Abstract
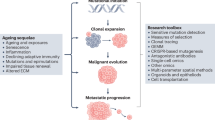
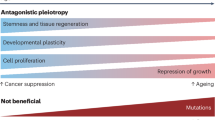
Ageing is an important risk factor for cancer incidence and augments cancer progression. A shared hallmark of ageing and cancer is metabolic reprogramming, which has been suggested to be not only a cause but also a consequence of ageing. Strikingly, many age-regulated pathways are known to also drive tumour progression, suggesting that metabolic reprogramming connects ageing and tumorigenic processes and shapes whether malignant phenotypes manifest, thrive and evolve. With the rising average age of the world population, understanding how age-related changes in the body influence cancer progression is of paramount importance. In this Perspective, we discuss the metabolic changes that occur with ageing and their potential links with tumour initiation and progression and the development of metastatic disease. Finally, we discuss age-induced metabolic divergences that cause racial disparities and their consequences for the tumorigenic process.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Chatsirisupachai, K., Lesluyes, T., Paraoan, L., Van Loo, P. & de Magalhães, J. P. An integrative analysis of the age-associated multi-omic landscape across cancers. Nat. Commun. 12, 2345 (2021).
Li, C. H., Haider, S. & Boutros, P. C. Age influences on the molecular presentation of tumours. Nat. Commun. 13, 208 (2022).
Shah, Y. et al. Pan-cancer analysis reveals molecular patterns associated with age. Cell Rep. 37, 110100 (2021).
Erbe, R. et al. Evaluating the impact of age on immune checkpoint therapy biomarkers. Cell Rep. 37, 110033 (2021).
Kaur, A. et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 532, 250–254 (2016).
Kaur, A. et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 9, 64–81 (2019).
Zabransky, D. J. et al. Fibroblasts in the aged pancreas drive pancreatic cancer progression. Cancer Res. 84, 1221–1236 (2024).
Chatsirisupachai, K., Lagger, C. & de Magalhaes, J. P. Age-associated differences in the cancer molecular landscape. Trends Cancer 8, 962–971 (2022).
Ghosh-Choudhary, S., Liu, J. & Finkel, T. Metabolic regulation of cell fate and function. Trends Cell Biol. 30, 201–212 (2020).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Pavlova, N. N., Zhu, J. & Thompson, C. B. The hallmarks of cancer metabolism: still emerging. Cell Metab. 34, 355–377 (2022).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011).
Gomes, A. P. et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638 (2013).
Serio, S. et al. Cardiac aging is promoted by pseudohypoxia increasing p300-induced glycolysis. Circ. Res. 133, 687–703 (2023).
Murao, N. et al. Increased glycolysis affects β-cell function and identity in aging and diabetes. Mol. Metab. 55, 101414 (2022).
Ravera, S. et al. Discrete changes in glucose metabolism define aging. Sci. Rep. 9, 10347 (2019).
Traxler, L. et al. Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer’s disease. Cell Metab. 34, 1248–1263.e6 (2022).
Wu, L. E., Gomes, A. P. & Sinclair, D. A. Geroncogenesis: metabolic changes during aging as a driver of tumorigenesis. Cancer Cell 25, 12–19 (2014).
Drapela, S., Ilter, D. & Gomes, A. P. Metabolic reprogramming: a bridge between aging and tumorigenesis. Mol. Oncol. 16, 3295–3318 (2022).
Zhang, Y. et al. Upregulation of antioxidant capacity and nucleotide precursor availability suffices for oncogenic transformation. Cell Metab. 33, 94–109.e8 (2021).
Morris, O., Deng, H., Tam, C. & Jasper, H. Warburg-like metabolic reprogramming in aging intestinal stem cells contributes to tissue hyperplasia. Cell Rep. 33, 108423 (2020).
Lu, C. & Thompson, C. B. Metabolic regulation of epigenetics. Cell Metab. 16, 9–17 (2012).
Schvartzman, J. M., Thompson, C. B. & Finley, L. W. S. Metabolic regulation of chromatin modifications and gene expression. J. Cell Biol. 217, 2247–2259 (2018).
Kinnaird, A., Zhao, S., Wellen, K. E. & Michelakis, E. D. Metabolic control of epigenetics in cancer. Nat. Rev. Cancer 16, 694–707 (2016).
Yang, J.-H. et al. Loss of epigenetic information as a cause of mammalian aging. Cell 186, 305–326.e27 (2023).
Wang, K. et al. Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 7, 374 (2022).
Feinberg, A. P. & Levchenko, A. Epigenetics as a mediator of plasticity in cancer. Science 379, eaaw3835 (2023).
Flavahan, W. A., Gaskell, E. & Bernstein, B. E. Epigenetic plasticity and the hallmarks of cancer. Science 357, eaal2380 (2017).
Nebbioso, A., Tambaro, F. P., Dell’Aversana, C. & Altucci, L. Cancer epigenetics: moving forward. PLoS Genet. 14, e1007362 (2018).
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
Moran-Crusio, K. et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20, 11–24 (2011).
Pan, X. et al. TET2 mutations contribute to adverse prognosis in acute myeloid leukemia (AML): results from a comprehensive analysis of 502 AML cases and the Beat AML public database. Clin. Exp. Med. 24, 35 (2024).
Pilley, S. E. et al. A metabolic atlas of mouse aging. Preprint at bioRxiv https://doi.org/10.1101/2024.05.04.592445 (2024).
Chin, R. M. et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 510, 397–401 (2014).
Kaur, P. et al. Combining stem cell rejuvenation and senescence targeting to synergistically extend lifespan. Aging 14, 8270–8291 (2022).
Asadi Shahmirzadi, A. et al. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 32, 447–456.e6 (2020).
McReynolds, M. R. et al. NAD+ flux is maintained in aged mice despite lower tissue concentrations. Cell Syst. 12, 1160–1172.e4 (2021).
McReynolds, M. R., Chellappa, K. & Baur, J. A. Age-related NAD+ decline. Exp. Gerontol. 134, 110888 (2020).
Manni, W., Jianxin, X., Weiqi, H., Siyuan, C. & Huashan, S. JMJD family proteins in cancer and inflammation. Signal Transduct. Target. Ther. 7, 304 (2022).
Demetriadou, C. & Kirmizis, A. Histone acetyltransferases in cancer: guardians or hazards? Crit. Rev. Oncog. 22, 195–218 (2017).
McCauley, B. S. et al. Altered chromatin states drive cryptic transcription in aging mammalian stem cells. Nat. Aging 1, 684–697 (2021).
Harries, L. W. et al. Human aging is characterized by focused changes in gene expression and deregulation of alternative splicing. Aging Cell 10, 868–878 (2011).
Holly, A. C. et al. Changes in splicing factor expression are associated with advancing age in man. Mech. Ageing Dev. 134, 356–366 (2013).
Debès, C. et al. Ageing-associated changes in transcriptional elongation influence longevity. Nature 616, 814–821 (2023).
Negrini, S., Gorgoulis, V. G. & Halazonetis, T. D. Genomic instability — an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 11, 220–228 (2010).
Jones, T. P. & McGranahan, N. Deciphering the landscape of transcriptional heterogeneity across cancer. Cancer Cell 41, 1548–1550 (2023).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 23, 56–73 (2022).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Crowell, P. D. et al. MYC is a regulator of androgen receptor inhibition-induced metabolic requirements in prostate cancer. Cell Rep. 42, 113221 (2023).
Kim, M. et al. Glioblastoma as an age-related neurological disorder in adults. Neurooncol. Adv. 3, vdab125 (2021).
Grassian, A. R. et al. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res. 74, 3317–3331 (2014).
Lenting, K. et al. Isocitrate dehydrogenase 1-mutated human gliomas depend on lactate and glutamate to alleviate metabolic stress. FASEB J. 33, 557–571 (2019).
Khurshed, M., Molenaar, R. J., Lenting, K., Leenders, W. P. & van Noorden, C. J. F. In silico gene expression analysis reveals glycolysis and acetate anaplerosis in IDH1 wild-type glioma and lactate and glutamate anaplerosis in IDH1-mutated glioma. Oncotarget 8, 49165–49177 (2017).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Grimm, A. & Eckert, A. Brain aging and neurodegeneration: from a mitochondrial point of view. J. Neurochem. 143, 418–431 (2017).
Kusi, M. et al. 2-Hydroxyglutarate destabilizes chromatin regulatory landscape and lineage fidelity to promote cellular heterogeneity. Cell Rep. 38, 110220 (2022).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011).
Zhuang, X. et al. Ageing limits stemness and tumorigenesis by reprogramming iron homeostasis. Nature 637, 184–194 (2025).
Patel, A. A. H. et al. Aging promotes lung cancer metastasis through epigenetic ATF4 induction. Preprint at bioRxiv https://doi.org/10.1101/2024.07.03.601209 (2024).
White, M. C. et al. Age and cancer risk: a potentially modifiable relationship. Am. J. Prev. Med. 46, S7–S15 (2014).
Tas, F., Ciftci, R., Kilic, L. & Karabulut, S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol. Lett. 6, 1507–1513 (2013).
de Visser, K. E. & Joyce, J. A. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell 41, 374–403 (2023).
Anderson, N. M. & Simon, M. C. The tumor microenvironment. Curr. Biol. 30, R921–R925 (2020).
Elia, I. & Haigis, M. C. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat. Metab. 3, 21–32 (2021).
Ding, J. et al. A metabolome atlas of the aging mouse brain. Nat. Commun. 12, 6021 (2021).
Duan, T. et al. Dietary restriction protects against diethylnitrosamine-induced hepatocellular tumorigenesis by restoring the disturbed gene expression profile. Sci. Rep. 7, 43745 (2017).
Ploeger, J. M., Manivel, J. C., Boatner, L. N. & Mashek, D. G. Caloric restriction prevents carcinogen-initiated liver tumorigenesis in mice. Cancer Prev. Res. 10, 660–670 (2017).
Murthy, V. K., Oredipe, O. A., Stiles, M. R. & Shipp, J. C. Increased fatty acid uptake, a factor in increased hepatic triacylglycerol synthesis in aging rats. Mech. Ageing Dev. 37, 49–54 (1986).
Sheedfar, F. et al. Increased hepatic CD36 expression with age is associated with enhanced susceptibility to nonalcoholic fatty liver disease. Aging 6, 281–295 (2014).
Slawik, M. & Vidal-Puig, A. J. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res. Rev. 5, 144–164 (2006).
Petrelli, F. et al. Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis: HCC and steatosis or steatohepatitis. Neoplasia 30, 100809 (2022).
Luo, X. et al. The fatty acid receptor CD36 promotes HCC progression through activating Src/PI3K/AKT axis-dependent aerobic glycolysis. Cell Death Dis. 12, 328 (2021).
Pascual, G. et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45 (2017).
Day, C. P. & James, O. F. W. Steatohepatitis: a tale of two “hits”? Gastroenterology 114, 842–845 (1998).
Dhar, D., Baglieri, J., Kisseleva, T. & Brenner, D. A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 245, 96–108 (2020).
Park, E. J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010).
Lipke, K., Kubis-Kubiak, A. & Piwowar, A. Molecular mechanism of lipotoxicity as an interesting aspect in the development of pathological states — current view of knowledge. Cells 11, 844 (2022).
Manukyan, L., Ubhayasekera, S. J., Bergquist, J., Sargsyan, E. & Bergsten, P. Palmitate-induced impairments of β-cell function are linked with generation of specific ceramide species via acylation of sphingosine. Endocrinology 156, 802–812 (2015).
Akoumi, A., Haffar, T., Mousterji, M., Kiss, R. S. & Bousette, N. Palmitate mediated diacylglycerol accumulation causes endoplasmic reticulum stress, Plin2 degradation, and cell death in H9C2 cardiomyoblasts. Exp. Cell Res. 354, 85–94 (2017).
Paul, B., Lewinska, M. & Andersen, J. B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 4, 100479 (2022).
Fekry, B. et al. C16-ceramide is a natural regulatory ligand of p53 in cellular stress response. Nat. Commun. 9, 4149 (2018).
Yin, X. et al. A ceramide-binding C1 domain mediates kinase suppressor of Ras membrane translocation. Cell. Physiol. Biochem. 24, 219–230 (2009).
Zhu, B., Shi, S., Ma, Y. G., Fan, F. & Yao, Z. Z. Lysophosphatidic acid enhances human hepatocellular carcinoma cell migration, invasion and adhesion through P38 MAPK pathway. Hepatogastroenterology 59, 785–789 (2012).
Angelidis, I. et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 10, 963 (2019).
Xiao, M. et al. Functional significance of cholesterol metabolism in cancer: from threat to treatment. Exp. Mol. Med. 55, 1982–1995 (2023).
Lee, J. Y., Kim, W. K., Bae, K. H., Lee, S. C. & Lee, E. W. Lipid metabolism and ferroptosis. Biology 10, 184 (2021).
Liu, W. et al. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat. Commun. 12, 5103 (2021).
Ma, X. et al. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 30, 143–156.e5 (2019).
Meunier, P., Aaron, J., Edouard, C. & Vlgnon, G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue: a quantitative study of 84 iliac bone biopsies. Clin. Orthop. Relat. Res. 80, 147–154 (1971).
Matsuzawa, Y. et al. Pathophysiology and pathogenesis of visceral fat obesity. Obes. Res. 3, 187s–194s (1995).
Olsen, T. S. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol. Microbiol. Scand. A 86a, 367–373 (1978).
Cree, M. G. et al. Intramuscular and liver triglycerides are increased in the elderly. J. Clin. Endocrinol. Metab. 89, 3864–3871 (2004).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Schmitt, C. A., Wang, B. & Demaria, M. Senescence and cancer — role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 19, 619–636 (2022).
Alimirah, F. et al. Cellular senescence promotes skin carcinogenesis through p38MAPK and p44/42MAPK signaling. Cancer Res. 80, 3606–3619 (2020).
Zhou, L. & Ruscetti, M. Senescent macrophages: a new “old” player in lung cancer development. Cancer Cell 41, 1201–1203 (2023).
Haston, S. et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell 41, 1242–1260.e6 (2023).
Ye, J. et al. Senescent CAFs mediate immunosuppression and drive breast cancer progression. Cancer Discov. 14, 1302–1323 (2024).
Belle, J. I. et al. Senescence defines a distinct subset of myofibroblasts that orchestrates immunosuppression in pancreatic cancer. Cancer Discov. 14, 1324–1355 (2024).
Takasugi, M., Yoshida, Y., Hara, E. & Ohtani, N. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 290, 1348–1361 (2023).
Aksoy, P., White, T. A., Thompson, M. & Chini, E. N. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun. 345, 1386–1392 (2006).
Zeidler, J. D. et al. The CD38 glycohydrolase and the NAD sink: implications for pathological conditions. Am. J. Physiol. Cell Physiol. 322, C521–C545 (2022).
Covarrubias, A. J. et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat. Metab. 2, 1265–1283 (2020).
Kilgour, M. K. et al. 1-Methylnicotinamide is an immune regulatory metabolite in human ovarian cancer. Sci. Adv. 7, eabe1174 (2021).
Ma, Y. et al. NNMT/1-MNA promote cell-cycle progression of breast cancer by targeting UBC12/Cullin-1-mediated degradation of P27 proteins. Adv. Sci. 11, e2305907 (2024).
Chini, C. et al. The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD+ decline. Biochem. Biophys. Res. Commun. 513, 486–493 (2019).
Maus, M. et al. Iron accumulation drives fibrosis, senescence and the senescence-associated secretory phenotype. Nat. Metab. 5, 2111–2130 (2023).
Shi, R., Hou, W., Wang, Z.-Q. & Xu, X. Biogenesis of iron–sulfur clusters and their role in DNA metabolism. Front. Cell Dev. Biol. 9, 735678 (2021).
Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 (2010).
Ward, N. P., Kang, Y. P., Falzone, A., Boyle, T. A. & DeNicola, G. M. Nicotinamide nucleotide transhydrogenase regulates mitochondrial metabolism in NSCLC through maintenance of Fe-S protein function. J. Exp. Med. 217, e20191689 (2020).
Winterbourn, C. C. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett. 82–83, 969–974 (1995).
Lee, P., Chandel, N. S. & Simon, M. C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268–283 (2020).
Wiley, C. D. et al. Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis. Cell Metab. 33, 1124–1136.e5 (2021).
Fernandez-Rebollo, E. et al. Senescence-associated metabolomic phenotype in primary and iPSC-derived mesenchymal stromal cells. Stem Cell Rep. 14, 201–209 (2020).
Xing, Y., Xuan, F., Wang, K. & Zhang, H. Aging under endocrine hormone regulation. Front. Endocrinol. 14, 1223529 (2023).
Franceschi, C. et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 (2000).
Gomes, A. P. et al. Age-induced accumulation of methylmalonic acid promotes tumour progression. Nature 585, 283–287 (2020).
Koelman, L., Pivovarova-Ramich, O., Pfeiffer, A. F. H., Grune, T. & Aleksandrova, K. Cytokines for evaluation of chronic inflammatory status in ageing research: reliability and phenotypic characterisation. Immun. Ageing 16, 11 (2019).
Giovannini, S. et al. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J. Am. Geriatr. Soc. 59, 1679–1685 (2011).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct. Target. Ther. 8, 200 (2023).
Senn, J. J., Klover, P. J., Nowak, I. A. & Mooney, R. A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 51, 3391–3399 (2002).
Mohallem, R. & Aryal, U. K. Regulators of TNFα mediated insulin resistance elucidated by quantitative proteomics. Sci. Rep. 10, 20878 (2020).
Niswender, K. D. et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52, 227–231 (2003).
Xuekai, L. et al. Advances in reprogramming of energy metabolism in tumor T cells. Front. Immunol. 15, 1347181 (2024).
Han, S., Georgiev, P., Ringel, A. E., Sharpe, A. H. & Haigis, M. C. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 35, 36–55 (2023).
Park, M. D. et al. Hematopoietic aging promotes cancer by fueling IL-1α–driven emergency myelopoiesis. Science 386, eadn0327 (2024).
Menjivar, R. E. et al. Arginase 1 is a key driver of immune suppression in pancreatic cancer. eLife 12, e80721 (2023).
Schofield, J. H. et al. Acod1 expression in cancer cells promotes immune evasion through the generation of inhibitory peptides. Cell Rep. 43, 113984 (2024).
Alaluf, E. et al. Heme oxygenase-1 orchestrates the immunosuppressive program of tumor-associated macrophages. JCI Insight 5, e133929 (2020).
Folkerd, E. J. & Dowsett, M. Influence of sex hormones on cancer progression. J. Clin. Oncol. 28, 4038–4044 (2010).
Sepich-Poore, G. D. et al. The microbiome and human cancer. Science 371, eabc4552 (2021).
Nagy, S., Petrosky, S. N., Demory Beckler, M. & Kesselman, M. M. The impact of modern dietary practices on cancer risk and progression: a systematic review. Cureus 15, e46639 (2023).
Bana, B. & Cabreiro, F. The microbiome and aging. Annu. Rev. Genet. 53, 239–261 (2019).
Kim, M. & Benayoun, B. A. The microbiome: an emerging key player in aging and longevity. Transl. Med. Aging 4, 103–116 (2020).
Wilmanski, T. et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 3, 274–286 (2021).
Pang, S. et al. Longevity of centenarians is reflected by the gut microbiome with youth-associated signatures. Nat. Aging 3, 436–449 (2023).
Covarrubias, A. J., Perrone, R., Grozio, A. & Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 22, 119–141 (2021).
Chellappa, K. et al. NAD precursors cycle between host tissues and the gut microbiome. Cell Metab. 34, 1947–1959.e5 (2022).
Shats, I. et al. Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 31, 564–579.e7 (2020).
Chowdhry, S. et al. NAD metabolic dependency in cancer is shaped by gene amplification and enhancer remodelling. Nature 569, 570–575 (2019).
Nagpal, R. et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr. Healthy Aging 4, 267–285 (2018).
Odamaki, T. et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 16, 90 (2016).
Hopkins, M. J., Sharp, R. & Macfarlane, G. T. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 48, 198–205 (2001).
Hou, K. et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 7, 135 (2022).
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Wong, L. M. et al. Comparative analysis of age- and gender-associated microbiome in lung adenocarcinoma and lung squamous cell carcinoma. Cancers 12, 1447 (2020).
Gomes, A. P. et al. Altered propionate metabolism contributes to tumour progression and aggressiveness. Nat. Metab. 4, 435–443 (2022).
Sahm, F. et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res. 73, 3225–3234 (2013).
Kesarwani, P. et al. Quinolinate promotes macrophage-induced immune tolerance in glioblastoma through the NMDAR/PPARγ signaling axis. Nat. Commun. 14, 1459 (2023).
Morris, M. S., Jacques, P. F., Rosenberg, I. H. & Selhub, J. Elevated serum methylmalonic acid concentrations are common among elderly Americans. J. Nutr. 132, 2799–2803 (2002).
Tejero, J., Lazure, F. & Gomes, A. P. Methylmalonic acid in aging and disease. Trends Endocrinol. Metab. 35, 188–200 (2024).
Wang, S. et al. Mitochondria-derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all-cause and cardiovascular mortality in the general population. Redox Biol. 37, 101741 (2020).
Riphagen, I. J. et al. Methylmalonic acid, vitamin B12, renal function, and risk of all-cause mortality in the general population: results from the prospective Lifelines-MINUTHE study. BMC Med. 18, 380 (2020).
Tejero, J. D. et al. Methylmalonic acid induces metabolic abnormalities and exhaustion in CD8+ T cells to suppress anti-tumor immunity. Oncogene 44, 105–114 (2025).
Singh, P. et al. Taurine deficiency as a driver of aging. Science 380, eabn9257 (2023).
Saleh, Z. et al. Alterations in metabolic pathways: a bridge between aging and weaker innate immune response. Front. Aging 5, 1358330 (2024).
Bae, M., Ahmed, K. & Yim, J. E. Beneficial effects of taurine on metabolic parameters in animals and humans. J. Obes. Metab. Syndr. 31, 134–146 (2022).
Kassis, A. et al. Nutritional and lifestyle management of the aging journey: a narrative review. Front. Nutr. 9, 1087505 (2022).
Gallagher, J. C. Vitamin D and aging. Endocrinol. Metab. Clin. N. Am. 42, 319–332 (2013).
Stover, P. J. Vitamin B12 and older adults. Curr. Opin. Clin. Nutr. Metab. Care 13, 24–27 (2010).
Gao, C.-F. et al. Proliferation and invasion: plasticity in tumor cells. Proc. Natl Acad. Sci. USA 102, 10528–10533 (2005).
Lewis, D. A. & Ly, T. Cell cycle entry control in naïve and memory CD8+ T cells. Front. Cell Dev. Biol. 9, 727441 (2021).
Williams, J. D. et al. Tumor autonomous effects of vitamin D deficiency promote breast cancer metastasis. Endocrinology 157, 1341–1347 (2016).
Mariean, C. R., Tiucă, O. M., Mariean, A. & Cotoi, O. S. Cancer cachexia: new insights and future directions. Cancers 15, 5590 (2023).
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 4, 17105 (2018).
Ali, S. & Garcia, J. M. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options — a mini-review. Gerontology 60, 294–305 (2014).
Geppert, J. et al. Aging aggravates cachexia in tumor-bearing mice. Cancers 14, 90 (2021).
Geppert, J. & Rohm, M. Cancer cachexia: biomarkers and the influence of age. Mol. Oncol. 18, 2070–2086 (2024).
Rohm, M., Zeigerer, A., Machado, J. & Herzig, S. Energy metabolism in cachexia. EMBO Rep. 20, e47258 (2019).
Pala, F. et al. Distinct metabolic states govern skeletal muscle stem cell fates during prenatal and postnatal myogenesis. J. Cell Sci. 131, jcs212977 (2018).
Gerstberger, S., Jiang, Q. & Ganesh, K. Metastasis. Cell 186, 1564–1579 (2023).
Drapela, S. & Gomes, A. P. Metabolic requirements of the metastatic cascade. Curr. Opin. Syst. Biol. 28, 100381 (2021).
Bergers, G. & Fendt, S. M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21, 162–180 (2021).
Fontana, R., Mestre-Farrera, A. & Yang, J. Update on epithelial-mesenchymal plasticity in cancer progression. Annu. Rev. Pathol. 19, 133–156 (2024).
Kai, F., Drain, A. P. & Weaver, V. M. The extracellular matrix modulates the metastatic journey. Dev. Cell 49, 332–346 (2019).
Larsen, M., Artym, V. V., Green, J. A. & Yamada, K. M. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 18, 463–471 (2006).
Marsico, G., Russo, L., Quondamatteo, F. & Pandit, A. Glycosylation and integrin regulation in cancer. Trends Cancer 4, 537–552 (2018).
Yki-Järvinen, H. et al. Increased glutamine:fructose-6-phosphate amidotransferase activity in skeletal muscle of patients with NIDDM. Diabetes 45, 302–307 (1996).
Clark, R. J. et al. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J. Biol. Chem. 278, 44230–44237 (2003).
Andres, R. Aging and diabetes. Med. Clin. N. Am. 55, 835–846 (1971).
Shimokata, H. et al. Age as independent determinant of glucose tolerance. Diabetes 40, 44–51 (1991).
Zavaroni, I. et al. Effect of age and environmental factors on glucose tolerance and insulin secretion in a worker population. J. Am. Geriatr. Soc. 34, 271–275 (1986).
Maneatis, T., Condie, R. & Reaven, G. Effect of age on plasma glucose and insulin responses to a test mixed meal. J. Am. Geriatr. Soc. 30, 178–182 (1982).
Kim, C. S., Park, S. & Kim, J. The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. J. Exerc. Nutr. Biochem. 21, 55–61 (2017).
Batkulwar, K. et al. Advanced glycation end products modulate amyloidogenic APP processing and tau phosphorylation: a mechanistic link between glycation and the development of Alzheimer’s disease. ACS Chem. Neurosci. 9, 988–1000 (2018).
Sasaki, N. et al. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am. J. Pathol. 153, 1149–1155 (1998).
Singh, V. P., Bali, A., Singh, N. & Jaggi, A. S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 18, 1–14 (2014).
Theodore, C. et al. Treatment of high-risk gestational trophoblastic disease with chemotherapy combinations containing cisplatin and etoposide. Cancer 64, 1824–1828 (1989).
Deng, R. et al. Glucose-derived AGEs promote migration and invasion of colorectal cancer by up-regulating Sp1 expression. Biochim. Biophys. Acta Gen. Subj. 1861, 1065–1074 (2017).
Pan, S. et al. Advanced glycation end products correlate with breast cancer metastasis by activating RAGE/TLR4 signaling. BMJ Open Diabetes Res. Care 10, e002697 (2022).
Alicea, G. M. et al. Age-related increases in IGFBP2 increase melanoma cell invasion and lipid synthesis. Cancer Res. Commun. 4, 1908–1918 (2024).
Podolsky, M. J. et al. Age-dependent regulation of cell-mediated collagen turnover. JCI Insight 5, e137519 (2020).
Stearns-Reider, K. M. et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 16, 518–528 (2017).
Poynard, T. et al. Prevalence of liver fibrosis and risk factors in a general population using non-invasive biomarkers (FibroTest). BMC Gastroenterol. 10, 40 (2010).
De Martino, D. & Bravo-Cordero, J. J. Collagens in cancer: structural regulators and guardians of cancer progression. Cancer Res. 83, 1386–1392 (2023).
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J. & Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120 (2020).
Lobel, G. P., Jiang, Y. & Simon, M. C. Tumor microenvironmental nutrients, cellular responses, and cancer. Cell Chem. Biol. 30, 1015–1032 (2023).
Olivares, O. et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat. Commun. 8, 16031 (2017).
Saha, M. et al. Sustained AMPK activation and proline metabolism play critical roles in the survival of matrix-deprived transformed cells. Front. Cell Dev. Biol. 9, 771366 (2021).
Elia, I. et al. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat. Commun. 8, 15267 (2017).
Kuehne, A. et al. An integrative metabolomics and transcriptomics study to identify metabolic alterations in aged skin of humans in vivo. BMC Genom. 18, 169 (2017).
Wang, X. et al. Targeted metabolomic profiling reveals association between altered amino acids and poor functional recovery after stroke. Front. Neurol. 10, 1425 (2019).
Choudhury, D. et al. Proline restores mitochondrial function and reverses aging hallmarks in senescent cells. Cell Rep. 43, 113738 (2024).
Uchitomi, R. et al. Metabolomic analysis of skeletal muscle in aged mice. Sci. Rep. 9, 10425 (2019).
Pilley, S. E. et al. Loss of attachment promotes proline accumulation and excretion in cancer cells. Sci. Adv. 9, eadh2023 (2023).
Loayza-Puch, F. et al. Tumour-specific proline vulnerability uncovered by differential ribosome codon reading. Nature 530, 490–494 (2016).
Piskounova, E. et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015).
Ubellacker, J. M. et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585, 113–118 (2020).
Ilter, D. et al. NADK-mediated de novo NADP(H) synthesis is a metabolic adaptation essential for breast cancer metastasis. Redox Biol. 61, 102627 (2023).
Carr, A. C. & Lykkesfeldt, J. Does aging affect vitamin C status relative to intake? Findings from NHANES 2017-2018. Nutrients 15, 892 (2023).
Yang, C. S., Chou, S. T., Liu, L., Tsai, P. J. & Kuo, J. S. Effect of ageing on human plasma glutathione concentrations as determined by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B Biomed. Appl. 674, 23–30 (1995).
Detcheverry, F., Senthil, S., Narayanan, S. & Badhwar, A. Changes in levels of the antioxidant glutathione in brain and blood across the age span of healthy adults: a systematic review. Neuroimage Clin. 40, 103503 (2023).
Hoey, L., Strain, J. J. & McNulty, H. Studies of biomarker responses to intervention with vitamin B-12: a systematic review of randomized controlled trials. Am. J. Clin. Nutr. 89, 1981s–1996s (2009).
Bansal, A. & Simon, M. C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 217, 2291–2298 (2018).
Cascio, G. et al. Transcriptional isoforms of NAD+ kinase regulate oxidative stress resistance and melanoma metastasis. Redox Biol. 76, 103289 (2024).
Leong, S. P., Naxerova, K., Keller, L., Pantel, K. & Witte, M. Molecular mechanisms of cancer metastasis via the lymphatic versus the blood vessels. Clin. Exp. Metastasis 39, 159–179 (2022).
Mann, J. et al. Ferroptosis inhibition by oleic acid mitigates iron-overload-induced injury. Cell Chem. Biol. 31, 249–264.e7 (2024).
Bekkhus, T., Olofsson, A., Sun, Y., Magnusson, P. U. & Ulvmar, M. H. Stromal transdifferentiation drives lipomatosis and induces extensive vascular remodeling in the aging human lymph node. J. Pathol. 259, 236–253 (2023).
Ali, S. et al. Age-associated changes in circulatory fatty acids: new insights on adults and long-lived individuals. Geroscience 45, 781–796 (2023).
Gao, Y. et al. Metastasis organotropism: redefining the congenial soil. Dev. Cell 49, 375–391 (2019).
Schild, T., Low, V., Blenis, J. & Gomes, A. P. Unique metabolic adaptations dictate distal organ-specific metastatic colonization. Cancer Cell 33, 347–354 (2018).
Crist, S. B. et al. Unchecked oxidative stress in skeletal muscle prevents outgrowth of disseminated tumour cells. Nat. Cell Biol. 24, 538–553 (2022).
Chen, M.-T. et al. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci. Rep. 7, 9254 (2017).
Frankel, T. L. et al. Predicting the development of brain metastases in patients with local/regional melanoma. J. Surg. Oncol. 109, 770–774 (2014).
Wu, A. M. L. et al. Aging and CNS myeloid cell depletion attenuate breast cancer brain metastasis. Clin. Cancer Res. 27, 4422–4434 (2021).
He, L., Ding, Y., Zhou, X., Li, T. & Yin, Y. Serine signaling governs metabolic homeostasis and health. Trends Endocrinol. Metab. 34, 361–372 (2023).
Amelio, I., Cutruzzolá, F., Antonov, A., Agostini, M. & Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 39, 191–198 (2014).
Ngo, B. et al. Limited environmental serine and glycine confer brain metastasis sensitivity to PHGDH inhibition. Cancer Discov. 10, 1352–1373 (2020).
Potier, B. et al. Contribution of the d-serine-dependent pathway to the cellular mechanisms underlying cognitive aging. Front. Aging Neurosci. 2, 1 (2010).
Calcia, M. A. et al. Plasma levels of D-serine in Brazilian individuals with schizophrenia. Schizophr. Res. 142, 83–87 (2012).
Mothet, J. P. et al. A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell 5, 267–274 (2006).
Madeira, C. et al. d-serine levels in Alzheimer’s disease: implications for novel biomarker development. Transl. Psychiatry 5, e561 (2015).
Shleper, M., Kartvelishvily, E. & Wolosker, H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J. Neurosci. 25, 9413–9417 (2005).
Liu, F. C. et al. Exploring the aging process of cognitively healthy adults by analyzing cerebrospinal fluid metabolomics using liquid chromatography-tandem mass spectrometry. BMC Geriatr. 23, 217 (2023).
St Paul, M. et al. Coenzyme A fuels T cell anti-tumor immunity. Cell Metab. 33, 2415–2427.e6 (2021).
Miallot, R. et al. The coenzyme A precursor pantethine enhances antitumor immunity in sarcoma. Life Sci. Alliance 6, e202302200 (2023).
Altea-Manzano, P. et al. A palmitate-rich metastatic niche enables metastasis growth via p65 acetylation resulting in pro-metastatic NF-κB signaling. Nat. Cancer 4, 344–364 (2023).
Kim, S. J., Cheresh, P., Jablonski, R. P., Williams, D. B. & Kamp, D. W. The role of mitochondrial DNA in mediating alveolar epithelial cell apoptosis and pulmonary fibrosis. Int. J. Mol. Sci. 16, 21486–21519 (2015).
Zhou, L., Zhang, H., Davies, K. J. A. & Forman, H. J. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol. 14, 35–40 (2018).
Korfei, M., MacKenzie, B. & Meiners, S. The ageing lung under stress. Eur. Respir. Rev. 29, 200126 (2020).
Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014).
Risson, E., Nobre, A. R., Maguer-Satta, V. & Aguirre-Ghiso, J. A. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. Cancer 1, 672–680 (2020).
Fane, M. E. et al. Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature 606, 396–405 (2022).
Turrell, F. K. et al. Age-associated microenvironmental changes highlight the role of PDGF-C in ER+ breast cancer metastatic relapse. Nat. Cancer 4, 468–484 (2023).
Fox, D. B. et al. NRF2 activation promotes the recurrence of dormant tumour cells through regulation of redox and nucleotide metabolism. Nat. Metab. 2, 318–334 (2020).
Harper, E. I. et al. Another wrinkle with age: aged collagen and intra-peritoneal metastasis of ovarian cancer. Aging Cancer 3, 116–129 (2022).
Thigpen, T. et al. Age as a prognostic factor in ovarian carcinoma. The Gynecologic Oncology Group experience. Cancer 71, 606–614 (1993).
Deng, F. et al. Age is associated with prognosis in serous ovarian carcinoma. J. Ovarian Res. 10, 36 (2017).
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 (2011).
Ladanyi, A. et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 37, 2285–2301 (2018).
Moraes, D. et al. The transcriptomic landscape of age-induced changes in human visceral fat and the predicted omentum-liver connectome in males. Biomedicines 11, 1446 (2023).
Aebi, M. Spinal metastasis in the elderly. Eur. Spine J. 12, S202–S213 (2003).
Hu, Z. et al. Prevalence and risk factors of bone metastasis and the development of bone metastatic prognostic classification system: a pan-cancer population study. Aging 15, 13134–13149 (2023).
Schwarcz, H. P., Agur, K. & Jantz, L. M. A new method for determination of postmortem interval: citrate content of bone. J. Forensic Sci. 55, 1516–1522 (2010).
Hu, Y. Y., Rawal, A. & Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl Acad. Sci. USA 107, 22425–22429 (2010).
Costello, L. C., Franklin, R. B., Reynolds, M. A. & Chellaiah, M. The important role of osteoblasts and citrate production in bone formation: “Osteoblast citration” as a new concept for an old relationship. Open Bone J. https://doi.org/10.2174/1876525401204010027 (2012).
Chen, H. et al. Bone and plasma citrate is reduced in osteoporosis. Bone 114, 189–197 (2018).
Lv, Z., Shi, W. & Zhang, Q. Role of essential amino acids in age-induced bone loss. Int. J. Mol. Sci. 23, 11281 (2022).
Pouikli, A. et al. Chromatin remodeling due to degradation of citrate carrier impairs osteogenesis of aged mesenchymal stem cells. Nat. Aging 1, 810–825 (2021).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 (2008).
Ambrosi, T. H. et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20, 771–784.e6 (2017).
Giaquinto, A. N. et al. Cancer statistics for African American/Black people 2022. CA Cancer J. Clin. 72, 202–229 (2022).
Levine, M. E. & Crimmins, E. M. Evidence of accelerated aging among African Americans and its implications for mortality. Soc. Sci. Med. 118, 27–32 (2014).
Augustus, G. J. & Ellis, N. A. Colorectal cancer disparity in African Americans: risk factors and carcinogenic mechanisms. Am. J. Pathol. 188, 291–303 (2018).
Yedjou, C. G. et al. Health and racial disparity in breast cancer. Adv. Exp. Med. Biol. 1152, 31–49 (2019).
Siddharth, S. & Sharma, D. Racial disparity and triple-negative breast cancer in African-American women: a multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers 10, 514 (2018).
Mitchell, S. L. et al. Characterization of mitochondrial haplogroups in a large population-based sample from the United States. Hum. Genet. 133, 861–868 (2014).
Wallace, D. C. A mitochondrial bioenergetic etiology of disease. J. Clin. Invest. 123, 1405–1412 (2013).
Tranah, G. J. et al. Mitochondrial DNA variation in human metabolic rate and energy expenditure. Mitochondrion 11, 855–861 (2011).
Lee, W. T. et al. Mitochondrial DNA haplotypes induce differential patterns of DNA methylation that result in differential chromosomal gene expression patterns. Cell Death Discov. 3, 17062 (2017).
Kelly, R. D. et al. Mitochondrial DNA haplotypes define gene expression patterns in pluripotent and differentiating embryonic stem cells. Stem Cell 31, 703–716 (2013).
Bilal, E. et al. Mitochondrial DNA haplogroup D4a is a marker for extreme longevity in Japan. PLoS ONE 3, e2421 (2008).
Kim, S., Myers, L., Ravussin, E., Cherry, K. E. & Jazwinski, S. M. Single nucleotide polymorphisms linked to mitochondrial uncoupling protein genes UCP2 and UCP3 affect mitochondrial metabolism and healthy aging in female nonagenarians. Biogerontology 17, 725–736 (2016).
Latorre-Pellicer, A. et al. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 535, 561–565 (2016).
Chattopadhyay, M. et al. The portrait of liver cancer is shaped by mitochondrial genetics. Cell Rep. 38, 110254 (2022).
Brinker, A. E. et al. Mitochondrial haplotype alters mammary cancer tumorigenicity and metastasis in an oncogenic driver-dependent manner. Cancer Res. 77, 6941–6949 (2017).
Ganly, I. et al. Mitonuclear genotype remodels the metabolic and microenvironmental landscape of Hürthle cell carcinoma. Sci. Adv. 8, eabn9699 (2022).
Mahmood, M. et al. Mitochondrial DNA mutations drive aerobic glycolysis to enhance checkpoint blockade response in melanoma. Nat. Cancer 5, 659–672 (2024).
Brinker, A. E. et al. Mitochondrial haplotype of the host stromal microenvironment alters metastasis in a non-cell autonomous manner. Cancer Res. 80, 1118–1129 (2020).
Picard, M. et al. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc. Natl Acad. Sci. USA 112, E6614–E6623 (2015).
Obradović, M. M. S. et al. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544 (2019).
He, X. Y. et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell 42, 474–486.e12 (2024).
Yiallouris, A. et al. Adrenal aging and its implications on stress responsiveness in humans. Front. Endocrinol. 10, 54 (2019).
Piazza, J. R., Almeida, D. M., Dmitrieva, N. O. & Klein, L. C. Frontiers in the use of biomarkers of health in research on stress and aging. J. Gerontol. B 65B, 513–525 (2010).
Choi, S. et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 592, 428–432 (2021).
Aguilera, G. HPA axis responsiveness to stress: implications for healthy aging. Exp. Gerontol. 46, 90–95 (2011).
Acknowledgements
We are grateful to members of the Gomes laboratory for the critical discussions on this topic and to J. Cleveland for the critical reading and editing of this Perspective. The Gomes laboratory is supported by the New Innovator Award from the Office of the Director of the National Institutes of Health (DP2AG0776980), an American Cancer Society Research Scholar Award (RSG-22-164-01-MM), the National Institute on Aging (R21AG083720), the National Cancer Institute (R01CA279023), the Florida Health Department Bankhead-Coley Research Program (24B03), the Florida Breast Cancer Research Foundation, the Phi Beta Psi Sorority, and the National Cancer Institute Comprehensive Cancer Center Grant P30 CA076292.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Christian Frezza, who co-reviewed along with Andromachi Pouikli, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Aerobic glycolysis
-
A metabolic process whereby cells favour glycolysis over oxidative phosphorylation, by converting glucose into lactate for energy production, even in the presence of oxygen.
- Conplastic mouse strains
-
Mouse strains that have identical genomic DNA but different mitochondrial DNA (mtDNA), often produced by repeatedly backcrossing females carrying the mtDNA of interest with males of the desired nuclear DNA background.
- Cryptic promoters
-
Promoter sequences that are normally hidden by heterochromatin, but which can become uncovered when chromatin is disrupted to initiate transcription, often driving the expression of alternative mRNA transcripts.
- Enantiomer
-
One of a pair of molecules that share the same molecular composition, but which are spatially arranged to be mirror images of each other.
- Endoplasmic reticulum (ER) stress
-
The accumulation of misfolded proteins within the ER caused by reactive oxygen species, hypoxia, various nutrient levels, or other factors that trigger the unfolded protein response to re-establish proteostasis.
- Epigenetic erosion
-
The decline in the stability and/or maintenance of epigenetic marks over time, often observed during ageing.
- Folate cycle
-
A metabolic pathway that utilizes dietary folate to support one-carbon metabolism, fueling nucleotide synthesis and methylation processes.
- Haplogroups
-
Groups of people defined by a set of genetic markers inherited from a common ancestor, usually associated with a specific geographical region.
- Haplotypes
-
Groups of genetic markers, such as single-nucleotide polymorphisms, that are usually inherited as a unit owing to their close proximity on a chromosome or mitochondrial DNA and are used to track genetic variation patterns within populations.
- Hyperinsulinaemia
-
A condition wherein insulin levels in the blood are chronically elevated, often associated with insulin resistance and diabetes.
- Hypothalamic–pituitary–adrenal axis
-
A communication system between the hypothalamus, the pituitary gland of the brain, and the adrenal glands that occurs via hormones to regulate the reaction of the body to stress.
- Iron–sulfur cluster
-
Molecules composed of iron and sulfur found in proteins that are essential for various biological processes such as the functioning of the electron transport chain and various enzymatic activities.
- Osteoporosis
-
A disease defined by reduced bone density often caused by an imbalance between bone formation and resorption and typically associated with older age.
- Oxidative phosphorylation
-
(OXPHOS). The cellular process that harnesses the reduction of oxygen to generate ATP.
- Single-nucleotide polymorphisms
-
(SNPs). DNA point mutations in a single nucleotide, which may or may not lead to phenotypic traits, and are commonly used as markers in population genetics to track inheritance.
- Transcriptional elongation
-
A step during gene expression wherein RNA polymerase II moves along the DNA template to create a nascent RNA transcript.
- Tricarboxylic acid cycle
-
A metabolic pathway that generates electron carriers NADH and flavin adenine dinucleotide (FADH2) by breaking down acetyl-coenzyme A derived from glucose and fatty acids, also known as the Krebs cycle or citric acid cycle.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lazure, F., Gomes, A.P. Cancer progression through the lens of age-induced metabolic reprogramming. Nat Rev Cancer 25, 801–817 (2025). https://doi.org/10.1038/s41568-025-00845-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41568-025-00845-4
This article is cited by
-
Ageing, immune fitness and cancer
Nature Reviews Cancer (2025)