Abstract
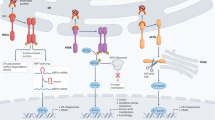
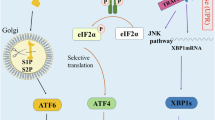
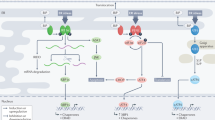
The endoplasmic reticulum (ER) has a central role in processes essential for mounting effective and durable antitumour immunity; this includes regulating protein synthesis, folding, modification and trafficking in immune cells. However, the tumour microenvironment imposes hostile conditions that disrupt ER homeostasis in both malignant and infiltrating immune cells, leading to chronic activation of the unfolded protein response (UPR). Dysregulated ER stress responses have emerged as critical modulators of cancer progression and immune escape, influencing the initiation, development and maintenance of antitumour immunity. In this Review, we examine how tumour-induced ER stress reshapes the functional landscape of immune cells within the tumour microenvironment. We highlight recent discoveries demonstrating how ER stress curtails endogenous antitumour immunity and reduces the efficacy of immunotherapies. Furthermore, we underscore novel therapeutic strategies targeting ER stress sensors or UPR components to restore immune function and enhance cancer immunotherapy outcomes. Together, this provides a comprehensive overview of the interplay between ER stress responses and antitumour immunity, emphasizing the potential of UPR-targeted interventions to improve immune control of cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Chen, X. & Cubillos-Ruiz, J. R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 21, 71–88 (2021).
Chen, X. et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 508, 103–107 (2014).
Oakes, S. A. Endoplasmic reticulum stress signaling in cancer cells. Am. J. Pathol. 190, 934–946 (2020).
Salvagno, C., Mandula, J. K., Rodriguez, P. C. & Cubillos-Ruiz, J. R. Decoding endoplasmic reticulum stress signals in cancer cells and antitumor immunity. Trends Cancer 8, 930–943 (2022).
Zhang, W. et al. Endoplasmic reticulum stress — a key guardian in cancer. Cell Death Discov. 10, 343 (2024).
Di Conza, G., Ho, P. C., Cubillos-Ruiz, J. R. & Huang, S. C. Control of immune cell function by the unfolded protein response. Nat. Rev. Immunol. 23, 546–562 (2023).
Yang, M., Cui, M., Sun, Y., Liu, S. & Jiang, W. Mechanisms, combination therapy, and biomarkers in cancer immunotherapy resistance. Cell Commun. Signal. 22, 338 (2024).
Marei, H. E., Hasan, A., Pozzoli, G. & Cenciarelli, C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 23, 64 (2023).
Ulianich, L. et al. ER stress impairs MHC Class I surface expression and increases susceptibility of thyroid cells to NK-mediated cytotoxicity. Biochim. Biophys. Acta 1812, 431–438 (2011).
Hwang, S. M. et al. Transgelin 2 guards T cell lipid metabolism and antitumour function. Nature 635, 1010–1018 (2024). This study demonstrated that ER stress-driven IRE1a–XBP1s silences transgelin 2, a cytoskeletal element that is critical for T cell lipid metabolism and antitumour function.
Song, M. et al. IRE1alpha-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 562, 423–428 (2018). This report uncovered that aberrant IRE1α–XBP1s activation curtails T cell mitochondrial respiration by inhibiting glutamine uptake and utilization.
Ma, X. et al. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 30, 143–156.e145 (2019). This study found that high cholesterol levels in the TME activate XBP1s-dependent PD1 induction in infiltrating T cells, thereby triggering exhaustion.
He, J., Zhou, Y. & Sun, L. Emerging mechanisms of the unfolded protein response in therapeutic resistance: from chemotherapy to immunotherapy. Cell Commun. Signal. 22, 89 (2024).
Bartoszewska, S., Sławski, J., Collawn, J. F. & Bartoszewski, R. Dual RNase activity of IRE1 as a target for anticancer therapies. J. Cell Commun. Signal. 17, 1145–1161 (2023).
Andrews, A. M., Tennant, M. D. & Thaxton, J. E. Stress relief for cancer immunotherapy: implications for the ER stress response in tumor immunity. Cancer Immunol. Immunother. 70, 1165–1175 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03950570 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04834778 (2021).
Stokes, M. E. et al. PERK inhibition by HC-5404 sensitizes renal cell carcinoma tumor models to antiangiogenic tyrosine kinase inhibitors. Clin. Cancer Res. 29, 4870–4882 (2023).
Acosta-Alvear, D., Harnoss, J. M., Walter, P. & Ashkenazi, A. Homeostasis control in health and disease by the unfolded protein response. Nat. Rev. Mol. Cell Biol. 26, 193–212 (2025).
Caine, J. J. & Geracioti, T. D. Taurine, energy drinks, and neuroendocrine effects. Cleve. Clin. J. Med. 83, 895–904 (2016).
Wojcik, O. P., Koenig, K. L., Zeleniuch-Jacquotte, A., Costa, M. & Chen, Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 208, 19–25 (2010).
Marcinkiewicz, J. & Kontny, E. Taurine and inflammatory diseases. Amino Acids 46, 7–20 (2014).
Seneff, S. & Kyriakopoulos, A. M. Taurine prevents mitochondrial dysfunction and protects mitochondria from reactive oxygen species and deuterium toxicity. Amino Acids 57, 6 (2025).
Surai, P. F., Earle-Payne, K. & Kidd, M. T. Taurine as a natural antioxidant: from direct antioxidant effects to protective action in various toxicological models. Antioxidants 10, 1876 (2021).
Wang, M., Wey, S., Zhang, Y., Ye, R. & Lee, A. S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid. Redox Signal. 11, 2307–2316 (2009).
Jong, C. J., Ito, T., Azuma, J. & Schaffer, S. Taurine depletion decreases GRP78 expression and downregulates perk-dependent activation of the unfolded protein response. Adv. Exp. Med. Biol. 803, 571–579 (2015).
Miyazaki, T. et al. Impaired bile acid metabolism with defectives of mitochondrial–tRNA taurine modification and bile acid taurine conjugation in the taurine depleted cats. Sci. Rep. 10, 4915 (2020).
Ridlon, J. M., Harris, S. C., Bhowmik, S., Kang, D. J. & Hylemon, P. B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7, 22–39 (2016).
Lee, M. H. et al. How bile acids and the microbiota interact to shape host immunity. Nat. Rev. Immunol. 24, 798–809 (2024).
Sharma, R., Quilty, F., Gilmer, J. F., Long, A. & Byrne, A. M. Unconjugated secondary bile acids activate the unfolded protein response and induce golgi fragmentation via a src-kinase-dependant mechanism. Oncotarget 8, 967–978 (2017).
Varanasi, S. K. et al. Bile acid synthesis impedes tumor-specific T cell responses during liver cancer. Science 387, 192–201 (2025). This manuscript demonstrates that targeting bile acid metabolism impacts the efficacy of immunotherapy in liver cancer models.
Cao, T. et al. Cancer SLC6A6-mediated taurine uptake transactivates immune checkpoint genes and induces exhaustion in CD8+ T cells. Cell 187, 2288–2304.e2227 (2024). This report showed that SLC6A6-driven taurine depletion by tumours triggers detrimental ER stress responses in infiltrating T cells, promoting exhaustion and immune evasion.
Ping, Y. et al. Taurine enhances the antitumor efficacy of PD-1 antibody by boosting CD8+ T cell function. Cancer Immunol. Immunother. 72, 1015–1027 (2023).
Dong, J. F., Zheng, X. Q. & Rui, H. B. Effect of taurine on immune function in mice with T-cell lymphoma during chemotherapy. Asian Pac. J. Trop. Med. 10, 1090–1094 (2017).
Maxfield, F. R. & Tabas, I. Role of cholesterol and lipid organization in disease. Nature 438, 612–621 (2005).
Li, Y. et al. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic–endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 279, 37030–37039 (2004).
Nam, G. H. et al. Statin-mediated inhibition of RAS prenylation activates ER stress to enhance the immunogenicity of KRAS mutant cancer. J. Immunother. Cancer 9, e002474 (2021).
Yang, S. et al. Role of endoplasmic reticulum stress in atherosclerosis and its potential as a therapeutic target. Oxid. Med. Cell Longev. 2020, 9270107 (2020).
Li, Z., Zhang, L., Liu, D. & Wang, C. Ceramide glycosylation and related enzymes in cancer signaling and therapy. Biomed. Pharmacother. 139, 111565 (2021).
Kumar, A. & Deep, G. Hypoxia in tumor microenvironment regulates exosome biogenesis: molecular mechanisms and translational opportunities. Cancer Lett. 479, 23–30 (2020).
Di Conza, G. et al. Tumor-induced reshuffling of lipid composition on the endoplasmic reticulum membrane sustains macrophage survival and pro-tumorigenic activity. Nat. Immunol. 22, 1403–1415 (2021).
Caldwell, R. W., Rodriguez, P. C., Toque, H. A., Narayanan, S. P. & Caldwell, R. B. Arginase: a multifaceted enzyme important in health and disease. Physiol. Rev. 98, 641–665 (2018).
Figueiredo-Pereira, C., Dias-Pedroso, D., Soares, N. L. & Vieira, H. L. A. CO-mediated cytoprotection is dependent on cell metabolism modulation. Redox Biol. 32, 101470 (2020).
Luu Hoang, K. N., Anstee, J. E. & Arnold, J. N. The diverse roles of heme oxygenase-1 in tumor progression. Front. Immunol. 12, 658315 (2021).
S, R. O., Queiroga, C. S. & Vieira, H. L. Mitochondria and carbon monoxide: cytoprotection and control of cell metabolism — a role for Ca2+ ? J. Physiol. 594, 4131–4138 (2016).
Ryter, S. W., Alam, J. & Choi, A. M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 86, 583–650 (2006).
Chakraborty, P. et al. Carbon monoxide activates PERK-regulated autophagy to induce immunometabolic reprogramming and boost antitumor T-cell function. Cancer Res. 82, 1969–1990 (2022).
Li, Y., Dang, J., Liang, Q. & Yin, L. Thermal-responsive carbon monoxide (CO) delivery expedites metabolic exhaustion of cancer cells toward reversal of chemotherapy resistance. ACS Cent. Sci. 5, 1044–1058 (2019).
Zhao, N. et al. Pharmacological targeting of MYC-regulated IRE1/XBP1 pathway suppresses MYC-driven breast cancer. J. Clin. Invest. 128, 1283–1299 (2018).
Tameire, F., Verginadis, I. I. & Koumenis, C. Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: mechanisms and targets for therapy. Semin. Cancer Biol. 33, 3–15 (2015).
Nguyen, H. G. et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci. Transl. Med. 10, eaar2036 (2018).
Unal, B. et al. Targeting IRE1α reprograms the tumor microenvironment and enhances anti-tumor immunity in prostate cancer. Nat. Commun. 15, 8895 (2024).
Granados, D. P. et al. ER stress affects processing of MHC class I-associated peptides. BMC Immunol. 10, 10 (2009).
Carlsten, M. et al. Bortezomib sensitizes multiple myeloma to NK cells via ER-stress-induced suppression of HLA-E and upregulation of DR5. OncoImmunology 8, e1534664 (2019).
Obiedat, A. et al. Transcription of the NKG2D ligand MICA is suppressed by the IRE1/XBP1 pathway of the unfolded protein response through the regulation of E2F1. FASEB J. 33, 3481–3495 (2019).
Meza Guzman, L. G., Keating, N. & Nicholson, S. E. Natural killer cells: tumor surveillance and signaling. Cancers 12, 952 (2020).
Sen Santara, S. et al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature 616, 348–356 (2023). This study identified cancer cell ecto-CRT as a primary trigger for NK cell recognition, providing a new therapeutic approach for enhancing NK cell antitumour immunity.
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Jiang, X. et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 18, 10 (2019).
He, X. & Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 30, 660–669 (2020).
Yang, Y. et al. Tumorous IRE1α facilitates CD8+T cells-dependent anti-tumor immunity and improves immunotherapy efficacy in melanoma. Cell Commun. Signal. 22, 83 (2024).
Chou, C. W. et al. The stabilization of PD-L1 by the endoplasmic reticulum stress protein GRP78 in triple-negative breast cancer. Am. J. Cancer Res. 10, 2621–2634 (2020).
Crowley, M. J. P. et al. Tumor-intrinsic IRE1α signaling controls protective immunity in lung cancer. Nat. Commun. 14, 120 (2023).
Gilardini Montani, M. S. et al. PGE2 released by pancreatic cancer cells undergoing ER stress transfers the stress to DCs impairing their immune function. Mol. Cancer Ther. 20, 934–945 (2021).
Logue, S. E. et al. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun. 9, 3267 (2018).
Harnoss, J. M. et al. IRE1α disruption in triple-negative breast cancer cooperates with antiangiogenic therapy by reversing ER stress adaptation and remodeling the tumor microenvironment. Cancer Res. 80, 2368–2379 (2020).
Mahadevan, N. R. et al. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc. Natl Acad. Sci. USA 108, 6561–6566 (2011).
Mahadevan, N. R. et al. Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8+ T cell priming. PLoS ONE 7, e51845 (2012).
Xu, L. et al. IRE1α silences dsRNA to prevent taxane-induced pyroptosis in triple-negative breast cancer. Cell 187, 7248–7266.e34 (2024). This study unveiled that disabling the IRE1α RNase activity in breast cancer cells unleashes the immunostimulatory effects of taxane treatment, enhancing antitumour immunity and the efficacy of ICB in preclinical models.
Galluzzi, L. et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 8, e000337 (2020).
Mandula, J. K. et al. Ablation of the endoplasmic reticulum stress kinase PERK induces paraptosis and type I interferon to promote anti-tumor T cell responses. Cancer Cell 40, 1145–1160.e1149 (2022). This study uncovered that melanoma cells exploit PERK activity to evade anti-tumour immunity. PERK was found to protect ER-stressed cancer cells from paraptosis-induced ICD, thereby limiting protective T cell responses.
Liu, L. et al. Ablation of ERO1A induces lethal endoplasmic reticulum stress responses and immunogenic cell death to activate anti-tumor immunity. Cell Rep. Med. 4, 101206 (2023).
Coleman, O. I. et al. Activated ATF6 induces intestinal dysbiosis and innate immune response to promote colorectal tumorigenesis. Gastroenterology 155, 1539–1552.e1512 (2018).
Lou, X., Gao, D., Yang, L., Wang, Y. & Hou, Y. Endoplasmic reticulum stress mediates the myeloid-derived immune suppression associated with cancer and infectious disease. J. Transl. Med. 21, 1 (2023).
Cubillos-Ruiz, J. R. et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161, 1527–1538 (2015). This study revealed that abnormal activation of IRE1α–XBP1s in intratumoural DCs blocked the development of adaptive immunity against ovarian cancer.
Guttman, O. et al. Antigen-derived peptides engage the ER stress sensor IRE1α to curb dendritic cell cross-presentation. J. Cell Biol. 221, e202111068 (2022).
Tang, R. et al. Tim-3 adapter protein Bat3 acts as an endogenous regulator of tolerogenic dendritic cell function. Sci. Immunol. 7, eabm0631 (2022).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Zhao, Y. et al. XBP1 regulates the protumoral function of tumor-associated macrophages in human colorectal cancer. Signal. Transduct. Target. Ther. 6, 357 (2021).
Kang, Y., Li, H., Liu, Y. & Li, Z. Regulation of VEGF-A expression and VEGF-A-targeted therapy in malignant tumors. J. Cancer Res. Clin. Oncol. 150, 221 (2024).
Little, A. C. et al. IL-4/IL-13 stimulated macrophages enhance breast cancer invasion via Rho-GTPase regulation of synergistic VEGF/CCL-18 signaling. Front. Oncol. 9, 456 (2019).
Mohamed, Aa. H. et al. Interleukin-6 serves as a critical factor in various cancer progression and therapy. Med. Oncol. 41, 182 (2024).
Raines, L. N. et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat. Immunol. 23, 431–445 (2022). This paper unveiled a new role for PERK in the regulation of metabolic and epigenetic circuits that support immunosuppressive macrophage activation in tumours.
De Leo, A. et al. Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma. Immunity 57, 1105–1123.e1108 (2024). This study showed that highly glycolytic monocyte-derived macrophages from glioblastoma tumours restrict antitumour T cell immunity via PERK-driven glucose metabolism that controls histone lactylation.
Kim, R. et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 612, 338–346 (2022).
Emmanuelli, A. et al. High-grade serous ovarian cancer development and anti-PD-1 resistance is driven by IRE1α activity in neutrophils. Oncoimmunology 13, 2411070 (2024).
Luo, H. et al. Tumor-associated neutrophils upregulate Nectin2 expression, creating the immunosuppressive microenvironment in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 43, 258 (2024).
Thevenot, P. T. et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity 41, 389–401 (2014).
Condamine, T. et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 1, aaf8943 (2016).
Veglia, F., Perego, M. & Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 19, 108–119 (2018).
Cubillos-Ruiz, J. R., Mohamed, E. & Rodriguez, P. C. Unfolding anti-tumor immunity: ER stress responses sculpt tolerogenic myeloid cells in cancer. J. Immunother. Cancer 5, 5 (2017).
Mohamed, E. et al. The unfolded protein response mediator PERK governs myeloid cell-driven immunosuppression in tumors through inhibition of STING signaling. Immunity 52, 668–682.e667 (2020). This study demonstrated that PERK deletion in MDSCs reduced NRF2 signalling, promoting STING-driven production of type I IFN that enhanced antitumour immunity.
Tcyganov, E. N. et al. Distinct mechanisms govern populations of myeloid-derived suppressor cells in chronic viral infection and cancer. J. Clin. Invest. 131, e145971 (2021).
Dong, H. et al. The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-Myc. Nat. Immunol. 20, 865–878 (2019). This paper showed that IRE1α–XBP1s regulates MYC signalling and mitochondrial respiration that supports NK cell expansion, supporting effective responses against viral infection and melanoma tumours.
Kim, S. H. et al. Enhancement of the anticancer ability of natural killer cells through allogeneic mitochondrial transfer. Cancers 15, 3225 (2023).
Slattery, K. et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J. Immunother. Cancer 9, e002044 (2021).
Tumino, N. et al. The tumor microenvironment drives NK cell metabolic dysfunction leading to impaired antitumor activity. Int. J. Cancer 152, 1698–1706 (2023).
Hunt, E. G., Andrews, A. M., Larsen, S. R. & Thaxton, J. E. The ER-mitochondria interface as a dynamic hub for T cell efficacy in solid tumors. Front. Cell Dev. Biol. 10, 867341 (2022).
Kaesler, S. et al. Effective T-cell recall responses require the taurine transporter Taut. Eur. J. Immunol. 42, 831–841 (2012).
Cao, Y. et al. ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression. Nat. Commun. 10, 1280 (2019).
Li, A., Song, N. J., Riesenberg, B. P. & Li, Z. The emerging roles of endoplasmic reticulum stress in balancing immunity and tolerance in health and diseases: mechanisms and opportunities. Front. Immunol. 10, 3154 (2019).
Hurst, K. E. et al. Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8+ T cells. Cancer Immunol. Res. 7, 476–486 (2019).
Guo, Q. et al. NF-kB in biology and targeted therapy: new insights and translational implications. Signal. Transduct. Target. Ther. 9, 53 (2024).
Riesenberg, B. P. et al. Stress-mediated attenuation of translation undermines T-cell activity in cancer. Cancer Res. 82, 4386–4399 (2022).
Liu, C. Y. et al. ER stress-related ATF6 upregulates CIP2A and contributes to poor prognosis of colon cancer. Mol. Oncol. 12, 1706–1717 (2018).
Sicari, D. et al. Mutant p53 improves cancer cells’ resistance to endoplasmic reticulum stress by sustaining activation of the UPR regulator ATF6. Oncogene 38, 6184–6195 (2019).
Liu, F., Chang, L. & Hu, J. Activating transcription factor 6 regulated cell growth, migration and inhibiteds cell apoptosis and autophagy via MAPK pathway in cervical cancer. J. Reprod. Immunol. 139, 103120 (2020).
MacFawn, I. P. et al. The activity of tertiary lymphoid structures in high grade serous ovarian cancer is governed by site, stroma, and cellular interactions. Cancer Cell 42, 1864–1881 e1865 (2024).
Lim, A. R., Rathmell, W. K. & Rathmell, J. C. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. eLife 9, e55185 (2020).
Yoo, Y. S., Han, H. G. & Jeon, Y. J. Unfolded protein response of the endoplasmic reticulum in tumor progression and immunogenicity. Oxid. Med. Cell Longev. 2017, 2969271 (2017).
Benhamron, S. et al. Regulated IRE1-dependent decay participates in curtailing immunoglobulin secretion from plasma cells. Eur. J. Immunol. 44, 867–876 (2014).
Tang, C. H. et al. Phosphorylation of IRE1 at S729 regulates RIDD in B cells and antibody production after immunization. J. Cell Biol. 217, 1739–1755 (2018).
Sheng, X. et al. IRE1α–XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat. Commun. 10, 323 (2019).
Papandreou, I. et al. Identification of an Ire1α endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood 117, 1311–1314 (2011).
Mimura, N. et al. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood 119, 5772–5781 (2012).
Vincenz, L., Jager, R., O’Dwyer, M. & Samali, A. Endoplasmic reticulum stress and the unfolded protein response: targeting the Achilles heel of multiple myeloma. Mol. Cancer Ther. 12, 831–843 (2013).
Xie, H. et al. IRE1α RNase-dependent lipid homeostasis promotes survival in Myc-transformed cancers. J. Clin. Invest. 128, 1300–1316 (2018).
Lin, J. et al. Targeting the IRE1α/XBP1s pathway suppresses CARM1-expressing ovarian cancer. Nat. Commun. 12, 5321 (2021).
Hetz, C., Axten, J. M. & Patterson, J. B. Pharmacological targeting of the unfolded protein response for disease intervention. Nat. Chem. Biol. 15, 764–775 (2019).
Harnoss, J. M. et al. Disruption of IRE1α through its kinase domain attenuates multiple myeloma. Proc. Natl Acad. Sci. USA 116, 16420–16429 (2019).
Moore, P. C. et al. Parallel signaling through IRE1α and PERK regulates pancreatic neuroendocrine tumor growth and survival. Cancer Res. 79, 6190–6203 (2019).
Pytel, D. et al. PERK is a haploinsufficient tumor suppressor: gene dose determines tumor-suppressive versus tumor promoting properties of PERK in melanoma. PLoS Genet. 12, e1006518 (2016).
Tameire, F. et al. ATF4 couples MYC-dependent translational activity to bioenergetic demands during tumour progression. Nat. Cell Biol. 21, 889–899 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06549790 (2024).
Dudek, A. Z. et al. A multicenter, open-label, phase 1a study of HC-5404 in patients with advanced solid tumors. J. Clin. Oncol. 42, e15118–e15118 (2024).
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016).
Grandjean, J. M. D. & Wiseman, R. L. Small molecule strategies to harness the unfolded protein response: where do we go from here? J. Biol. Chem. 295, 15692–15711 (2020).
Alsterda, A. et al. Salubrinal exposes anticancer properties in inflammatory breast cancer cells by manipulating the endoplasmic reticulum stress pathway. Front. Oncol. 11, 654940 (2021).
Bastola, P., Neums, L., Schoenen, F. J. & Chien, J. VCP inhibitors induce endoplasmic reticulum stress, cause cell cycle arrest, trigger caspase-mediated cell death and synergistically kill ovarian cancer cells in combination with Salubrinal. Mol. Oncol. 10, 1559–1574 (2016).
Nagamine, B. S., Godil, J. & Dolan, B. P. The unfolded protein response reveals eIF2α phosphorylation as a critical factor for direct MHC class I antigen presentation. Immunohorizons 5, 135–146 (2021).
Gallagher, C. M. et al. Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6α branch. eLife 5, e11878 (2016).
Ramirez, M. U., Hernandez, S. R., Soto-Pantoja, D. R. & Cook, K. L. Endoplasmic reticulum stress pathway, the unfolded protein response, modulates immune function in the tumor microenvironment to impact tumor progression and therapeutic response. Int. J. Mol. Sci. 21, 169 (2019).
Li, C., Imai, M., Hasegawa, S., Yamasaki, M. & Takahashi, N. Growth inhibition of refractory human gallbladder cancer cells by retinol, and its mechanism of action. Biol. Pharm. Bull. 40, 495–503 (2017).
Masciarelli, S. et al. Retinoic acid and arsenic trioxide sensitize acute promyelocytic leukemia cells to ER stress. Leukemia 32, 285–294 (2018).
Roy, A. et al. Multiple roles of RARRES1 in prostate cancer: autophagy induction and angiogenesis inhibition. PLoS ONE 12, e0180344 (2017).
Schoenhacker-Alte, B. et al. Sensitivity towards the GRP78 inhibitor KP1339/IT-139 is characterized by apoptosis induction via caspase 8 upon disruption of ER homeostasis. Cancer Lett. 404, 79–88 (2017).
Flocke, L. S., Trondl, R., Jakupec, M. A. & Keppler, B. K. Molecular mode of action of NKP-1339 — a clinically investigated ruthenium-based drug — involves ER- and ROS-related effects in colon carcinoma cell lines. Invest. New Drugs 34, 261–268 (2016).
Wernitznig, D. et al. First-in-class ruthenium anticancer drug (KP1339/IT-139) induces an immunogenic cell death signature in colorectal spheroids in vitro. Metallomics 11, 1044–1048 (2019).
Cerezo, M. et al. Compounds triggering ER stress exert anti-melanoma effects and overcome BRAF inhibitor resistance. Cancer Cell 29, 805–819 (2016).
Farshbaf, M. et al. Cell surface GRP78: an emerging imaging marker and therapeutic target for cancer. J. Control. Release 328, 932–941 (2020).
Arap, M. A. et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 6, 275–284 (2004).
Rauschert, N. et al. A new tumor-specific variant of GRP78 as target for antibody-based therapy. Lab. Invest. 88, 375–386 (2008).
Wang, S. et al. Chimeric antigen receptor T cells targeting cell surface GRP78 efficiently kill glioblastoma and cancer stem cells. J. Transl. Med. 21, 493 (2023).
Li, Y. et al. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5−/− mice. Nat. Commun. 10, 1492 (2019).
Ming, J. et al. A novel chemical, STF-083010, reverses tamoxifen-related drug resistance in breast cancer by inhibiting IRE1/XBP1. Oncotarget 6, 40692–40703 (2015).
Rimawi, M. F. et al. Early efficacy evaluation of ORIN1001, a first in class IRE1 alpha inhibitor, in advanced solid tumors. J. Clin. Oncol. 41, 1092–1092 (2023).
Li, X. et al. Population pharmacokinetic model for oral ORIN1001 in Chinese patients with advanced solid tumors. Front. Pharmacol. 15, 1322557 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05027594 (2021).
Burris, H. A. et al. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: a first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open 1, e000154 (2016).
Acknowledgements
We apologize to colleagues whose work could not be included in this review due to space limitations. We are grateful to all past and present members of the Rodriguez and Cubillos-Ruiz laboratories for their scientific contributions. ER stress-related research in the Rodriguez lab has been supported by NIH grants R01-CA233512, R01-CA262121, R01-CA27303 and P01-CA250984 Project #4 to P.C.R. ER stress-related research in the Cubillos-Ruiz lab has been supported by NIH R01 grants NS114653, CA271619 and CA282072; US Department of Defense grants W81XWH-16-1-0438, W81XWH-21-1-0478 and W81XWH-22-OCRP-IIRA; The Mark Foundation for Cancer Research, The Pershing Square Sohn Cancer Research Alliance, AACR-Stand Up to Cancer, The Cancer Research Institute, and the Ovarian Cancer Research Alliance. S.-M.H. was supported by the AACR-Bristol Myers Squibb Immuno-Oncology Research Fellowship.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
P.C.R. and J.R.C.-R. hold patents on the targeting of ER stress responses for the treatment of disease, as well as on the use of immune modulators for cancer therapy. J.R.C.-R. is a scientific consultant for Autoimmunity Biologic Solutions, Inc., and Emerald Bioventures, LLC, and holds stock options in Vescor Therapeutics.
Peer review
Peer review information
Nature Reviews Cancer thanks Eric Chevet, Jessica Thaxton who co-reviewed with Annaleigh Powe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Autophagy
-
A conserved lysosomal degradation pathway that recycles cellular components, regulating metabolism, stress adaptation and immune responses in health and disease.
- Immunogenic cell death
-
(ICD). A regulated form of cell death that elicits an adaptive immune response by releasing damage-associated molecular patterns and promoting antigen presentation.
- Major histocompatibility complex
-
(MHC). A group of cell surface molecules responsible for antigen presentation to T cells, enabling immune recognition and activation in both innate and adaptive immunity.
- Paraptosis
-
A caspase-independent, non-apoptotic form of programmed cell death distinguished by cytoplasmic vacuolization and mitochondrial swelling, often linked to ER stress and protein aggregation.
- Polymorphonuclear myeloid-derived suppressor cells
-
(PMN-MDSCs). A subset of immunosuppressive myeloid cells with neutrophil-like features that inhibit T cell activation and promote immune evasion within the tumour microenvironment.
- Pyroptosis
-
A lytic, pro-inflammatory form of programmed cell death triggered by inflammasome activation, characterized by gasdermin-mediated membrane pore formation and cytokine release.
- Tertiary lymphoid structures
-
(TLSs). Ectopic lymphoid aggregates that develop in non-lymphoid tissues during chronic inflammation or cancer, serving as local hubs for immune activation and adaptive responses.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hwang, SM., Chang, S., Rodriguez, P.C. et al. Endoplasmic reticulum stress responses in anticancer immunity. Nat Rev Cancer 25, 684–702 (2025). https://doi.org/10.1038/s41568-025-00836-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41568-025-00836-5
This article is cited by
-
ER stress sparks nerve pain
Nature Reviews Cancer (2026)
-
Disulfidptosis in cancer: from redox stress to therapeutic strategy
Cancer Gene Therapy (2026)
-
A Novel Strategy for Enhancing Lung Cancer Immunotherapy Efficacy: Traditional Chinese Medicine Formula Shuyu Pills Enhances Anti-PD-1 Efficacy Via the “GM-ERS-Immunity” Axis
Biological Procedures Online (2025)
-
Targeting the unfolded protein response for cancer therapy: mitigating tumor adaptation and immune suppression
Biomarker Research (2025)
-
Engineering endoplasmic reticulum targeted metal–polyphenol curcumin nanomicelles for melanoma therapy
Journal of Nanobiotechnology (2025)