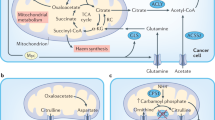
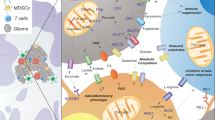
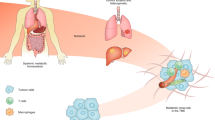
Abstract
Brain metastases remain a major clinical challenge, characterized by high mortality rates and often limited therapeutic options. The cellular and molecular processes that drive brain metastases are highly intricate, underscored by dynamic metabolic adaptations that enable tumour cells to thrive in the unique microenvironment of the brain. Emerging clinical and preclinical evidence reveals that these metabolic adaptations are not uniform but vary based on the tumour’s tissue of origin, oncogenomic landscape and capacity to endure nutrient stress. Notably, proliferative and dormant metastatic cells within the brain exhibit distinct metabolic profiles, highlighting the complexity of targeting these cells. Key metabolic pathways, including glucose, fatty acid and amino acid metabolism, are co-opted not only to sustain cancer cell survival and growth but also to modulate interactions with resident brain cells, reshaping their function to support metastasis. Importantly, this metabolic heterogeneity underscores the inadequacy of a one-size-fits-all therapeutic approach. Here, we review the adaptive metabolic reprogramming that facilitates brain metastases and discuss emerging strategies to tailor interventions aimed at preventing and treating overt brain metastases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kotecha, R., Gondi, V., Ahluwalia, M. S., Brastianos, P. K. & Mehta, M. P. Recent advances in managing brain metastasis. F1000Res 7, F1000 (2018).
Lv, Y., Ma, X., Du, Y. & Feng, J. Understanding patterns of brain metastasis in triple-negative breast cancer and exploring potential therapeutic targets. Onco Targets Ther. 14, 589–607 (2021).
Zou, Y. et al. Polyunsaturated fatty acids from astrocytes activate PPARgamma signaling in cancer cells to promote brain metastasis. Cancer Discov. 9, 1720–1735 (2019). This study is the first to reveal that astrocytes in the brain microenvironment supply polyunsaturated fatty acids, which are taken up by cancer cells to activate PPARγ, thereby driving metastatic cancer cell proliferation and outgrowth, highlighting PPARγ blockade as a promising therapeutic strategy for advanced brain metastases.
Parida, P. K. et al. Limiting mitochondrial plasticity by targeting DRP1 induces metabolic reprogramming and reduces breast cancer brain metastases. Nat. Cancer 4, 893–907 (2023). This study uncovered a metabolic interaction between dormant brain metastases and astrocytes, in which astrocyte-derived fatty acids are oxidized by latent cells with DRP1-driven fragmented mitochondria to sustain bioenergetics and maintain redox balance, presenting DRP1 as a therapeutic target to prevent latency and metastatic relapse.
Cagney, D. N. et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 19, 1511–1521 (2017).
Witzel, I., Oliveira-Ferrer, L., Pantel, K., Muller, V. & Wikman, H. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res. 18, 8 (2016).
Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 13, 4429–4434 (2007).
Zimmer, A. S., Van Swearingen, A. E. D. & Anders, C. K. HER2-positive breast cancer brain metastasis: a new and exciting landscape. Cancer Rep. 5, e1274 (2022).
Khan, M. et al. Tumor primary site and histology subtypes role in radiotherapeutic management of brain metastases. Front. Oncol. 10, 781 (2020).
Darlix, A. et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br. J. Cancer 121, 991–1000 (2019).
Lin, N. U., Amiri-Kordestani, L., Palmieri, D., Liewehr, D. J. & Steeg, P. S. CNS metastases in breast cancer: old challenge, new frontiers. Clin. Cancer Res. 19, 6404–6418 (2013).
Aversa, C. et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast 23, 623–628 (2014).
Anderson, W. F., Rosenberg, P. S. & Katki, H. A. Tracking and evaluating molecular tumor markers with cancer registry data: HER2 and breast cancer. J. Natl Cancer Inst. 106, dju093 (2014).
Mesa-Eguiagaray, I. et al. Distinct temporal trends in breast cancer incidence from 1997 to 2016 by molecular subtypes: a population-based study of Scottish cancer registry data. Br. J. Cancer 123, 852–859 (2020).
Kuksis, M. et al. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 23, 894–904 (2021).
Ho, V. K. et al. Survival of breast cancer patients with synchronous or metachronous central nervous system metastases. Eur. J. Cancer 51, 2508–2516 (2015).
Tan, X. L. et al. Burden and risk factors of brain metastases in melanoma: a systematic literature review. Cancers 14, 6108 (2022).
Chukwueke, U., Batchelor, T. & Brastianos, P. Management of brain metastases in patients with melanoma. J. Oncol. Pract. 12, 536–542 (2016).
Takemura, K. et al. Outcomes of patients with brain metastases from renal cell carcinoma receiving first-line therapies: results from the international metastatic renal cell carcinoma database consortium. Eur. Urol. 86, 488–492 (2024).
Bianchi, M. et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann. Oncol. 23, 973–980 (2012).
Hirsch, L. et al. Clinical activity and safety of cabozantinib for brain metastases in patients with renal cell carcinoma. JAMA Oncol. 7, 1815–1823 (2021).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Lyle, L. T. et al. Alterations in pericyte subpopulations are associated with elevated blood–tumor barrier permeability in experimental brain metastasis of breast cancer. Clin. Cancer Res. 22, 5287–5299 (2016).
Ngo, B. et al. Limited environmental serine and glycine confer brain metastasis sensitivity to PHGDH inhibition. Cancer Discov. 10, 1352–1373 (2020). This study showed that brain metastatic cells upregulate PHGDH to overcome serine deficiency in the brain microenvironment and that PHGDH inhibition reduces brain metastasis by inducing DNA damage and disrupting nucleotide synthesis.
Altea-Manzano, P., Cuadros, A. M., Broadfield, L. A. & Fendt, S. M. Nutrient metabolism and cancer in the in vivo context: a metabolic game of give and take. EMBO Rep. 21, e50635 (2020).
Chen, J. et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 75, 554–565 (2015). This study demonstrated that breast cancer brain metastases enhance gluconeogenesis and utilize glutamine and leucine to sustain purine synthesis under glucose limitation, with FBP silencing reducing metastasis.
Valiente, M. et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014).
Karimi, E. et al. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 614, 555–563 (2023).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
de Visser, K. E. & Joyce, J. A. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell 41, 374–403 (2023).
Dey, P., Kimmelman, A. C. & DePinho, R. A. Metabolic codependencies in the tumor microenvironment. Cancer Discov. 11, 1067–1081 (2021).
Bergers, G. & Fendt, S. M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21, 162–180 (2021).
Elia, I., Doglioni, G. & Fendt, S. M. Metabolic hallmarks of metastasis formation. Trends Cell Biol. 28, 673–684 (2018).
Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20, 26–41 (2020).
Kadry, H., Noorani, B. & Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17, 69 (2020).
O’Brown, N. M., Pfau, S. J. & Gu, C. Bridging barriers: a comparative look at the blood–brain barrier across organisms. Genes Dev. 32, 466–478 (2018).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Parida, P. K. et al. Metabolic diversity within breast cancer brain-tropic cells determines metastatic fitness. Cell Metab. 34, 90–105.e7 (2022). This study highlights the distinct metabolic profiles of synchronous, latent or dormant, and metachronous breast cancer brain metastatic cells and how these differences influence metastatic fitness.
Roshanzamir, F., Robinson, J. L., Cook, D., Karimi-Jafari, M. H. & Nielsen, J. Metastatic triple negative breast cancer adapts its metabolism to destination tissues while retaining key metabolic signatures. Proc. Natl Acad. Sci. USA 119, e2205456119 (2022).
Mergenthaler, P., Lindauer, U., Dienel, G. A. & Meisel, A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36, 587–597 (2013).
Chen, E. I. et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 67, 1472–1486 (2007).
Duan, W. et al. Warburg effect enhanced by AKR1B10 promotes acquired resistance to pemetrexed in lung cancer-derived brain metastasis. J. Transl. Med. 21, 547 (2023).
Kim, H. M., Jung, W. H. & Koo, J. S. Site-specific metabolic phenotypes in metastatic breast cancer. J. Transl. Med. 12, 354 (2014). This study revealed that glycolysis-related proteins, including glucose transporter 1, hexokinase II, carbonic anhydrase IX and monocarboxylate transporter 4, are significantly elevated in triple-negative breast cancer brain metastases compared with bone and liver metastases, which display a less glycolytic phenotype.
Palmieri, D. et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 7, 1438–1445 (2009).
Schneegans, S. et al. HERC5 downregulation in non-small cell lung cancer is associated with altered energy metabolism and metastasis. J. Exp. Clin. Cancer Res. 43, 110 (2024).
Li, X. et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol. Cell 61, 705–719 (2016).
Marin-Valencia, I. et al. Glucose metabolism via the pentose phosphate pathway, glycolysis and Krebs cycle in an orthotopic mouse model of human brain tumors. NMR Biomed. 25, 1177–1186 (2012).
Li, A. M. et al. Metabolic profiling reveals a dependency of human metastatic breast cancer on mitochondrial serine and one-carbon unit metabolism. Mol. Cancer Res. 18, 599–611 (2020).
Jin, X. et al. A metastasis map of human cancer cell lines. Nature 588, 331–336 (2020). This study showed that PIK3CA mutations and ERBB2 gene amplifications in breast cancer are associated with a lipid-synthesis signature in brain metastasis, and targeting lipid metabolism genes such as SREBF1 can reduce metastasis.
Liu, W. et al. Glutathione peroxidase 4-dependent glutathione high-consumption drives acquired platinum chemoresistance in lung cancer-derived brain metastasis. Clin. Transl. Med. 11, e517 (2021).
Cha, Y. J., Jung, W. H. & Koo, J. S. Differential site-based expression of pentose phosphate pathway-related proteins among breast cancer metastases. Dis. Markers 2017, 7062517 (2017).
Scheid, A. D., Beadnell, T. C. & Welch, D. R. Roles of mitochondria in the hallmarks of metastasis. Br. J. Cancer 124, 124–135 (2021).
Zhang, Z. et al. MFN1-dependent alteration of mitochondrial dynamics drives hepatocellular carcinoma metastasis by glucose metabolic reprogramming. Br. J. Cancer 122, 209–220 (2020).
Porporato, P. E., Filigheddu, N., Pedro, J. M. B., Kroemer, G. & Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 28, 265–280 (2018).
Sabharwal, S. S. & Schumacker, P. T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 14, 709–721 (2014).
Ashton, T. M., McKenna, W. G., Kunz-Schughart, L. A. & Higgins, G. S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 24, 2482–2490 (2018).
Nolfi-Donegan, D., Braganza, A. & Shiva, S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 37, 101674 (2020).
Greene, J., Segaran, A. & Lord, S. Targeting OXPHOS and the electron transport chain in cancer; molecular and therapeutic implications. Semin. Cancer Biol. 86, 851–859 (2022).
Kamer, I. et al. Predicting brain metastasis in early stage non-small cell lung cancer patients by gene expression profiling. Transl. Lung Cancer Res. 9, 682–692 (2020).
Xiao, L. et al. RNA sequence profiling reveals unique immune and metabolic features of breast cancer brain metastases. Front. Oncol. 11, 679262 (2021).
Fischer, G. M. et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 9, 628–645 (2019). This study revealed that melanoma BrMs exhibit increased OXPHOS levels, contributing to resistance to MAPK inhibitors, and demonstrated that combining OXPHOS inhibitors with MAPK-targeted therapies enhances treatment efficacy and reduces BrMs in mouse models.
Fukumura, K. et al. Multi-omic molecular profiling reveals potentially targetable abnormalities shared across multiple histologies of brain metastasis. Acta Neuropathol. 141, 303–321 (2021).
Blackman, M. et al. Mitochondrial protein Cox7b is a metabolic sensor driving brain-specific metastasis of human breast cancer cells. Cancers 14, 4371 (2022).
Neman, J. et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl Acad. Sci. USA 111, 984–989 (2014).
Kleffman, K. et al. Melanoma-secreted amyloid beta suppresses neuroinflammation and promotes brain metastasis. Cancer Discov. 12, 1314–1335 (2022).
Fischer, G. M. et al. Clinical, molecular, metabolic, and immune features associated with oxidative phosphorylation in melanoma brain metastases. Neurooncol. Adv. 3, vdaa177 (2021).
Sundstrom, T. et al. Inhibition of mitochondrial respiration prevents BRAF-mutant melanoma brain metastasis. Acta Neuropathol. Commun. 7, 55 (2019).
Liu, B. & Zhang, X. Metabolic reprogramming underlying brain metastasis of breast cancer. Front. Mol. Biosci. 8, 791927 (2021).
O’Kane, R. L. & Hawkins, R. A. Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood–brain barrier. Am. J. Physiol. Endocrinol. Metab. 285, E1167–E1173 (2003).
Dolgodilina, E. et al. Brain interstitial fluid glutamine homeostasis is controlled by blood–brain barrier SLC7A5/LAT1 amino acid transporter. J. Cereb. Blood Flow. Metab. 36, 1929–1941 (2016).
Basun, H. et al. Amino acid concentrations in cerebrospinal fluid and plasma in Alzheimer’s disease and healthy control subjects. J. Neural Transm. Park. Dis. Dement. Sect. 2, 295–304 (1990).
Cutruzzola, F. et al. The emerging role of amino acids of the brain microenvironment in the process of metastasis formation. Cancers 13, 2891 (2021).
Fernstrom, J. D. Branched-chain amino acids and brain function. J. Nutr. 135, 1539S–1546S (2005).
Kalita-de Croft, P. et al. Proteomic analysis of the breast cancer brain metastasis microenvironment. Int. J. Mol. Sci. 20, 2524 (2019).
Papin-Michault, C. et al. Study of LAT1 expression in brain metastases: towards a better understanding of the results of positron emission tomography using amino acid tracers. PLoS ONE 11, e0157139 (2016).
El Ansari, R. et al. The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative breast cancer subtypes and is a key therapeutic target in luminal B tumours. Breast Cancer Res. 20, 21 (2018).
Mao, L. et al. Branch chain amino acid metabolism promotes brain metastasis of NSCLC through EMT occurrence by regulating ALKBH5 activity. Int. J. Biol. Sci. 20, 3285–3301 (2024).
Knott, S. R. V. et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378–381 (2018).
Tanaka, K. et al. Glioma cells require one-carbon metabolism to survive glutamine starvation. Acta Neuropathol. Commun. 9, 16 (2021).
Natarajan, S. K. & Venneti, S. Glutamine metabolism in brain tumors. Cancers 11, 1628 (2019).
Cheng, Y. J., Fan, F., Zhang, Z. & Zhang, H. J. Lipid metabolism in malignant tumor brain metastasis: reprogramming and therapeutic potential. Expert Opin. Ther. Targets 27, 861–878 (2023).
Fu, Y. et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2, 27–59 (2021).
Martin-Perez, M., Urdiroz-Urricelqui, U., Bigas, C. & Benitah, S. A. The role of lipids in cancer progression and metastasis. Cell Metab. 34, 1675–1699 (2022).
Vogel, F. C. E., Chaves-Filho, A. B. & Schulze, A. Lipids as mediators of cancer progression and metastasis. Nat. Cancer 5, 16–29 (2024).
Luo, X. et al. Emerging roles of lipid metabolism in cancer metastasis. Mol. Cancer 16, 76 (2017).
Ferraro, G. B. et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat. Cancer 2, 414–428 (2021). This study highlights that breast cancer brain metastases rely on fatty acid synthesis driven by FASN, and both genetic and pharmacological inhibition of FASN impairs brain metastasis growth.
Vogel, F. C. E. & Schulze, A. Fatty acid synthesis enables brain metastasis. Nat. Cancer 2, 374–376 (2021).
Savino, A. M. et al. Metabolic adaptation of acute lymphoblastic leukemia to the central nervous system microenvironment is dependent on stearoyl CoA desaturase. Nat. Cancer 1, 998–1009 (2020). This study demonstrated that central nervous system (CNS)-derived acute lymphoblastic leukaemia cells upregulate fatty acid synthesis, particularly SCD1, to enhance CNS infiltration, and targeting SCD1 through genetic or pharmacological inhibition reduces CNS metastasis.
Li, Y. Q. et al. RARRES2 regulates lipid metabolic reprogramming to mediate the development of brain metastasis in triple negative breast cancer. Mil. Med. Res. 10, 34 (2023). This study showed that reduced RARRES2 in triple-negative breast cancer enhances glycerophospholipid levels and modulates the PTEN–mTOR–SREBP1 pathway, promoting tumour adaptation to the brain.
Santana-Codina, N. et al. GRP94 is involved in the lipid phenotype of brain metastatic cells. Int. J. Mol. Sci. 20, 3883 (2019).
Wei, C. et al. LPCAT1 promotes brain metastasis of lung adenocarcinoma by up-regulating PI3K/AKT/MYC pathway. J. Exp. Clin. Cancer Res. 38, 95 (2019).
Cordero, A. et al. FABP7 is a key metabolic regulator in HER2+ breast cancer brain metastasis. Oncogene 38, 6445–6460 (2019).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014). For the first time, this study demonstrated that acetate metabolism, driven by acetyl-CoA synthetase 2 overexpression, is a critical adaptation in brain metastases from diverse primary tumours, enabling them to thrive in the brain’s unique microenvironment.
Ciraku, L. et al. O-GlcNAc transferase regulates glioblastoma acetate metabolism via regulation of CDK5-dependent ACSS2 phosphorylation. Oncogene 41, 2122–2136 (2022).
Kim, K., Marquez-Palencia, M. & Malladi, S. Metastatic latency, a veiled threat. Front. Immunol. 10, 1836 (2019).
Massague, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
Risson, E., Nobre, A. R., Maguer-Satta, V. & Aguirre-Ghiso, J. A. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. Cancer 1, 672–680 (2020).
Fares, J., Fares, M. Y., Khachfe, H. H., Salhab, H. A. & Fares, Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal. Transduct. Target. Ther. 5, 28 (2020).
Er, E. E. et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat. Cell Biol. 20, 966–978 (2018).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Sunderland, A. et al. Biglycan and reduced glycolysis are associated with breast cancer cell dormancy in the brain. Front. Oncol. 13, 1191980 (2023).
Kuo, M. H. et al. Glucose transporter 3 is essential for the survival of breast cancer cells in the brain. Cells 8, 1568 (2019).
Viale, A. et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514, 628–632 (2014).
Woldmar, N. et al. Proteomic analysis of brain metastatic lung adenocarcinoma reveals intertumoral heterogeneity and specific alterations associated with the timing of brain metastases. ESMO Open 8, 100741 (2023).
Xie, Q. et al. Mitochondrial control by DRP1 in brain tumor initiating cells. Nat. Neurosci. 18, 501–510 (2015).
Klemm, F. et al. Compensatory CSF2-driven macrophage activation promotes adaptive resistance to CSF1R inhibition in breast-to-brain metastasis. Nat. Cancer 2, 1086–1101 (2021).
Dai, J. et al. Astrocytic laminin-211 drives disseminated breast tumor cell dormancy in brain. Nat. Cancer 3, 25–42 (2022).
Kagawa, Y. et al. Role of FABP7 in tumor cell signaling. Adv. Biol. Regul. 71, 206–218 (2019).
Sun, Y. et al. Polyunsaturated fatty acid-binding protein FABP7, an attractive metabolic target for inhibition of glioblastoma stem cells. Neuro Oncol. 26, 587–589 (2024).
Aizawa, F. et al. Astrocytes release polyunsaturated fatty acids by lipopolysaccharide stimuli. Biol. Pharm. Bull. 39, 1100–1106 (2016).
Moore, S. A. Polyunsaturated fatty acid synthesis and release by brain-derived cells in vitro. J. Mol. Neurosci. 16, 195–200 (2001).
Cisternas, P. & Inestrosa, N. C. Brain glucose metabolism: role of Wnt signaling in the metabolic impairment in Alzheimer’s disease. Neurosci. Biobehav. Rev. 80, 316–328 (2017).
Blazquez, R. et al. LEF1 supports metastatic brain colonization by regulating glutathione metabolism and increasing ROS resistance in breast cancer. Int. J. Cancer 146, 3170–3183 (2020).
Contreras-Zarate, M. J. et al. Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene 38, 4685–4699 (2019).
Fong, M. Y. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015). For the first time, this study revealed that cancer cells secrete miR-122-containing vesicles to the pre-metastatic niche, in which miR-122 reduces glucose uptake by downregulating PKM, enhancing nutrient availability and promoting metastasis in triple-negative breast cancers.
Stevens, L. E. et al. Extracellular matrix receptor expression in subtypes of lung adenocarcinoma potentiates outgrowth of micrometastases. Cancer Res. 77, 1905–1917 (2017).
Ciminera, A. K., Jandial, R. & Termini, J. Metabolic advantages and vulnerabilities in brain metastases. Clin. Exp. Metastasis 34, 401–410 (2017).
Chen, Q. et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016).
Spranger, S. et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 5, 200ra116 (2013).
Herrera-Rios, D. et al. Macrophages/microglia represent the major source of indolamine 2,3-dioxygenase expression in melanoma metastases of the brain. Front. Immunol. 11, 120 (2020).
Biermann, J. et al. Dissecting the treatment-naive ecosystem of human melanoma brain metastasis. Cell 185, 2591–2608.e30 (2022). This study utilized single-nucleus RNA sequencing and spatial transcriptomics to uncover that melanoma brain metastases adopt a neuronal-like state, exhibiting unique metabolic adaptations with elevated oxidative phosphorylation and glycolysis, setting them apart from extracranial metastases.
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Nygaard, V., Prasmickaite, L., Vasiliauskaite, K., Clancy, T. & Hovig, E. Melanoma brain colonization involves the emergence of a brain-adaptive phenotype. Oncoscience 1, 82–94 (2014).
Horska, A. & Barker, P. B. Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin. N. Am. 20, 293–310 (2010).
Gambhir, S. S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2, 683–693 (2002).
Santos, L., Moreira, J. N., Abrunhosa, A. & Gomes, C. Brain metastasis: an insight into novel molecular targets for theranostic approaches. Crit. Rev. Oncol. Hematol. 198, 104377 (2024).
Xu, X. et al. Imaging brain metastasis patients with 18F-(2S,4R)-4-fluoroglutamine. Clin. Nucl. Med. 43, e392–e399 (2018).
Dunphy, M. P. S. et al. In vivo PET assay of tumor glutamine flux and metabolism: in-human trial of 18F-(2S,4R)-4-fluoroglutamine. Radiology 287, 667–675 (2018).
Ishibashi, K., Miura, Y., Wagatsuma, K., Kameyama, M. & Ishii, K. Brain 11C-ITMM PET to longitudinally assess type 1 metabotropic glutamate receptor availability in Alzheimer’s disease. J. Neuroimaging 31, 864–868 (2021).
Ishibashi, K. et al. Astrocyte-induced mGluR1 activates human lung cancer brain metastasis via glutamate-dependent stabilization of EGFR. Dev. Cell 59, 579–594.e6 (2024).
Larkin, J. R. et al. Early diagnosis of brain metastases using a biofluids-metabolomics approach in mice. Theranostics 6, 2161–2169 (2016).
Marin-Valencia, I. et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 15, 827–837 (2012).
Dong, T. et al. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci. Rep. 7, 6069 (2017).
Anami, S. et al. Serum lactate dehydrogenase predicts survival in small-cell lung cancer patients with brain metastases that were treated with whole-brain radiotherapy. J. Radiat. Res. 60, 257–263 (2019).
Wang, F. X. et al. Cerebrospinal fluid-based metabolomics to characterize different types of brain tumors. J. Neurol. 267, 984–993 (2020).
Ozer, O. et al. Liquid biopsy markers for early diagnosis of brain metastasis patients with breast cancer by metabolomics. Eur. J. Mass Spectrom. 28, 56–64 (2022).
Endo, S. et al. Synthesis of potent and selective inhibitors of aldo-keto reductase 1B10 and their efficacy against proliferation, metastasis, and cisplatin resistance of lung cancer cells. J. Med. Chem. 60, 8441–8455 (2017).
Vashisht Gopal, Y. N. et al. A novel mitochondrial inhibitor blocks MAPK pathway and overcomes MAPK inhibitor resistance in melanoma. Clin. Cancer Res. 25, 6429–6442 (2019).
Cheng, G. et al. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nat. Commun. 10, 2205 (2019).
Luttman, J. H. et al. ABL allosteric inhibitors synergize with statins to enhance apoptosis of metastatic lung cancer cells. Cell Rep. 37, 109880 (2021).
Serhan, H. A. et al. Targeting fatty acid synthase in preclinical models of TNBC brain metastases synergizes with SN-38 and impairs invasion. npj Breast Cancer 10, 43 (2024).
Esquea, E. M. et al. Selective and brain-penetrant ACSS2 inhibitors target breast cancer brain metastatic cells. Front. Pharmacol. 15, 1394685 (2024).
Kollareddy, M. et al. Regulation of nucleotide metabolism by mutant p53 contributes to its gain-of-function activities. Nat. Commun. 6, 7389 (2015).
Kieliszek, A. M. et al. De novo GTP synthesis is a metabolic vulnerability for the interception of brain metastases. Cell Rep. Med. 5, 101755 (2024).
Lassman, A. B. et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J. Neurooncol 78, 255–260 (2006).
Kim, S. K. et al. A national consensus survey for current practice in brain tumor management III: brain metastasis and primary central nervous system lymphoma. Brain Tumor Res. Treat. 8, 20–28 (2020).
Abramson, J. S. et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 116, 4283–4290 (2010).
Gross, M. I. et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 13, 890–901 (2014).
Yap, T. A. et al. Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: phase I trials. Nat. Med. 29, 115–126 (2023).
Krukowski, K. et al. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain 158, 1126–1137 (2017).
Laussel, C. & Leon, S. Cellular toxicity of the metabolic inhibitor 2-deoxyglucose and associated resistance mechanisms. Biochem. Pharmacol. 182, 114213 (2020).
Wicks, R. T. et al. Local delivery of cancer-cell glycolytic inhibitors in high-grade glioma. Neuro Oncol. 17, 70–80 (2015).
Anders, C. K. et al. Consortium for Intracranial Metastasis Academic Research (CIMARa): global interdisciplinary collaborations to improve outcomes of patient with brain metastases. Neurooncol. Adv. 7, vdaf049 (2025).
Christensen, T. D., Spindler, K. L., Palshof, J. A. & Nielsen, D. L. Systematic review: brain metastases from colorectal cancer — incidence and patient characteristics. BMC Cancer 16, 260 (2016).
Berghoff, A. S. et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open 1, e000024 (2016).
Massara, M. et al. The bacterial microbiome modulates the initiation of brain metastasis by impacting the gut-to-brain axis. iScience 28, 111874 (2025).
Ducker, G. S. et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc. Natl Acad. Sci. USA 114, 11404–11409 (2017).
Nagpal, A. et al. Neoadjuvant neratinib promotes ferroptosis and inhibits brain metastasis in a novel syngeneic model of spontaneous HER2+ve breast cancer metastasis. Breast Cancer Res. 21, 94 (2019).
Thiel, E. et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 11, 1036–1047 (2010).
Mishima, K. et al. Randomized phase III study of high-dose methotrexate and whole-brain radiotherapy with/without temozolomide for newly diagnosed primary CNS lymphoma: JCOG1114C. Neuro Oncol. 25, 687–698 (2023).
Biswas, A. K. et al. Targeting S100A9–ALDH1A1–retinoic acid signaling to suppress brain relapse in EGFR-mutant lung cancer. Cancer Discov. 12, 1002–1021 (2022). This study identified the S100A9–ALDH1A1–retinoic acid signalling axis as a key driver of BrM and osimertinib resistance in EGFR-mutant lung cancer, suggesting that targeting retinoic acid metabolism could improve treatment efficacy and prevent brain relapses.
Hebert, J. D., Neal, J. W. & Winslow, M. M. Dissecting metastasis using preclinical models and methods. Nat. Rev. Cancer 23, 391–407 (2023).
Acknowledgements
P.K.P. acknowledges support from the Anusandhan National Research Foundation (ANRF/ECRG/2024/002550/LS) and the seed grant from IISER Berhampur. S.M. acknowledges support from ACS (RSG-20-47-01-CSM), Susan G. Komen Career Catalyst Grant (CCR22902470) and NIH/NCI R01CA292390.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Jan Remsik and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
PEACE: https://clinicaltrials.gov/study/NCT0300475
RENACER: https://renacerbrainmet.org/
TRACERx: http://tracerx.co.uk/
Glossary
- [1H]NMR spectroscopy
-
A powerful analytical technique that uses proton nuclear magnetic resonance to provide detailed insights into molecular structure, distinguishing nearly all hydrogen atoms in organic compounds based on their characteristic chemical shifts.
- Adipokine
-
Cell signalling molecules primarily secreted by adipose tissue that have key roles in regulating the body’s energy and metabolic status.
- Autophagic cell death
-
A regulated form of cell death in which cells degrade their own organelles and cytoplasmic components through the lysosomal pathways.
- Blood–brain barrier
-
A highly selective, dynamic interface that regulates the exchange of molecules between the bloodstream and the central nervous system.
- Brain microenvironment
-
(BME). Complex ecosystem characterized by the presence of specified cell types (astrocytes, microglia and neurons), molecular components (such as neurotransmitters, chemokines and cytokines), metabolic dependencies (high oxygen and glucose demand) and tissue architecture.
- Disseminated tumour cells
-
Cancer cells that break off the primary tumour and travel through the lymph and blood circulation to distant organs within the body.
- Fragmented mitochondria
-
Smaller mitochondrial units resulting from increased mitochondrial fission that is often associated with metabolic reprogramming and adaptation to stress within tumour microenvironment.
- Glial cells
-
Non-neuronal cells in the central nervous system including astrocytes, microglia and oligodendrocytes provide structural and functional support to neurons and help regulate immune and metabolic responses in the brain.
- Kynurenine pathway
-
The primary route of tryptophan catabolism that generates immunosuppressive metabolites such as kynurenine and quinolinic acid, which modulate immune responses.
- Metachronous BrM
-
Refers to metastatic tumours that are diagnosed typically more than 6 months after diagnosis and treatment start of the primary tumour.
- Metastatic dormancy
-
The period during which disseminated tumour cells in distal organs remain dormant or inactive before forming detectable overt metastatic lesions.
- Metastatic niche
-
A dynamic and specialized microenvironment within a distal organ that is shaped by complex interactions among tumour cells, resident stromal cells, immune components, extracellular matrix remodelling and secreted factors and supports the survival, adaptation and potential outgrowth of disseminated tumour cells.
- Mitochondrial biogenesis
-
A process by which new mitochondria are formed within cells through the division of existing mitochondria in response to cellular energy demands and is essential to maintain mitochondrial DNA, mitochondrial mass and energy homeostasis and support cellular functions.
- One-carbon metabolism
-
A network of biochemical reactions comprising both the folate and methionine cycles allows cells to generate one-carbon units (for example, methyl groups) that are essential for biosynthesis of important anabolic precursors and for methylation reactions.
- Oxidative carboxylation
-
A metabolic reaction that occurs under oxidative conditions and requires energy (typically from NAD+ or FAD) to add CO2 to a substrate, often involving decarboxylation-coupled reactions.
- Pentose phosphate pathway
-
A glucose-oxidizing pathway that runs in parallel to glycolysis that generates NADPH and ribose-5-phosphate for anabolic reactions, including nucleotide synthesis and redox homeostasis.
- Peripheral neuropathy
-
A condition resulting from damage to peripheral nerves, often manifesting as pain, tingling or weakness, and can also be induced by chemotherapy.
- Pseudotriad synapses
-
Specialized neuronal synaptic structures involving complex interactions among the presynaptic neuron, postsynaptic neuron and cancer cells that overexpress NMDA receptors.
- Reactive astrocytes
-
Astrocytes in an activated state that is triggered by injury, inflammation or tumour invasion and characterized by morphological changes and upregulation of markers such as glial fibrillary acidic protein.
- Redox homeostasis
-
Dynamic process that maintains the balance between the production and elimination of reactive oxygen species and antioxidants within the cells that are critical to prevent cells from oxidative damage and support cellular function.
- Reductive carboxylation
-
A metabolic reaction that uses reducing equivalents (typically NADPH) to add carbon dioxide (CO2) to a molecule, often a keto acid.
- Synchronous BrM
-
Refers to metastatic tumours that are diagnosed at the same time or within a short period (3–6 months) after the primary tumour is diagnosed.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parida, P.K., Malladi, S. Metabolic adaptations of brain metastasis. Nat Rev Cancer 25, 723–739 (2025). https://doi.org/10.1038/s41568-025-00848-1
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41568-025-00848-1