that curtails AKT activation to prevent insulin hyperresponsiveness. Cell Metab. 35, 1009–1021.e1009 (2023).
Wang, J. et al. A non-metabolic function of hexokinase 2 in small cell lung cancer: promotes cancer cell stemness by increasing USP11-mediated CD133 stability. Cancer Commun. 42, 1008–1027 (2022).
Ma, Q. et al. The moonlighting function of glycolytic enzyme enolase-1 promotes choline phospholipid metabolism and tumor cell proliferation. Proc. Natl Acad. Sci. USA 120, e2209435120 (2023).
Yang, W. & Lu, Z. Pyruvate kinase M2 at a glance. J. Cell Sci. 128, 1655–1660 (2015).
Oslund, R. C. et al. Bisphosphoglycerate mutase controls serine pathway flux via 3-phosphoglycerate. Nat. Chem. Biol. 13, 1081–1087 (2017).
Hitosugi, T. et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell 22, 585–600 (2012).
Cai, X. et al. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol. Cell 83, 3904–3920.e7 (2023).
Li, F. et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat. Cell Biol. 22, 728–739 (2020).
Dou, X. et al. PDK4-dependent hypercatabolism and lactate production of senescent cells promotes cancer malignancy. Nat. Metab. 5, 1887–1910 (2023).
Wei, Y. et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat. Commun. 8, 14041 (2017).
Hou, P. P. et al. Ectosomal PKM2 promotes HCC by inducing macrophage differentiation and remodeling the tumor microenvironment. Mol. Cell 78, 1192–1206.e10 (2020).
Funasaka, T., Hogan, V. & Raz, A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial and mesenchymal phenotype conversions in breast cancer. Cancer Res. 69, 5349–5356 (2009).
Han, J. et al. GPI is a prognostic biomarker and correlates with immune infiltrates in lung adenocarcinoma. Front. Oncol. 11, 752642 (2021).
Tsutsumi, S., Hogan, V., Nabi, I. R. & Raz, A. Overexpression of the autocrine motility factor/phosphoglucose isomerase induces transformation and survival of NIH-3T3 fibroblasts. Cancer Res. 63, 242–249 (2003).
Lu, Z., Ghosh, S., Wang, Z. & Hunter, T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 4, 499–515 (2003).
Lay, A. J. et al. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature 408, 869–873 (2000).
Colbert, L. E. et al. Tumor-resident Lactobacillus iners confer chemoradiation resistance through lactate-induced metabolic rewiring. Cancer Cell 41, 1945–1962.e11 (2023). This study reveals that tumour-resident probiotic bacterial species produce lactate, thereby regulating tumour metabolism and gene expression.
Wolf, A. J. et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166, 624–636 (2016).
Jia, J. et al. Protection of extraribosomal RPL13a by GAPDH and dysregulation by S-nitrosylation. Mol. Cell 47, 656–663 (2012).
Wang, X. et al. A GAPDH serotonylation system couples CD8(+) T cell glycolytic metabolism to antitumor immunity. Mol. Cell 84, 760–775.e7 (2024).
Khan, F. et al. Lactate dehydrogenase A regulates tumor-macrophage symbiosis to promote glioblastoma progression. Nat. Commun. 15, 1987 (2024).
Terrasse, R. et al. Human and pneumococcal cell surface glyceraldehyde-3-phosphate dehydrogenase (GAPDH) proteins are both ligands of human C1q protein. J. Biol. Chem. 287, 42620–42633 (2012).
Chen, T. et al. NSUN2 is a glucose sensor suppressing cGAS/STING to maintain tumorigenesis and immunotherapy resistance. Cell Metab. 35, 1782–1798.e8 (2023).
Huang, T. Y. et al. Phosphoenolpyruvate regulates the Th17 transcriptional program and inhibits autoimmunity. Cell Rep. 42, 112205 (2023).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015). This study reveals that phosphoenolpyruvate promotes the antitumour functions of CD4+ and CD8+ T cells by regulating calcium signalling and gene expression.
Zhang, W. et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 178, 176–189.e15 (2019).
Feng, T. et al. Adipocyte-derived lactate is a signalling metabolite that potentiates adipose macrophage inflammation via targeting PHD2. Nat. Commun. 13, 5208 (2022).
Goswami, S., Zhang, Q., Celik, C. E., Reich, E. M. & Yilmaz, O. H. Dietary fat and lipid metabolism in the tumor microenvironment. Biochim. Biophys. Acta Rev. Cancer 1878, 188984 (2023).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014).
Chen, Y. D., Varasteh, B. B. & Reaven, G. M. Plasma lactate concentration in obesity and type 2 diabetes. Diabete Metab. 19, 348–354 (1993).
Stine, Z. E., Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 21, 141–162 (2022).
Mellinghoff, I. K. et al. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. N. Engl. J. Med. 389, 589–601 (2023).
Li, H. et al. FBP1 regulates proliferation, metastasis, and chemoresistance by participating in C-MYC/STAT3 signaling axis in ovarian cancer. Oncogene 40, 5938–5949 (2021).
Wenes, M. et al. The mitochondrial pyruvate carrier regulates memory T cell differentiation and antitumor function. Cell Metab. 34, 731–746.e9 (2022).
Cao, Z. et al. Lactate oxidase nanocapsules boost T cell immunity and efficacy of cancer immunotherapy. Sci. Transl. Med. 15, eadd2712 (2023). This study presents a potential strategy to modulate lactate levels in the tumour microenvironment to enhance T cell function for more effective cancer immunotherapy.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (82188102 and 82030074 to Z.L.; 82372816 to D.G.; 82203553 to Y.M.; 82002445 to G.Z.), the Ministry of Science and Technology of the People’s Republic of China (2020YFA0803300 to Z.L.) and the National Center of Technology Innovation for Biopharmaceuticals (NCTIB2022HS02006 to Z.L.). Z.L. is the Kuancheng Wang Distinguished Chair.
Author information
Authors and Affiliations
Contributions
D.G., Y.M., G.Z. and Z.L. researched data for the article. D.G., Y.M. and G.Z. contributed substantially to the discussion of the content. Z.L. wrote the article. D.G., Y.M., Q.W. and Z.L. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Sara Sdelci and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Ataxia telangiectasia
-
A rare autosomal recessive genetic disorder characterized by progressive neurological degeneration (ataxia), telangiectasias (visible blood vessel abnormalities), immune deficiencies, increased cancer risk and hypersensitivity to ionizing radiation due to impaired DNA repair.
- Autophagosome
-
A double-membrane vesicle that engulfs cellular components for degradation during autophagy.
- Cytokinesis
-
The final stage of cell division in which the cytoplasm divides, resulting in two daughter cells.
- Damage-associated molecular pattern
-
Molecules released by stressed or damaged cells owing to trauma or an infection by a pathogen, acting as endogenous danger signals and triggering an immune response.
- Ectosomes
-
Extracellular vesicles released directly from the plasma membrane, made up of proteins, mRNAs and microRNAs, and involved in intercellular communication.
- Homologous recombination
-
A DNA repair pathway that uses a homologous sequence as a template to accurately repair double-strand breaks.
- Inflammasome
-
A cytosolic multiprotein complex that activates inflammatory responses and cell death by promoting cytokine maturation and pyroptosis.
- Isomerization
-
A chemical reaction that rearranges molecules to form isomers, altering their structure without changing their molecular formula.
- Kinetochore
-
A protein complex structure on the centromere of a chromosome that serves as the attachment site for spindle microtubules during cell division.
- Lactylation
-
A post-translational modification involving the addition of a lactyl group to lysine residues on proteins, using lactyl-coenzyme A or lactate as an acylation donor.
- Lipid droplet
-
Cellular organelles that store neutral lipids, such as triglycerides and cholesterol esters, for energy storage, membrane formation and protection from lipotoxicity.
- Lipolysis
-
The breakdown of stored lipids to release free fatty acids and glycerol for energy metabolism.
- Liquid–liquid phase separation
-
The process by which biomolecules spontaneously separate into highly concentrated liquid-like droplets, giving rise to membraneless organelles.
- Mitochondrial fission
-
The division of a mitochondrion into two separate mitochondrial organelles, essential for cellular energy regulation and quality control.
- Mitophagy
-
A selective form of autophagy in which damaged or dysfunctional mitochondria are degraded to maintain cellular health.
- Non-homologous end joining
-
(NHEJ). A DNA repair pathway that fixes double-strand breaks by directly ligating the broken DNA ends without requiring a homologous sequence as a template.
- Oxidative phosphorylation
-
A mitochondrial process in which ATP is produced using energy from electrons transferred through the electron transport chain.
- Pattern recognition receptor
-
Immune receptors that recognize pathogen-associated molecular patterns or damage-associated molecular patterns to initiate immune responses.
- Pentose phosphate pathway
-
(PPP). A metabolic pathway parallel to glycolysis that generates NADPH and ribose-5-phosphate for antioxidant reactions and nucleotide biosynthesis.
- Serotonylation
-
A post-translational modification in which serotonin is covalently attached to glutamine residues within proteins, driven by transglutaminases, and affecting protein function and cellular signalling.
- S-nitrosylation
-
A post-translational modification that occurs when nitric oxide covalently attaches to a cysteine thiol group on a protein to form an S-nitrosothiol, regulating protein function and cellular signalling.
- Succinylation
-
A post-translational modification in which a succinyl group is added to lysine residues, driven both enzymatically and non-enzymatically, altering protein function and activity.
- Sulfhydration
-
A modification involving the addition of hydrogen sulfide to cysteine residues in proteins, affecting their activity and stability.
- Sumoylation
-
A post-translational modification in which small ubiquitin-like modifier (SUMO) proteins are attached to lysine residues of target proteins, influencing their activity and stability.
- Telomere
-
Protective DNA–protein structures at the ends of chromosomes that prevent genomic instability during replication.
- Transnitrosylation
-
The transfer of a nitric oxide group between proteins, driven both enzymatically and non-enzymatically, regulating protein conformational changes, activity, intracellular trafficking and protein–protein interactions.
- Tricarboxylic acid (TCA) cycle
-
A central metabolic pathway that generates energy by oxidizing acetyl-coenzyme A into carbon dioxide and producing NADH and FADH2 for ATP synthesis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, D., Meng, Y., Zhao, G. et al. Moonlighting functions of glucose metabolic enzymes and metabolites in cancer. Nat Rev Cancer 25, 426–446 (2025). https://doi.org/10.1038/s41568-025-00800-3
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41568-025-00800-3
This article is cited by
-
Immune evasion by macrophage-derived lactate
Nature Cell Biology (2026)
-
Deacetylation of TALDO1 by HDAC6 promotes glycolysis and nasopharyngeal carcinoma progression through a moonlighting function
Cell Death & Disease (2025)
-
Nucleus-translocated glucokinase functions as a protein kinase to phosphorylate TAZ and promote tumour growth
Nature Communications (2025)
-
Nuclear-localized metabolic enzymes: emerging key players in tumor epigenetic regulation
Molecular and Cellular Biochemistry (2025)