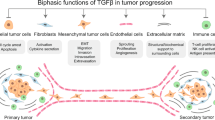
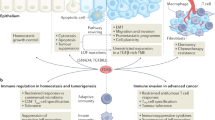
Abstract
The transforming growth factor-β (TGFβ) paradox refers to the well-established role of TGFβ in suppressing cancer in healthy tissues yet promoting malignancy in established cancers. Although this positioned TGFβ inhibitors as a potential therapeutic strategy for malignancy, therapuetic blockade has failed in multiple clinical trials. The general lack of selection principles for defining which patients would most benefit from the addition of a TGFβ inhibitor has probably hindered its deployment. Here, we highlight the therapeutic potential in TGFβ regulation of DNA repair using human papillomavirus (HPV)-driven head and neck squamous cell carcinoma (HNSCC) as an illustrative example. HPV inhibits TGFβ signalling, which in turn reduces DNA damage repair, ultimately conferring sensitivity to cancer treatments and thus contributing to the favourable prognosis of HPV-positive HNSCC. Here, we review the DNA repair deficit caused by a loss of TGFβ signalling and how this could be targeted to induce synthetic lethality. Moreover, we explore its role in predicting response to immune checkpoint inhibitors and the potential of biomarkers to select which patients with cancer could ultimately benefit from TGFβ inhibition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Massagué, J. & Sheppard, D. TGF-β signaling in health and disease. Cell 186, 4007–4037 (2023).
Nixon, B. G., Gao, S., Wang, X. & Li, M. O. TGFβ control of immune responses in cancer: a holistic immuno-oncology perspective. Nat. Rev. Immunol. 23, 346–362 (2022).
Batlle, E. & Massague, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 (2019).
Chen, J. et al. Targeting transforming growth factor-β signaling for enhanced cancer chemotherapy. Theranostics 11, 1345–1363 (2021).
Derynck, R., Turley, S. J. & Akhurst, R. J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 18, 9–34 (2021).
Moreau, J. M., Velegraki, M., Bolyard, C., Rosenblum, M. D. & Li, Z. Transforming growth factor-β1 in regulatory T cell biology. Sci. Immunol. 7, eabi4613 (2022).
Tian, M. & Schiemann, W. P. The TGF-β paradox in human cancer: an update. Future Oncol. 5, 259–271 (2009).
Glick, A. B. et al. Loss of expression of transforming growth factor β in skin and skin tumors is associated with hyperproliferation and a high risk for malignant conversion. Proc. Natl Acad. Sci. USA 90, 6076–6080 (1993).
Glick, A. B. et al. Targeted deletion of the TGF-β1 gene causes rapid progression to squamous cell carcinoma. Genes. Dev. 8, 2429–2440 (1994).
Korkut, A. et al. A pan-cancer analysis reveals high-frequency genetic alterations in mediators of signaling by the TGF-β superfamily. Cell Syst. 7, 422–437.e7 (2018).
Barcellos-Hoff, M. H. The radiobiology of TGFβ. Semin. Cancer Biol. 86, 857–867 (2022).
Teicher, B. A., Ikebe, M., Ara, G., Keyes, S. R. & Herbst, R. S. Transforming growth factor-β1 overexpression produces drug resistance in vivo: reversal by decorin. In Vivo 11, 463–472 (1997).
Teicher, B. A., Holden, S. A., Ara, G. & Chen, G. Transforming growth factor-β in in vivo resistance. Cancer Chemother. Pharmacol. 37, 601–609 (1996).
Liu, P., Menon, K., Alvarez, E., Lu, K. & Teicher, B. A. Transforming growth factor-β and response to anticancer therapies in human liver and gastric tumors in vitro and in vivo. Int. J. Oncol. 16, 599–610 (2000).
Ehrhart, E. J., Carroll, A., Segarini, P., Tsang, M. L.-S. & Barcellos-Hoff, M. H. Latent transforming growth factor-β activation in situ: quantitative and functional evidence following low dose irradiation. FASEB J. 11, 991–1002 (1997).
Barcellos-Hoff, M. H. & Dix, T. A. Redox-mediated activation of latent transforming growth factor-β1. Molec Endocrin 10, 1077–1083 (1996).
Barcellos-Hoff, M. H. Radiation-induced transforming growth factor β and subsequent extracellular matrix reorganization in murine mammary gland. Cancer Res. 53, 3880–3886 (1993).
Vanpouille-Box, C. et al. Transforming growth factor (TGF) β is a master regulator of radiotherapy-induced anti-tumor immunity. Cancer Res. 75, 2232–2242 (2015).
Holmgaard, R. B. et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J. Immunother. Cancer 6, 47 (2018).
Dodagatta-Marri, E. et al. α-PD-1 therapy elevates Treg/TH balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J. Immunother. Cancer 7, 62 (2019).
Lind, H. et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J. Immunother. Cancer 8, e000433 (2020).
Martin, C. J. et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci. Transl. Med. 12, eaay8456 (2020).
Ciardiello, D., Elez, E., Tabernero, J. & Seoane, J. Clinical development of therapies targeting TGFβ: current knowledge and future perspectives. Ann. Oncol. 31, 1336–1349 (2020).
Anido, J. et al. TGF-β receptor inhibitors target the CD44high/Id1high glioma-initiating cell population in human glioblastoma. Cancer Cell 18, 655–668 (2010).
Rodon, J. et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin. Cancer Res. 21, 553–560 (2015).
Wick, A. et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-β receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Invest. N. Drugs 38, 1570–1579 (2020).
Sharma, P. et al. The next decade of immune checkpoint therapy. Cancer Discov. 11, 838–857 (2021).
Huang, C.-Y. et al. Recent progress in TGF-β inhibitors for cancer therapy. Biomed. Pharmacother. 134, 111046 (2021).
Metropulos, A. E., Munshi, H. G. & Principe, D. R. The difficulty in translating the preclinical success of combined TGFβ and immune checkpoint inhibition to clinical trial. eBioMedicine 86, 104380 (2022).
Ang, K. K. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010).
Lohaus, F. et al. HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother. Oncol. 113, 317–323 (2014).
Liu, Q., Lopez, K., Murnane, J., Humphrey, T. & Barcellos-Hoff, M. H. Misrepair in context: TGFβ regulation of DNA repair. Front. Oncol. 9, 799 (2019).
Kirshner, J. et al. Inhibition of transforming growth factor-β1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res. 66, 10861–10869 (2006).
Bornstein, S. et al. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J. Clin. Invest. 119, 3408–3419 (2009).
Haeger, S. M. et al. Smad4 loss promotes lung cancer formation but increases sensitivity to DNA topoisomerase inhibitors. Oncogene 35, 577–586 (2016).
Liu, Q. et al. Loss of TGFβ signaling increases alternative end-joining DNA repair that sensitizes to genotoxic therapies across cancer types. Sci. Transl. Med. 13, eabc4465 (2021).
Liu, Q. et al. Subjugation of TGFβ signaling by human papilloma virus in head and neck squamous cell carcinoma shifts DNA repair from homologous recombination to alternative end joining. Clin. Cancer Res. 24, 6001–6014 (2018).
Guix, I. et al. Validation of anticorrelated TGFβ signaling and alternative end-joining DNA repair signatures that predict response to genotoxic cancer therapy. Clin. Cancer Res. 28, 1372–1382 (2022).
Sandhu, C. et al. TGF-β stabilizes p15INK4B protein, increases p15INK4B/cdk4 complexes and inhibits cyclin D1/cdk4 association in human mammary epithelial cells. Mol. Cell. Biol. 17, 2458–2467 (1997).
Moody, C. A. & Laimins, L. A. Human papillomavirus oncoproteins: pathways to transformation. Nat. Rev. Cancer 10, 550–560 (2010).
Levovitz, C. et al. TGFβ receptor 1: an immune susceptibility gene in HPV-associated cancer. Cancer Res. 74, 6833–6844 (2014).
Ceccaldi, R., Rondinelli, B. & D’Andrea, A. D. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 26, 52–64 (2016).
Glick, A. B., Weinberg, W. C., Wu, I. H., Quan, W. & Yuspa, S. H. Transforming growth factor β1 suppresses genomic instability independent of a G1 arrest, p53, and Rb. Cancer Res. 56, 3645–3650 (1996).
Maxwell, C. A. et al. Targeted and nontargeted effects of ionizing radiation that impact genomic instability. Cancer Res. 68, 8304–8311 (2008).
Kanamoto, T., Hellman, U., Heldin, C. H. & Souchelnytskyi, S. Functional proteomics of transforming growth factor-β1-stimulated Mv1Lu epithelial cells: Rad51 as a target of TGFβ1-dependent regulation of DNA repair. EMBO J. 21, 1219–1230 (2002).
Kim, M. R. et al. TGFβ protects cells from gamma-IR by enhancing the activity of the NHEJ repair pathway. Mol. Cancer Res. 13, 319–329 (2015).
Lee, J., Kim, M. R., Kim, H. J., An, Y. S. & Yi, J. Y. TGF-β1 accelerates the DNA damage response in epithelial cells via Smad signaling. Biochem. Biophys. Res. Commun. 476, 420–425 (2016).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3, 155–168 (2003).
Ewan, K. B. et al. Transforming growth factor-β1 mediates cellular response to DNA damage in situ. Cancer Res. 62, 5627–5631 (2002).
Tsai, W.-B., Chung, Y. M., Takahashi, Y., Xu, Z. & Hu, M. C. T. Functional interaction between FOXO3a and ATM regulates DNA damage response. Nat. Cell Biol. 10, 460–467 (2008).
Chung, Y. M. et al. FOXO3 signalling links ATM to the p53 apoptotic pathway following DNA damage. Nat. Commun. 3, 1000 (2012).
Martinez-Ruiz, H. et al. A TGFβ–miR-182–BRCA1 axis controls the mammary differentiation hierarchy. Sci. Signal. 9, ra118 (2016).
Deng, Y. et al. Transcriptional down-regulation of Brca1 and E-cadherin by CtBP1 in breast cancer. Mol. Carcinog. 51, 500–507 (2012).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Bennardo, N., Cheng, A., Huang, N. & Stark, J. M. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 4, e1000110 (2008).
Iliakis, G., Murmann, T. & Soni, A. Alternative end-joining repair pathways are the ultimate backup for abrogated classical non-homologous end-joining and homologous recombination repair: implications for the formation of chromosome translocations. Mutat. Res. Genet. Toxicol. Env. Mutagen. 793, 166–175 (2015).
Ceccaldi, R. et al. Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature 518, 258–262 (2015).
Wood, R. D. & Doublie, S. DNA polymerase θ (POLQ), double-strand break repair, and cancer. DNA Repair. 44, 22–32 (2016).
Ramsden, D. A., Carvajal-Garcia, J. & Gupta, G. P. Mechanism, cellular functions and cancer roles of polymerase-θ-mediated DNA end joining. Nat. Rev. Mol. Cell Biol. 23, 125–140 (2021).
Sallmyr, A. & Tomkinson, A. E. Repair of DNA double-strand breaks by mammalian alternative end-joining pathways. J. Biol. Chem. 293, 10536–10546 (2018).
Espín, R. et al. Harnessing transcriptional regulation of alternative end-joining to predict cancer treatment. NAR Cancer 7, zcaf007 (2025).
Du, S. et al. Attenuation of the DNA damage response by TGFβ inhibitors enhances radiation sensitivity of NSCLC cells in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 91, 91–99 (2014).
Hardee, M. E. et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 72, 4119–4129 (2012).
Bouquet, S. F. et al. Transforming growth factor β1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin. Cancer Res. 17, 6754–6765 (2011).
Zhang, M. et al. Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 71, 7155–7167 (2011).
Zhang, M. et al. Trimodal glioblastoma treatment consisting of concurrent radiotherapy, temozolomide, and the novel TGF-β receptor I kinase inhibitor LY2109761. Neoplasia 13, 537–549 (2011).
Young, K. H. et al. TGFβ inhibition prior to hypofractionated radiation enhances efficacy in preclinical models. Cancer Immunol. Res. 2, 1011–1022 (2014).
Jank, B. J. et al. Radiosensitizing effect of galunisertib, a TGF-β receptor I inhibitor, on head and neck squamous cell carcinoma in vitro. Invest. N. Drugs 40, 478–486 (2022).
Liberzon, A. et al. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Taube, J. H. et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc. Natl Acad. Sci. USA 107, 15449–15454 (2010).
Andarawewa, K. L. et al. Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor β induced epithelial to mesenchymal transition. Cancer Res. 67, 8662–8670 (2007).
Chowdhury, S. et al. Proteogenomic analysis of chemo-refractory high-grade serous ovarian cancer. Cell 186, 3476–3498.e35 (2023).
Cleary, J. M., Aguirre, A. J., Shapiro, G. I. & D’Andrea, A. D. Biomarker-guided development of DNA repair inhibitors. Mol. Cell 78, 1070–1085 (2020).
Morris, Z. S. et al. Proceedings of the National Cancer Institute Workshop on combining immunotherapy with radiotherapy: challenges and opportunities for clinical translation. Lancet Oncol. 26, e152–e170 (2025).
Roberts, A. B. & Wakefield, L. M. The two faces of transforming growth factor β in carcinogenesis. Proc. Natl Acad. Sci. USA 100, 8621–8623 (2003).
Yang, Y. et al. The outcome of TGFβ antagonism in metastatic breast cancer models in vivo reflects a complex balance between tumor-suppressive and proprogression activities of TGFβ. Clin. Cancer Res. 26, 643–656 (2020).
Guo, W. et al. Targeting the TGF-β signaling pathway: an updated patent review (2021–present). Expert. Opin. Ther. Pat. 34, 99–126 (2024).
Danielpour, D. Advances and challenges in targeting TGF-β isoforms for therapeutic intervention of cancer: a mechanism-based perspective. Pharmaceuticals 17, 533 (2024).
Flanders, K. C. et al. Quantitation of TGF-β proteins in mouse tissues shows reciprocal changes in TGF-β1 and TGF-β3 in normal vs neoplastic mammary epithelium. Oncotarget 7, 38164–38179 (2016).
Terabe, M. et al. Blockade of only TGF-β1 and 2 is sufficient to enhance the efficacy of vaccine and PD-1 checkpoint blockade immunotherapy. Oncoimmunology 6, e1308616 (2017).
Canè, S., Van Snick, J., Uyttenhove, C., Pilotte, L. & Van den Eynde, B. J. TGFβ1 neutralization displays therapeutic efficacy through both an immunomodulatory and a non-immune tumor-intrinsic mechanism. J. Immunother. Cancer 9, e001798 (2021).
Yom, S. S. et al. Reduced-dose radiation therapy for HPV-associated oropharyngeal carcinoma (NRG Oncology HN002). J. Clin. Oncol. 39, 956–965 (2021).
Drew, Y., Zenke, F. T. & Curtin, N. J. DNA damage response inhibitors in cancer therapy: lessons from the past, current status and future implications. Nat. Rev. Drug Discov. 24, 19–39 (2025).
Zhang, M., Lahn, M. & Huber, P. E. Translating the combination of TGFβ blockade and radiotherapy into clinical development in glioblastoma. Oncoimmunology 1, 943–945 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02688712 (2025).
Yamazaki, T. et al. Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a single-arm, phase 2 trial. Lancet Oncol. 23, 1189–1200 (2022).
Feng, W., Smith, C. M., Simpson, D. A. & Gupta, G. P. Targeting non-homologous and alternative end joining repair to enhance cancer radiosensitivity. Semin. Radiat. Oncol. 32, 29–41 (2022).
Zatreanu, D. et al. Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance. Nat. Commun. 12, 3636 (2021).
Zhou, J. et al. A first-in-class polymerase θ inhibitor selectively targets homologous-recombination-deficient tumors. Nat. Cancer 2, 598–610 (2021).
Hoppe, M. M., Sundar, R., Tan, D. S. P. & Jeyasekharan, A. D. Biomarkers for homologous recombination deficiency in cancer. J. Natl Cancer Inst. 110, 704–713 (2018).
Gonzalez-Martin, A. et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 381, 2391–2402 (2019).
Mellman, I., Chen, D. S., Powles, T. & Turley, S. J. The cancer-immunity cycle: indication, genotype, and immunotype. Immunity 56, 2188–2205 (2023).
Lynch, C., Pitroda, S. P. & Weichselbaum, R. R. Radiotherapy, immunity, and immune checkpoint inhibitors. Lancet Oncol. 25, e352–e362 (2024).
Gulley, J. L. et al. Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment. Mol. Oncol. 16, 2117–2134 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02517398 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03427411 (2023).
Strauss, J. et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J. Immunother. Cancer 8, e001395 (2020).
Gameiro, S. R., Strauss, J., Gulley, J. L. & Schlom, J. Preclinical and clinical studies of bintrafusp alfa, a novel bifunctional anti-PD-L1/TGFβRII agent: current status. Exp. Biol. Med. 247, 1124–1134 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03631706 (2025).
Barcellos-Hoff, M. H. & Gulley, J. L. Molecular pathways and mechanisms of TGFβ in cancer therapy. Clin. Cancer Res. 29, 2025–2033 (2023).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Lake, R. A. & Robinson, B. W. Immunotherapy and chemotherapy—a practical partnership. Nat. Rev. Cancer 5, 397–405 (2005).
Lu, C. et al. DNA sensing in mismatch repair-deficient tumor cells is essential for anti-tumor immunity. Cancer Cell 39, 96–108.e106 (2021).
Turajlic, S. et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 18, 1009–1021 (2017).
Moore, J. et al. Identification of a conserved subset of cold tumors responsive to immune checkpoint blockade. J. Immunother. Cancer 13, e010528 (2025).
Hugo, W. et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 165, 35–44 (2016).
Dhainaut, M. et al. Spatial CRISPR genomics identifies regulators of the tumor microenvironment. Cell 185, 1223–1239.e20 (2022).
Kim, B.-G., Malek, E., Choi, S. H., Ignatz-Hoover, J. J. & Driscoll, J. J. Novel therapies emerging in oncology to target the TGF-β pathway. J. Hematol. Oncol. 14, 55 (2021).
Böttinger, E. P., Letterio, J. J. & Roberts, A. B. Biology of TGF-β in knockout and transgenic mouse models. Kidney Int. 51, 1355–1360 (1997).
Kitisin, K. et al. TGF-β signaling in development. Sci. STKE https://doi.org/10.1126/stke.3992007cm1 (2007).
Shah, M., Foreman, D. M. & Ferguson, M. W. J. Control of scarring in adult wounds by neutralizing antibody to transforming growth factor β. Lancet 339, 213–214 (1992).
Beck, L. S. et al. TGF-β1 accelerates wound healing: reversal of steroid-impaired healing in rats and rabbits. Growth Factors 5, 295–304 (1991).
Liarte, S., Bernabé-García, Á. & Nicolás, F. J. Role of TGF-β in skin chronic wounds: a keratinocyte perspective. Cells 9, 306 (2020).
Sánchez-Capelo, A. Dual role for TGF-β1 in apoptosis. Cytokine Growth Factor. Rev. 16, 15–34 (2005).
Acknowledgements
The authors acknowledge J. Gkantalis for draft figures. They also thank the research contributions of collaborators and laboratory members on which this Perspective is based. The authors also acknowledge funding from the University of California, San Francisco (UCSF) Department of Radiation Oncology, the Genentech imCORE network and National Institutes of Health (NIH) grants R01CA239235 and R01CA270332 (to M.H.B.-H.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to the article.
Corresponding author
Ethics declarations
Competing interests
M.H.B.-H. reports grants and non-financial support from Roche/Genentech; personal fees and non-financial support from Innovation Pathways, Inc.; consulting fees from Scholar Rock and Vericyte; non-financial support from Bicara during the conduct of the study; spoke at a meeting organized by for-profit Hanson-Wade in 2022 and 2023; has a patent pending for PCT/US2021/037078; and is a co-inventor on a patent pending (18/009,885) and a provisional patent (18/602,978) related to the βAlt signature owned by University of California, San Francisco (UCSF). S.S.Y. reports research support from Bristol-Myers Squibb, Merck, EMD Serono, Biomimetix and Nanobiotix.
Peer review
Peer review information
Nature Reviews Cancer thanks L. Wakefield, C.-H. Heldin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barcellos-Hoff, M.H., Yom, S.S. Revisiting the TGFβ paradox: insights from HPV-driven cancer and the DNA damage response. Nat Rev Cancer 25, 534–544 (2025). https://doi.org/10.1038/s41568-025-00819-6
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41568-025-00819-6