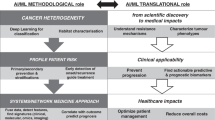
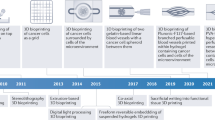
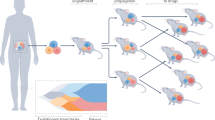
Abstract
Development of acquired therapeutic resistance limits the efficacy of cancer treatments and accounts for therapeutic failure in most patients. How resistance arises, varies across cancer types and differs depending on therapeutic modalities is incompletely understood. Novel strategies that address and overcome the various and complex resistance mechanisms necessitate a deep understanding of the underlying dynamics. We are at a crucial time when innovative technologies applied to patient-relevant tumour models have the potential to bridge the gap between fundamental research into mechanisms and timing of acquired resistance and clinical applications that translate these findings into actionable strategies to extend therapy efficacy. Unprecedented spatial and time-resolved high-throughput platforms generate vast amounts of data, from which increasingly complex information can be extracted and analysed through artificial intelligence and machine learning-based approaches. This Roadmap outlines key mechanisms that underlie the acquisition of therapeutic resistance in cancer and explores diverse modelling strategies. Clinically relevant, tractable models of disease and biomarker-driven precision approaches are poised to transform the landscape of acquired therapy resistance in cancer and its clinical management. Here, we propose an integrated strategy that leverages next-generation technologies to dissect the complexities of therapy resistance, shifting the paradigm from reactive management to predictive and proactive prevention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
13 June 2025
In the version of the article initially published, the Fig. 1 legend inadvertently omitted to state that the figure is adapted from Laisné, M., Lupien, M. & Vallot, C. Epigenomic heterogeneity as a source of tumour evolution. Nat. Rev. Cancer 25, 7–26 (2025). This has now been corrected in the HTML and PDF versions of the article.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Gerstung, M. et al. The evolutionary history of 2,658 cancers. Nature 578, 122–128 (2020).
ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature 578, 82–93 (2020).
Klein, C. A. Selection and adaptation during metastatic cancer progression. Nature 501, 365–372 (2013).
Dentro, S. C. et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 184, 2239–2254.e39 (2021).
Bhandari, V. et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 51, 308–318 (2019).
Bhandari, V., Li, C. H., Bristow, R. G. & Boutros, P. C. Divergent mutational processes distinguish hypoxic and normoxic tumours. Nat. Commun. 11, 737 (2020).
De Palma, M., Biziato, D. & Petrova, T. V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Emert, B. L. et al. Variability within rare cell states enables multiple paths toward drug resistance. Nat. Biotechnol. 39, 865–876 (2021).
Taplin, M. E. et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N. Engl. J. Med. 332, 1393–1398 (1995).
Cahill, D. P. et al. Mutations of mitotic checkpoint genes in human cancers. Nature 392, 300–303 (1998).
Watanabe, T. et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 344, 1196–1206 (2001).
Robey, R. W. et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 18, 452–464 (2018).
Dhanyamraju, P. K., Schell, T. D., Amin, S. & Robertson, G. P. Drug-tolerant persister cells in cancer therapy resistance. Cancer Res. 82, 2503–2514 (2022).
Morin, P. J. Drug resistance and the microenvironment: nature and nurture. Drug Resist. Updat. 6, 169–172 (2003).
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J. & Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120 (2020).
Almeida, F. V., Douglass, S. M., Fane, M. E. & Weeraratna, A. T. Bad company: microenvironmentally mediated resistance to targeted therapy in melanoma. Pigment Cell Melanoma Res. 32, 237–247 (2019).
National Cancer Institute. Cancer Moonshot Blue Ribbon Panel Report 2016 https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/history/blue-ribbon-panel-report-2016.pdf (2025).
West, J. et al. Towards Multidrug Adaptive Therapy. Cancer Res. 80, 1578–1589 (2020).
Marine, J.-C., Dawson, S.-J. & Dawson, M. A. Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Rev. Cancer 20, 743–756 (2020).
Whiting, F. J. H., Househam, J., Baker, A. M., Sottoriva, A. & Graham, T. A. Phenotypic noise and plasticity in cancer evolution. Trends Cell Biology. 34, 451–464 (2024).
Terekhanova, N. V. et al. Epigenetic regulation during cancer transitions across 11 tumour types. Nature 623, 432–441 (2023).
Hagenbeek, T. J. et al. An allosteric pan-TEAD inhibitor blocks oncogenic YAP/TAZ signaling and overcomes KRAS G12C inhibitor resistance. Nat. Cancer 4, 812–828 (2023).
Zhang, Y. et al. Metabolic switch regulates lineage plasticity and induces synthetic lethality in triple-negative breast cancer. Cell Metab. 36, 193–208.e8 (2024).
Hu, Q. et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat. Immunol. 20, 835–851 (2019).
Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010).
Wang, H. et al. Antiandrogen treatment induces stromal cell reprogramming to promote castration resistance in prostate cancer. Cancer Cell 41, 1345–1362.e9 (2023).
Denmeade, S. R. et al. TRANSFORMER: a randomized phase II study comparing bipolar androgen therapy versus enzalutamide in asymptomatic men with castration-resistant metastatic prostate cancer. J. Clin. Oncol. 39, 1371–1382 (2021).
Sena, L. A. et al. Bipolar androgen therapy sensitizes castration-resistant prostate cancer to subsequent androgen receptor ablative therapy. Eur. J. Cancer 144, 302–309 (2021).
Gao, A. & Guo, M. Epigenetic based synthetic lethal strategies in human cancers. Biomark. Res. 8, 44 (2020).
Boudreau, A. et al. Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat. Chem. Biol. 12, 779–786 (2016).
Stine, Z. E., Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 21, 141–162 (2022).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Olou, A. A., King, R. J., Yu, F. & Singh, P. K. MUC1 oncoprotein mitigates ER stress via CDA-mediated reprogramming of pyrimidine metabolism. Oncogene 39, 3381–3395 (2020).
Cheung, E. C. & Vousden, K. H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 22, 280–297 (2022).
Osman, A. A. et al. Dysregulation and epigenetic reprogramming of NRF2 signaling axis promote acquisition of cisplatin resistance and metastasis in head and neck squamous cell carcinoma. Clin. Cancer Res. 29, 1344–1359 (2023). This study identifies that acquired cisplatin resistance in head and neck squamous cell carcinoma is driven by dysregulation of the KEAP1–NRF2 signalling axis, where epigenetic reprogramming and mutations in KEAP1 lead to enhanced NRF2 activation.
Yu, W. et al. Evolution of cisplatin resistance through coordinated metabolic reprogramming of the cellular reductive state. Br. J. Cancer 128, 2013–2024 (2023).
Tadros, S. et al. De novo lipid synthesis facilitates gemcitabine resistance through endoplasmic reticulum stress in pancreatic cancer. Cancer Res. 77, 5503–5517 (2017).
Shukla, S. K. et al. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell 32, 392 (2017).
Mullen, N. J. & Singh, P. K. Nucleotide metabolism: a pan-cancer metabolic dependency. Nat. Rev. Cancer 23, 275–294 (2023).
Murthy, D. et al. Cancer-associated fibroblast-derived acetate promotes pancreatic cancer development by altering polyamine metabolism via the ACSS2-SP1-SAT1 axis. Nat. Cell Biol. 26, 613–627 (2024).
Murthy, D. et al. The MUC1-HIF-1α signaling axis regulates pancreatic cancer pathogenesis through polyamine metabolism remodeling. Proc. Natl Acad. Sci. USA 121, e2315509121 (2024).
King, R. J. et al. CD73 induces GM-CSF/MDSC-mediated suppression of T cells to accelerate pancreatic cancer pathogenesis. Oncogene 41, 971–982 (2022).
He, C. et al. Vitamin B6 competition in the tumor microenvironment hampers antitumor functions of NK cells. Cancer Discov. 14, 176–193 (2024).
Mehta, A. & Stanger, B. Z. Lineage plasticity: the new cancer hallmark on the block Cancer Res. 84, 184–191 (2024).
Beltran, H. et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin. Cancer Res. 25, 6916–6924 (2019).
Cannell, I. G. et al. FOXC2 promotes vasculogenic mimicry and resistance to anti-angiogenic therapy. Cell Rep. 42, 112791 (2023).
He, J. et al. Drug tolerant persister cell plasticity in cancer: a revolutionary strategy for more effective anticancer therapies. Signal Transduct. Target. Ther. 9, 209 (2024).
Mancini, C., Lori, G., Pranzini, E. & Taddei, M. L. Metabolic challengers selecting tumor-persistent cells. Trends Endocrinol. Metab. 35, 263–276 (2024).
Russo, M. et al. Cancer drug-tolerant persister cells: from biological questions to clinical opportunities. Nat. Rev. Cancer 24, 694–717 (2024).
Marsolier, J. et al. H3K27me3 conditions chemotolerance in triple-negative breast cancer. Nat. Genet. 54, 459–468 (2022). By identifying how chromatin landscapes influence drug-resistant persister cells in triple-negative breast cancer, this study highlights H3K27me3 as a therapeutic target to delay acquired resistance and tumour recurrence.
França, G. S. et al. Cellular adaptation to cancer therapy along a resistance continuum. Nature 631, 876–883 (2024). This study introduces the concept of resistance continuum, whereby cancer cells undergo multiple state transitions encompassing mutational, epigenetic and transcriptional changes.
Guler, G. D. et al. Repression of stress-Induced LINE-1 expression protects cancer cell subpopulations from lethal drug exposure. Cancer Cell 32, 221–237.e13 (2017).
National Comprehensive Cancer Network. Treatment by cancer type. nccn.org https://www.nccn.org/guidelines/category_1 (2025).
Vasan, N., Baselga, J. & Hyman, D. M. A view on drug resistance in cancer. Nature 575, 299–309 (2019).
Misale, S. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486, 532–536 (2012).
André Fabrice, et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Braun, T. P., Eide, C. A. & Druker, B. J. Response and resistance to BCR-ABL1-targeted therapies. Cancer Cell 37, 530–542 (2020).
Matulonis, U. A. et al. Ovarian cancer. Nat. Rev. Dis. Prim. 2, 16061 (2016).
Christie, E. L. & Bowtell, D. D. L. Acquired chemotherapy resistance in ovarian cancer. Ann. Oncol. 28, viii13–viii15 (2017).
Osman, A. A. et al. Evolutionary action score of TP53 coding variants is predictive of platinum response in head and neck cancer patients. Cancer Res. 75, 1205–1215 (2015).
Pal Choudhuri, S. et al. Acquired cross-resistance in small cell lung cancer due to extrachromosomal DNA amplification of MYC paralogs. Cancer Discov. 14, 804–827 (2024).
Prasanna, P. G. et al. Therapy-induced senescence: opportunities to improve anticancer therapy. J. Natl Cancer Inst. 113, 1285–1298 (2021).
Schmitt, C. A., Wang, B. & Demaria, M. Senescence and cancer — role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 19, 619–636 (2022).
Vander Velde, R. et al. Resistance to targeted therapies as a multifactorial, gradual adaptation to inhibitor specific selective pressures. Nat. Commun. 11, 2393 (2020).
O’Dwyer, P. J. et al. The NCI-MATCH trial: lessons for precision oncology. Nat. Med. 29, 1349–1357 (2023).
Sicklick, J. K. et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med. 25, 744–750 (2019). The I-PREDICT study demonstrates that personalized, multi-drug regimens based on molecular profiling can improve disease control in patients with advanced cancers, highlighting the potential for personalized combination therapies over single-agent approaches.
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Tyner, J. W. et al. Understanding drug sensitivity and tackling resistance in cancer. Cancer Res. 82, 1448–1460 (2022).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Gouda, M. A. & Subbiah, V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open 8, 100788 (2023).
Hanrahan, A. J., Chen, Z., Rosen, N. & Solit, D. B. BRAF - a tumour-agnostic drug target with lineage-specific dependencies. Nat. Rev. Clin. Oncol. 21, 224–247 (2024).
Knudsen, E. S., Witkiewicz, A. K. & Rubin, S. M. Cancer takes many paths through G1/S. Trends Cell Biol. 34, 636–645 (2024).
Gerosa, R. et al. Cyclin-dependent kinase 2 (CDK2) inhibitors and others novel CDK inhibitors (CDKi) in breast cancer: clinical trials, current impact, and future directions. Crit. Rev. Oncol. Hematol. 196, 104324 (2024).
Kollmann, K. et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell 24, 167–181 (2013).
Singhal, A., Li, B. T. & O’Reilly, E. M. Targeting KRAS in cancer. Nat. Med. 30, 969–983 (2024).
Awad, M. M. et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N. Engl. J. Med. 384, 2382–2393 (2021).
Schoenfeld, A. J. & Hellmann, M. D. Acquired resistance to immune checkpoint inhibitors. Cancer Cell 37, 443–455 (2020).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016). This study shows that acquired resistance to PD1 blockade in melanoma is driven by mutations affecting interferon-receptor signalling (JAK1 and JAK2) and antigen presentation.
Chowell, D. et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359, 582–587 (2018).
Sade-Feldman, M. et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8, 1136 (2017).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Gao, J. et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167, 397–404.e9 (2016).
Shin, D. S. et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 7, 188–201 (2017).
Pulanco, M. C., Madsen, A. T., Tanwar, A., Corrigan, D. T. & Zang, X. Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies. Cell Mol. Immunol. 20, 694–713 (2023).
Hugo, W. et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 168, 542 (2017).
Lee, J. H. et al. Transcriptional downregulation of MHC class I and melanoma de- differentiation in resistance to PD-1 inhibition. Nat. Commun. 11, 1897 (2020).
Wang, L. et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat. Commun. 9, 3503 (2018).
O’Hare, T. et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 16, 401–412 (2009).
Khorashad, J. S. et al. BCR-ABL1 compound mutations in tyrosine kinase inhibitor-resistant CML: frequency and clonal relationships. Blood 121, 489–498 (2013).
Joshi, S. K. et al. The AML microenvironment catalyzes a stepwise evolution to gilteritinib resistance. Cancer Cell 39, 999–1014.e8 (2021).
Intlekofer, A. M. et al. Acquired resistance to IDH inhibition through trans or cis dimer-interface mutations. Nature 559, 125–129 (2018).
Perner, F. et al. MEN1 mutations mediate clinical resistance to menin inhibition. Nature 615, 913–919 (2023).
Furman, R. R. et al. Ibrutinib resistance in chronic lymphocytic leukemia. N. Engl. J. Med. 370, 2352–2354 (2014).
Blombery, P. et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 9, 342–353 (2019).
McMahon, C. M. et al. Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov. 9, 1050–1063 (2019). Emergence of gilteritinib resistance, a FLT3 inhibitor, in FLT3-mutated AML is driven by diverse clonal adaptations and evolution, highlighting the need for combination therapies to overcome complex resistance patterns upon FLT3 kinase inhibition.
Zhang, H. et al. Clinical resistance to crenolanib in acute myeloid leukemia due to diverse molecular mechanisms. Nat. Commun. 10, 244 (2019).
Woyach, J. A. et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 370, 2286–2294 (2014).
Harding, J. J. et al. Isoform switching as a mechanism of acquired resistance to mutant isocitrate dehydrogenase inhibition. Cancer Discov. 8, 1540–1547 (2018).
Bonnet, D. & Dick, J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730–737 (1997).
Sachs, K. et al. Single-cell gene expression analyses reveal distinct self-renewing and proliferating subsets in the leukemia stem cell compartment in acute myeloid leukemia. Cancer Res. 80, 458–470 (2020).
Hope, K. J., Jin, L. & Dick, J. E. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat. Immunol. 5, 738–743 (2004).
Kuusanmäki, H. et al. Erythroid/megakaryocytic differentiation confers BCL-XL dependency and venetoclax resistance in acute myeloid leukemia. Blood 141, 1610–1625 (2023).
Kuusanmäki, H. et al. Phenotype-based drug screening reveals association between venetoclax response and differentiation stage in acute myeloid leukemia. Haematologica 105, 708–720 (2020).
Majumder, M. M. et al. Multi-parametric single cell evaluation defines distinct drug responses in healthy hematologic cells that are retained in corresponding malignant cell types. Haematologica 105, 1527–1538 (2020).
Pei, S. et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 10, 536–551 (2020).
Zhang, H. et al. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat. Cancer 1, 826–839 (2020).
Nuno, K. et al. Convergent epigenetic evolution drives relapse in acute myeloid leukemia. eLife 13, e93019 (2024).
Zeng, A. G. X. et al. A cellular hierarchy framework for understanding heterogeneity and predicting drug response in acute myeloid leukemia. Nat. Med. 28, 1212–1223 (2022).
Bottomly, D. et al. Integrative analysis of drug response and clinical outcome in acute myeloid leukemia. Cancer Cell 40, 850–864.e9 (2022).
Kopper, O. et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 25, 838–849 (2019).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Phan, N. et al. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol. 2, 78 (2019).
Wang, H.-M. et al. Using patient-derived organoids to predict locally advanced or metastatic lung cancer tumor response: A real-world study. Cell Rep. Med. 4, 100911 (2023).
Al Shihabi, A. et al. The landscape of drug sensitivity and resistance in sarcoma. Cell Stem Cell 31, 1524–1542.e4 (2024). This paper demonstrates feasibility of establishing PDOs from serially collected samples of rare cancers and performing large-scale drug screenings within a clinically actionable timeframe.
Zhao, Z. et al. Organoids. Nat. Rev. Methods Prim. 2, 94 (2022).
Drost, J. & Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 18, 407–418 (2018).
Pine, A. R. et al. Tumor microenvironment is critical for the maintenance of cellular states found in primary glioblastomas. Cancer Discov. 10, 964–979 (2020).
Nguyen, H. T. L. et al. A platform for rapid patient-derived cutaneous neurofibroma organoid establishment and screening. Cell Rep. Methods 4, 100772 (2024).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
Öhlund, D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 (2017).
Langer, E. M. et al. Modeling tumor phenotypes in vitro with three-dimensional bioprinting. Cell Rep. 26, 608–623.e6 (2019).
Tebon, P. J. et al. Drug screening at single-organoid resolution via bioprinting and interferometry. Nat. Commun. 14, 3168 (2023).
Polak, R., Zhang, E. T. & Kuo, C. J. Cancer organoids 2.0: modelling the complexity of the tumour immune microenvironment. Nat. Rev. Cancer 24, 523–539 (2024).
Zhou, Z. et al. Harnessing 3D in vitro systems to model immune responses to solid tumours: a step towards improving and creating personalized immunotherapies. Nat. Rev. Immunol. 24, 18–32 (2024).
Ding, S. et al. Patient-derived micro-organospheres enable clinical precision oncology. Cell Stem Cell 29, 905–917.e6 (2022).
Dekkers, J. F. et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat. Biotechnol. 41, 60–69 (2023).
Alieva, M. et al. BEHAV3D: a 3D live imaging platform for comprehensive analysis of engineered T cell behavior and tumor response. Nat. Protoc. 19, 2052–2084 (2024).
Dekkers, J. F. et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14, 1756–1771 (2019).
Sufi, J. et al. Multiplexed single-cell analysis of organoid signaling networks. Nat. Protoc. 16, 4897–4918 (2021).
Qin, X. et al. Cell-type-specific signaling networks in heterocellular organoids. Nat. Methods 17, 335–342 (2020). This study presents a multiplexed mass cytometry protocol enabling single-cell analysis of PTMs within organoids co-cultured with stromal cells, illustrating how cellular signalling networks and cell states adapt to therapeutic resistance.
Song, H. et al. Epitranscriptomics and epiproteomics in cancer drug resistance: therapeutic implications. Signal Transduct. Target. Ther. 5, 193 (2020).
Steiner, S. et al. Ex vivo modeling of acquired drug resistance in BRAF - mutated pancreatic cancer organoids uncovers individual therapeutic vulnerabilities. Cancer Lett. 584, 216650 (2024).
Tang, M. et al. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 30, 833–853 (2020).
Paek, J. et al. Microphysiological engineering of self-assembled and perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS Nano 13, 7627–7643 (2019).
Jing, X. et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 18, 157 (2019).
Ingber, D. E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 23, 467–491 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06064682 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05890781 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03890614 (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06406608 (2024).
Wang, H. et al. Pharmacogenomic profiling of pediatric acute myeloid leukemia to identify therapeutic vulnerabilities and inform functional precision medicine Blood Cancer Discov. 3, 516–535 (2022).
Pemovska, T. et al. Individualized systems medicine strategy to tailor treatments for patients with chemorefractory acute myeloid leukemia. Cancer Discov. 3, 1416–1429 (2013). Leveraging clinical specimens, this paper uses a personalized functional testing approach to identify effective regimens and predict disease evolution.
Tognon, C. E., Sears, R. C., Mills, G. B., Gray, J. W. & Tyner, J. W. Ex vivo analysis of primary tumor specimens for evaluation of cancer therapeutics. Annu. Rev. Cancer Biol. 5, 39–57 (2021).
Tyner, J. W. et al. Kinase pathway dependence in primary human leukemias determined by rapid inhibitor screening. Cancer Res. 73, 285–296 (2013).
Barbaglio, F. et al. Three-dimensional co-culture model of chronic lymphocytic leukemia bone marrow microenvironment predicts patient-specific response to mobilizing agents. Haematologica 106, 2334–2344 (2021).
Kurtz, S. E. et al. Dual inhibition of JAK1/2 kinases and BCL2: a promising therapeutic strategy for acute myeloid leukemia. Leukemia 32, 2025–2028 (2018).
Eide, C. A. et al. Simultaneous kinase inhibition with ibrutinib and BCL2 inhibition with venetoclax offers a therapeutic strategy for acute myeloid leukemia. Leukemia 34, 2342–2353 (2020).
Eldfors, S. et al. Monosomy 7/del(7q) cause sensitivity to inhibitors of nicotinamide phosphoribosyltransferase in acute myeloid leukemia. Blood Adv. 8, 1621–1633 (2024).
Nechiporuk, T. et al. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov. 9, 910–925 (2019).
Jafari, M. et al. Bipartite network models to design combination therapies in acute myeloid leukaemia. Nat. Commun. 13, 2128 (2022).
Mirzaie, M. et al. Designing patient-oriented combination therapies for acute myeloid leukemia based on efficacy/toxicity integration and bipartite network modeling. Oncogenesis 13, 11 (2024).
Kytölä, S. et al. Ex vivo venetoclax sensitivity predicts clinical response in acute myeloid leukemia in the prospective VenEx trial. Blood 145, 409–421 (2025).
Kuusanmäki, H. et al. Ex vivo venetoclax sensitivity testing predicts treatment response in acute myeloid leukemia. Haematologica 108, 1768–1781 (2023).
Bray, L. J. et al. A three-dimensional ex vivo tri-culture model mimics cell-cell interactions between acute myeloid leukemia and the vascular niche. Haematologica 102, 1215–1226 (2017).
de Janon, A., Mantalaris, A. & Panoskaltsis, N. Three-dimensional human bone marrow organoids for the study and application of normal and abnormal hematoimmunopoiesis. J. Immunol. 210, 895–904 (2023).
Khan, A. O. et al. Human bone marrow organoids for disease modeling, discovery, and validation of therapeutic targets in hematologic malignancies Cancer Discov. 13, 364–385 (2023).
Olijnik, A. A. et al. Generating human bone marrow organoids for disease modeling and drug discovery. Nat. Protoc. 19, 2117–2146 (2024).
Sommerkamp, P., Mercier, F. E., Wilkinson, A. C., Bonnet, D. & Bourgine, P. E. Engineering human hematopoietic environments through ossicle and bioreactor technologies exploitation. Exp. Hematol. 94, 20–25 (2021).
Bourgine, P. E. et al. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc. Natl Acad. Sci. USA 115, E5688–e5695 (2018).
Dozzo, A. et al. Modelling acute myeloid leukemia (AML): What’s new? A transition from the classical to the modern. Drug Deliv. Transl. Res. 13, 2110–2141 (2023).
García-García, A. et al. Culturing patient-derived malignant hematopoietic stem cells in engineered and fully humanized 3D niches. Proc. Natl Acad. Sci. USA 118, e2114227118 (2021).
Borella, G. et al. Targeting the plasticity of mesenchymal stromal cells to reroute the course of acute myeloid leukemia. Blood 138, 557–570 (2021).
Giallongo, S. et al. Engagement of mesenchymal stromal cells in the remodeling of the bone marrow microenvironment in hematological cancers. Biomolecules 13, 1701 (2023).
Barozzi, D. & Scielzo, C. Emerging strategies in 3D culture models for hematological cancers. Hemasphere 7, e932 (2023).
Koc, S. et al. PDXNet portal: patient-derived xenograft model, data, workflow and tool discovery. NAR Cancer 4, zcac014 (2022).
Liu, Y. et al. Patient-derived xenograft models in cancer therapy: technologies and applications. Signal Transduct. Target. Ther. 8, 160 (2023).
Meric-Bernstam, F. et al. Assessment of patient-derived xenograft growth and antitumor activity: the NCI PDXNet consensus recommendations. Mol. Cancer Ther. 23, 924–938 (2024).
Guillen, K. P. et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat. Cancer 3, 232–250 (2022).
Knudsen, E. S. et al. Pancreatic cancer cell lines as patient-derived avatars: genetic characterisation and functional utility. Gut 67, 508–520 (2018).
Sun, H. et al. Comprehensive characterization of 536 patient-derived xenograft models prioritizes candidatesfor targeted treatment. Nat. Commun. 12, 5086 (2021).
Yao, Y. et al. Clinical utility of PDX cohorts to reveal biomarkers of intrinsic resistance and clonal architecture changes underlying acquired resistance to cetuximab in HNSCC. Signal Transduct. Target. Ther. 7, 73 (2022).
Zanella, E. R., Grassi, E. & Trusolino, L. Towards precision oncology with patient-derived xenografts. Nat. Rev. Clin. Oncol. 19, 719–732 (2022).
Kahounová, Z. et al. Circulating tumor cell-derived preclinical models: current status and future perspectives. Cell Death Dis. 14, 530 (2023).
Dietrich, C. et al. INX-315, a selective CDK2 inhibitor, induces cell cycle arrest and senescence in solid tumors. Cancer Discov. 14, 446–467 (2024).
Gao, H. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 21, 1318–1325 (2015).
Knudsen, E. S. et al. Targeting dual signalling pathways in concert with immune checkpoints for the treatment of pancreatic cancer. Gut 70, 127–138 (2021).
Witkiewicz, A. K. et al. Integrated patient-derived models delineate individualized therapeutic vulnerabilities of pancreatic cancer. Cell Rep. 16, 2017–2031 (2016). This study finds that PDX models can recapitulate unique drug sensitivities in pancreatic cancer that cannot be predicted by genetic analysis alone, highlighting the potential of functional drug sensitivity testing to guide individualized therapies.
Chuprin, J. et al. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 20, 192–206 (2023).
Jeon, S. H. et al. Anti-4-1BB×PD-L1 bispecific antibody reinvigorates tumor-specific exhausted CD8+ T cells and enhances the efficacy of anti-PD-1 blockade. Clin. Cancer Res. 30, 4155–4166 (2024).
Knorr, D. A. et al. FcγRIIB Is an immune checkpoint limiting the activity of Treg-targeting antibodies in the tumor microenvironment. Cancer Immunol. Res. 12, 322–333 (2024).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9, 279–286 (2003).
Kochenderfer, J. N. & Rosenberg, S. A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 10, 267–276 (2013).
Wei, Y. et al. KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci. Immunol. 6, eabf9792 (2021).
Ren, X. et al. Blockade of the immunosuppressive KIR2DL5/PVR pathway elicits potent human NK cell-mediated antitumor immunity. J. Clin. Invest. 132, e163620 (2022).
Ren, X., Corrigan, D. T. & Zang, X. Protocol for evaluating antitumor activity of KIR3DL3 blockade in an NK cell-based xenogeneic lung tumor model. STAR Protoc. 3, 101818 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05958199 (2025).
Odunsi, A. et al. Fidelity of human ovarian cancer patient-derived xenografts in a partially humanized mouse model for preclinical testing of immunotherapies. J. Immunother. Cancer 8, e001237 (2020).
Ashizawa, T. et al. Antitumor effect of programmed death-1 (PD-1) blockade in humanized the NOG-MHC double knockout mouse. Clin. Cancer Res. 23, 149–158 (2017).
Jia, Q., Chu, H., Jin, Z., Long, H. & Zhu, B. High-throughput single-сell sequencing in cancer research. Signal Transduct. Target. Ther. 7, 145 (2022).
Tan, Y., Lin, H. & Cheng, J. X. Profiling single cancer cell metabolism via high-content SRS imaging with chemical sparsity. Sci. Adv. 9, eadg6061 (2023).
Blakely, C. M. et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat. Genet. 49, 1693–1704 (2017). This study uncovers the intrinsic and acquired heterogeneity of the gene alteration landscapes in advanced-stage non-small-cell lung cancers.
Cao, L. et al. Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell 184, 5031–5052.e26 (2021).
Zhang, K. et al. Longitudinal single-cell RNA-seq analysis reveals stress-promoted chemoresistance in metastatic ovarian cancer. Sci. Adv. 8, eabm1831 (2022).
Kim, C. et al. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell 173, 879–893.e13 (2018).
Zaidi, S. et al. Single-cell analysis of treatment-resistant prostate cancer: implications of cell state changes for cell surface antigen–targeted therapies. Proc. Natl Acad. Sci. USA 121, e2322203121 (2024).
Maynard, A. et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell 182, 1232–1251.e22 (2020). In this study, the authors apply scRNA-seq to longitudinally collected lung cancer biopsies and identify key transcriptional signatures distinctive of the residual disease and resistant tumours.
Bleijs, M., van de Wetering, M., Clevers, H. & Drost, J. Xenograft and organoid model systems in cancer research. EMBO J. 38, e101654 (2019).
Wang, E., Xiang, K., Zhang, Y. & Wang, X.-F. Patient-derived organoids (PDOs) and PDO-derived xenografts (PDOXs): new opportunities in establishing faithful pre-clinical cancer models. J. Natl Cancer Cent. 2, 263–276 (2022).
Zhang, Y. et al. Sample-multiplexing approaches for single-cell sequencing. Cell Mol. Life Sci. 79, 466 (2022).
Adil, A., Kumar, V., Jan, A. T. & Asger, M. Single-cell transcriptomics: current methods and challenges in data acquisition and analysis. Front. Neurosci. 15, 591122 (2021).
Sinha, S. et al. PERCEPTION predicts patient response and resistance to treatment using single-cell transcriptomics of their tumors. Nat. Cancer 5, 938–952 (2024). This study develops a machine learning platform based on scRNA-seq data that can help predict acquired resistance to therapy and identifying novel sensitivities.
Zhu, Y. et al. Spatially resolved proteome mapping of laser capture microdissected tissue with automated sample transfer to nanodroplets. Mol. Cell Proteom. 17, 1864–1874 (2018).
Espina, V. et al. Laser-capture microdissection. Nat. Protoc. 1, 586–603 (2006).
Ctortecka, C. et al. An automated nanowell-array workflow for quantitative multiplexed single-cell proteomics sample preparation at high sensitivity. Mol. Cell Proteom. 22, 100665 (2023).
Zhu, Y. et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10-100 mammalian cells. Nat. Commun. 9, 882 (2018).
van Galen, P. et al. Single-cell RNA-seq reveals AML hierarchies relevant to disease progression and immunity. Cell 176, 1265–1281.e24 (2019).
Eckert, S. et al. Decrypting the molecular basis of cellular drug phenotypes by dose-resolved expression proteomics. Nat. Biotechnol. 43, 406–415 (2024).
Bandyopadhyay, S. et al. Mapping the cellular biogeography of human bone marrow niches using single-cell transcriptomics and proteomic imaging. Cell 187, 3120–3140.e29 (2024).
Bosc, C. et al. Mitochondrial inhibitors circumvent adaptive resistance to venetoclax and cytarabine combination therapy in acute myeloid leukemia. Nat. Cancer 2, 1204–1223 (2021). This article shows how in AML, adaptive resistance to combination therapy is linked to mitochondrial adaptations, particularly oxidative phosphorylation, which can be targeted to delay relapse.
Hoyt, C. C. Multiplex immunofluorescence and multispectral imaging: forming the basis of a clinical test platform for immuno-oncology. Front. Mol. Biosci. 8, 674747 (2021).
Proietto, M. et al. Tumor heterogeneity: preclinical models, emerging technologies, and future applications. Front. Oncol. 13, 1164535 (2023).
Gaglia, G. et al. Temporal and spatial topography of cell proliferation in cancer. Nat. Cell Biol. 24, 316–326 (2022).
Pearson, J. D. et al. Binary pan-cancer classes with distinct vulnerabilities defined by pro- or anti-cancer YAP/TEAD activity. Cancer Cell 39, 1115–1134.e12 (2021).
Black, S. et al. CODEX multiplexed tissue imaging with DNA-conjugated antibodies. Nat. Protoc. 16, 3802–3835 (2021).
Kuett, L. et al. Three-dimensional imaging mass cytometry for highly multiplexed molecular and cellular mapping of tissues and the tumor microenvironment. Nat. Cancer 3, 122–133 (2022).
Marx, V. Method of the Year: spatially resolved transcriptomics. Nat. Methods 18, 9–14 (2021).
Sans, M. et al. Spatial transcriptomics of intraductal papillary mucinous neoplasms of the pancreas identifies NKX6-2 as a driver of gastric differentiation and indolent biological potential. Cancer Discov. 13, 1844–1861 (2023).
Denisenko, E. et al. Spatial transcriptomics reveals discrete tumour microenvironments and autocrine loops within ovarian cancer subclones. Nat. Commun. 15, 2860 (2024).
Izumi, M. et al. Integrative single-cell RNA-seq and spatial transcriptomics analyses reveal diverse apoptosis-related gene expression profiles in EGFR-mutated lung cancer. Cell Death Dis. 15, 580 (2024).
Kumarasamy, V., Vail, P., Nambiar, R., Witkiewicz, A. K. & Knudsen, E. S. Functional determinants of cell cycle plasticity and sensitivity to CDK4/6 inhibition. Cancer Res. 81, 1347–1360 (2021).
Witkiewicz, A. K. et al. Determinants of response to CDK4/6 inhibitors in the real-world setting. npj Precis. Oncol. 7, 90 (2023).
Kumarasamy, V. et al. The extracellular niche and tumor microenvironment enhance KRAS inhibitor efficacy in pancreatic cancer. Cancer Res. 84, 1115–1132 (2024).
Hickey, J. W. et al. T cell-mediated curation and restructuring of tumor tissue coordinates an effective immune response. Cell Rep. 42, 113494 (2023).
Hickey, J. W. et al. Integrating multiplexed imaging and multiscale modeling identifies tumor phenotype conversion as a critical component of therapeutic T cell efficacy. Cell Syst. 15, 322–338.e5 (2024).
Phillips, D. et al. Immune cell topography predicts response to PD-1 blockade in cutaneous T cell lymphoma. Nat. Commun. 12, 6726 (2021).
Wang, X. Q. et al. Spatial predictors of immunotherapy response in triple-negative breast cancer. Nature 621, 868–876 (2023).
Quek, C. et al. Single-cell spatial multiomics reveals tumor microenvironment vulnerabilities in cancer resistance to immunotherapy. Cell Rep. 43, 114392 (2024).
Taube, J. M. et al. Multi-institutional TSA-amplified multiplexed immunofluorescence reproducibility evaluation (MITRE) study. J. Immunother. Cancer 9, e002197 (2021).
Lord, C. J., Quinn, N. & Ryan, C. J. Integrative analysis of large-scale loss-of-function screens identifies robust cancer-associated genetic interactions. eLife 9, e58925 (2020).
McFarland, J. M. et al. Improved estimation of cancer dependencies from large-scale RNAi screens using model-based normalization and data integration. Nat. Commun. 9, 4610 (2018).
Pacini, C. et al. Integrated cross-study datasets of genetic dependencies in cancer. Nat. Commun. 12, 1661 (2021).
Kampmann, M. CRISPRi and CRISPRa screens in mammalian cells for precision biology and medicine. ACS Chem. Biol. 13, 406–416 (2018).
Li, S. et al. Gain-of-function genetic screening identifies the antiviral function of TMEM120A via STING activation. Nat. Commun. 13, 105 (2022).
Schmidt, R. et al. CRISPR activation and interference screens decode stimulation responses in primary human T cells. Science 375, eabj4008 (2022).
Liu, S. J. et al. In vivo perturb-seq of cancer and microenvironment cells dissects oncologic drivers and radiotherapy responses in glioblastoma. Genome Biol. 25, 256 (2024).
Han, K. et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature 580, 136–141 (2020). CRISPR screens in 3D cancer spheroids reveal vulnerabilities specific to the 3D growth environment, highlighting the importance of using physiologically accurate models for therapy prediction.
McLean, B. et al. A CRISPR path to finding vulnerabilities and solving drug resistance: targeting the diverse cancer landscape and its ecosystem. Adv. Genet. Hoboken 3, 2200014 (2022).
Coelho, M. A. et al. Base editing screens define the genetic landscape of cancer drug resistance mechanisms. Nat. Genet. 56, 2479–2492 (2024).
Gallo, D. et al. CCNE1 amplification is synthetic lethal with PKMYT1 kinase inhibition. Nature 604, 749–756 (2022).
Pfeifer, M. et al. Genome-wide CRISPR screens identify the YAP/TEAD axis as a driver of persister cells in EGFR mutant lung cancer. Commun. Biol. 7, 497 (2024).
Jost, M. et al. Combined CRISPRi/a-based chemical genetic screens reveal that rigosertib is a microtubule-destabilizing agent. Mol. Cell 68, 210–223.e6 (2017).
Wei, L. et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for sorafenib resistance in HCC. Nat. Commun. 10, 4681 (2019).
Zeng, H. et al. Genome-wide CRISPR screening reveals genetic modifiers of mutant EGFR dependence in human NSCLC. eLife 8, e50223 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05147272 (2024).
Hustedt, N. et al. A consensus set of genetic vulnerabilities to ATR inhibition. Open Biol. 9, 190156 (2019).
Benada, J. et al. Synthetic lethal interaction between WEE1 and PKMYT1 is a target for multiple low-dose treatment of high-grade serous ovarian carcinoma. NAR Cancer 5, zcad029 (2023).
Shirani-Bidabadi, S. et al. CRISPR technology: a versatile tool to model, screen, and reverse drug resistance in cancer. Eur. J. Cell Biol. 102, 151299 (2023).
Quintanal-Villalonga, A. et al. Multiomic analysis of lung tumors defines pathways activated in neuroendocrine transformation. Cancer Discov. 11, 3028–3047 (2021).
Chan, J. M. et al. Lineage plasticity in prostate cancer depends on JAK/STAT inflammatory signaling. Science 377, 1180–1191 (2022).
Lewis, S. M. et al. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods 18, 997–1012 (2021).
Jones, M. G., Yang, D. & Weissman, J. S. New tools for lineage tracing in cancer in vivo. Annu. Rev. Cancer Biol. 7, 111–129 (2023).
Quinn, J. J. et al. Single-cell lineages reveal the rates, routes, and drivers of metastasis in cancer xenografts. Science 371, eabc1944 (2021).
Simeonov, K. P. et al. Single-cell lineage tracing of metastatic cancer reveals selection of hybrid EMT states. Cancer Cell 39, 1150–1162.e9 (2021).
Erickson, A. et al. Spatially resolved clonal copy number alterations in benign and malignant tissue. Nature 608, 360–367 (2022).
Miller, T. E. et al. Mitochondrial variant enrichment from high-throughput single-cell RNA sequencing resolves clonal populations. Nat. Biotechnol. 40, 1030–1034 (2022).
Cotton, J. L. et al. Expressed barcoding enables high-resolution tracking of the evolution of drug tolerance. Cancer Res. 83, 3611–3623 (2023).
Kester, L. & van Oudenaarden, A. Single-cell transcriptomics meets lineage tracing. Cell Stem Cell 23, 166–179 (2018).
Yang, D. et al. Lineage tracing reveals the phylodynamics, plasticity, and paths of tumor evolution. Cell 185, 1905–1923.e25 (2022).
Fazzari, E. et al. Stem-22. Single-cell lineage tracing in primary glioblastoma reveals distinct progenitor subtypes driving intratumoral heterogeneity. Neuro Oncol. 25, v37 (2023).
De Las Rivas, J. et al. Cancer drug resistance induced by EMT: novel therapeutic strategies. Arch. Toxicol. 95, 2279–2297 (2021).
Lin, L. et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat. Genet. 47, 250–256 (2015).
Sommer, E. R., Napoli, G. C., Chau, C. H., Price, D. K. & Figg, W. D. Targeting the metastatic niche: single-cell lineage tracing in prime time. iScience 26, 106174 (2023).
Genovese, G. et al. Synthetic vulnerabilities of mesenchymal subpopulations in pancreatic cancer. Nature 542, 362–366 (2017).
Kim, D. H., Kim, W. D., Kim, S. K., Moon, D. H. & Lee, S. J. TGF-β1-mediated repression of SLC7A11 drives vulnerability to GPX4 inhibition in hepatocellular carcinoma cells. Cell Death Dis. 11, 406 (2020).
Haderk, F. et al. Focal adhesion kinase-YAP signaling axis drives drug-tolerant persister cells and residual disease in lung cancer. Nat. Commun. 15, 3741 (2024).
Chen, H. Y. et al. Regulation of neuroendocrine plasticity by the RNA-binding protein ZFP36L1. Nat. Commun. 13, 4998 (2022).
Davies, A. et al. An androgen receptor switch underlies lineage infidelity in treatment-resistant prostate cancer. Nat. Cell Biol. 23, 1023–1034 (2021).
Ku, S. Y. et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83 (2017).
Alix-Panabières, C. & Pantel, K. Liquid biopsy: from discovery to clinical application. Cancer Discov. 11, 858–873 (2021).
Ignatiadis, M., Sledge, G. W. & Jeffrey, S. S. Liquid biopsy enters the clinic — implementation issues and future challenges. Nat. Rev. Clin. Oncol. 18, 297–312 (2021).
Goossens, N., Nakagawa, S., Sun, X. & Hoshida, Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 4, 256–269 (2015).
Passaro, A. et al. Cancer biomarkers: emerging trends and clinical implications for personalized treatment. Cell 187, 1617–1635 (2024).
Khleif, S. N., Doroshow, J. H., Hait, W. N. & AACR-FDA-NCI Cancer Biomarkers Collaborative. AACR-FDA-NCI Cancer Biomarkers Collaborative consensus report: advancing the use of biomarkers in cancer drug development. Clin. Cancer Res. 16, 3299–3318 (2010).
Duffy, M. J. et al. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). Eur. J. Cancer 75, 284–298 (2017).
Albain, K. S., Paik, S. & Van’t Veer, L. Prediction of adjuvant chemotherapy benefit in endocrine responsive, early breast cancer using multigene assays. Breast 18, S141–S145 (2009).
Tarabichi, M. et al. A practical guide to cancer subclonal reconstruction from DNA sequencing. Nat. Methods 18, 144–155 (2021).
Salcedo, A. et al. A community effort to create standards for evaluating tumor subclonal reconstruction. Nat. Biotechnol. 38, 97–107 (2020).
Al Bakir, M. et al. The evolution of non-small cell lung cancer metastases in TRACERx. Nature 616, 534–542 (2023).
Caputo, V. et al. Diagnostic value of liquid biopsy in the era of precision medicine: 10 years of clinical evidence in cancer. Explor. Target. Antitumor Ther. 4, 102–138 (2023).
Amirouchene-Angelozzi, N., Swanton, C. & Bardelli, A. Tumor evolution as a therapeutic target. Cancer Discov. 7, 805–817 (2017).
Jamshidi, A. et al. Evaluation of cell-free DNA approaches for multi-cancer early detection. Cancer Cell 40, 1537–1549.e12 (2022).
Liu, M. C. et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 31, 745–759 (2020).
Bergqvist, M. et al. Thymidine kinase activity levels in serum can identify HR+ metastatic breast cancer patients with a low risk of early progression (SWOG S0226). Biomarkers 28, 313–322 (2023).
McCartney, A. et al. Plasma thymidine kinase activity as a biomarker in patients with luminal metastatic breast cancer treated with palbociclib within the TREnd trial. Clin. Cancer Res. 26, 2131–2139 (2020).
Nanni, C., Farolfi, A., Castellucci, P. & Fanti, S. Total body positron emission tomography/computed tomography: current status in oncology. Semin. Nucl. Med. 55, 31–40 (2025).
Pai, S. et al. Foundation model for cancer imaging biomarkers. Nat. Mach. Intell. 6, 354–367 (2024).
Prelaj, A. et al. Artificial intelligence for predictive biomarker discovery in immuno-oncology: a systematic review. Ann. Oncol. 35, 29–65 (2024).
Vazquez-Levin, M. H., Reventos, J. & Zaki, G. Editorial: Artificial intelligence: a step forward in biomarker discovery and integration towards improved cancer diagnosis and treatment. Front. Oncol. 13, 1161118 (2023).
Danielli, S. G. et al. Single cell transcriptomic profiling identifies tumor-acquired and therapy-resistant cell states in pediatric rhabdomyosarcoma. Nat. Commun. 15, 6307 (2024).
Wegmann, R. et al. Single-cell landscape of innate and acquired drug resistance in acute myeloid leukemia. Nat. Commun. 15, 9402 (2024).
Caswell, D. R. et al. The role of APOBEC3B in lung tumor evolution and targeted cancer therapy resistance. Nat. Genet. 56, 60–73 (2024).
Lipkova, J. et al. Artificial intelligence for multimodal data integration in oncology. Cancer Cell 40, 1095–1110 (2022).
Morris, S. R. & Carey, L. A. Gene expression profiling in breast cancer. Curr. Opin. Oncol. 19, 547–551 (2007).
Kim, C. & Paik, S. Gene-expression-based prognostic assays for breast cancer. Nat. Rev. Clin. Oncol. 7, 340–347 (2010).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03460977 (2025).
Gini, B., Thomas, N. & Blakely, C. M. Impact of concurrent genomic alterations in epidermal growth factor receptor (EGFR)-mutated lung cancer. J. Thorac. Dis. 12, 2883–2895 (2020).
Johnson, B. E. et al. An omic and multidimensional spatial atlas from serial biopsies of an evolving metastatic breast cancer. Cell Rep. Med. 3, 100525 (2022). This publication showcases analysis of serial biopsy samples taken from a patient with breast cancer and serves as an example of how integrative data analysis can reveal potential mechanisms of response and resistance and suggest novel therapeutic vulnerabilities.
Labrie, M. et al. Multiomics analysis of serial PARP inhibitor treated metastatic TNBC inform on rational combination therapies. NPJ Precis. Oncol. 5, 92 (2021).
Wirth, A.-K. et al. In vivo PDX CRISPR/Cas9 screens reveal mutual therapeutic targets to overcome heterogeneous acquired chemo-resistance. Leukemia 36, 2863–2874 (2022).
Kim, M. et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 10, 3991 (2019).
Al Shihabi, A. et al. Personalized chordoma organoids for drug discovery studies. Sci. Adv. 8, eabl3674 (2022).
Gutmann, D. H. et al. Precision preclinical modeling to advance cancer treatment. J. Natl Cancer Inst. 117, 586–594 (2025).
Meric-Bernstam, F. et al. National Cancer Institute Combination Therapy Platform Trial with Molecular Analysis for Therapy Choice (ComboMATCH). Clin. Cancer Res. 29, 1412–1422 (2023).
O’Donnell, P. H. & Dolan, M. E. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin. Cancer Res. 15, 4806–4814 (2009).
Marcu, L. G. & Marcu, D. C. Pharmacogenomics and Big Data in medical oncology: developments and challenges. Ther. Adv. Med. Oncol. 16, 17588359241287658 (2024).
American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. https://cancerprogressreport.aacr.org/disparities/cdpr24-contents/cdpr24-disparities-in-clinical-research-and-cancer-treatment/ (2024).
Habibzadeh, F. Disparity in the selection of patients in clinical trials. Lancet 399, 1048 (2022).
Letai, A. Functional precision cancer medicine-moving beyond pure genomics. Nat. Med. 23, 1028–1035 (2017).
Han, J. J. FDA Modernization Act 2.0 allows for alternatives to animal testing. Artif. Organs 47, 449–450 (2023).
Chang, T. G. et al. LORIS robustly predicts patient outcomes with immune checkpoint blockade therapy using common clinical, pathologic and genomic features. Nat. Cancer 5, 1158–1175 (2024).
Laisné, M., Lupien, M. & Vallot, C. Epigenomic heterogeneity as a source of tumour evolution. Nat. Rev. Cancer 25, 7–26 (2025).
Acknowledgements
The authors thank M. Espey and J. Hildesheim for helpful discussions. They acknowledge the following grant support: R01CA244729 and R01CA244729-03S (to A.S. and P.C.B.); R01CA240718 and R01CA264248 (to A.S.); P30CA016042, U2CCA271894, U24CA248265 and DOD W81XWH2210751 (to P.C.B.); U01CA223976 and U01CA223976-03S1 (to C.D.W.); R01CA247362 and R01CA267467 (to E.S.K. and A.K.W.); U24CA274159, R01CA234162 and PCF 22CHAL13 (to D.W.G.); U54CA274220 (to D.J. and S.T.G.); U54CA224019 (to C.E.T. and J.W.T.); U01CA271412 (to C.E.T.); U54CA224081 (to T.G.B.); R01CA175495 (to X.Z.); U54CA274321 (to V.C.S., A.A.O. and J.N.M.); R01CA280980 (to V.C.S.); R01CA270234, R01CA163649, R01CA256911 and U54CA274329 (to P.K.S.); R37CA276924 (to K.M.); and R01CA175397-07S1 (to K.S.C.).
Author information
Authors and Affiliations
Consortia
Contributions
E.S.K., T.N.O., A.K.W. and A.S. researched data for the article. E.S.K., T.N.O., C.E.T., J.W.T., B.G., T.G.B., D.W.G., P.K.S., K.S.C., H.M. and A.S. contributed substantially to discussion of the content. E.S.K., C.E.T., J.W.T., B.G., T.G.B., X.Z., A.K.W., D.W.G., D.J., S.T.G., C.D.W., P.C.B., V.C.S., A.A.O., J.N.M., P.K.S., D.K., K.S.C. and A.S. wrote the article. E.S.K., T.N.O., B.G., T.G.B., X.Z., C.D.W., V.C.S., A.A.O. and A.S. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no direct competing interests. For full disclosure, C.D.W. has received research funding from Varian Medical Systems, AACR-Novocure and OMS Foundation, clinical trial support from MuReva and Tactile Medical, and consultancy/honoraria from LifeNet Health, Guidepoint Global and EMD Serono; A.S. sits on the Board of the Society for Functional Precision Medicine; P.C.B. sits on the Scientific Advisory Boards of Intersect Diagnostics Inc., BioSymetrics Inc. and previously sat on the board of Sage Bionetworks; E.S.K. has sponsored research funded by Blueprint Medicines and Bristol Myers Squibb and is a member of the Cancer Cell Cyclse–LLC consulting enterprise; A.K.W. has sponsored research funded by Blueprint Medicines and Bristol Myers Squibb; C.E.T. has received funding from AstraZeneca; J.W.T. has received research support from Acerta, Agios, Aptose, Array, AstraZeneca, Constellation, Genentech, Gilead, Incyte, Janssen, Kronos, Meryx, Petra, Schrodinger, Seattle Genetics, Syros, Takeda and Tolero and serves on the advisory board for Recludix Pharm, AmMax Bio and Ellipses Pharma; V.C.S. is a consultant for and equity holder in Femtovox Inc.
Peer review
Peer review information
Nature Reviews Cancer thanks J. Marine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Innovative Methodologies and New Data for Predictive Oncology Model Evaluation (IMPROVE) | CBIIT: https://datascience.cancer.gov/collaborations/nci-department-energy-collaborations/improve
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soragni, A., Knudsen, E.S., O’Connor, T.N. et al. Acquired resistance in cancer: towards targeted therapeutic strategies. Nat Rev Cancer 25, 613–633 (2025). https://doi.org/10.1038/s41568-025-00824-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41568-025-00824-9
This article is cited by
-
Dynamic biomarkers in hormone receptor-positive/HER2-negative breast cancer trials: a new hope for precision oncology
npj Breast Cancer (2026)
-
Molecular analysis of tumor recurrence using established cancer stem cell-line and drug discovery
International Journal of Clinical Oncology (2026)
-
Lineage plasticity: a new dilemma in lung cancer treatment
Molecular Biology Reports (2026)
-
Drug resistance in cancer: molecular mechanisms and emerging treatment strategies
Molecular Biomedicine (2025)
-
The application of organoids in treatment decision-making for digestive system cancers: progress and challenges
Molecular Cancer (2025)