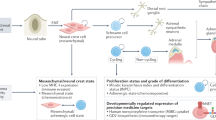
Abstract
Neurotoxicity is a common and potentially severe adverse effect from conventional and novel cancer therapy. The mechanisms that underlie clinical symptoms of central and peripheral nervous system injury remain incompletely understood. For conventional cytotoxic chemotherapy or radiotherapy, direct toxicities to brain structures and neurovascular damage may result in myelin degradation and impaired neurogenesis, which eventually translates into delayed neurodegeneration accompanied by cognitive symptoms. Chemotherapy-induced peripheral neuropathy is one of the most prevalent adverse events of chemotherapy, seen specifically with platinum- and taxane-based regimens, vinca alkaloids, thalidomide and bortezomib, and is also emerging as a concerning feature of novel targeted therapies and immunotherapies. In patients treated with molecularly targeted compounds or immune-activating agents, on-target but off-tumour effects and systemic inflammation characterize a distinct clinical profile with predominantly acute neurological symptoms with a phenotype defined by the specific antigen target. The development of mechanistically driven treatment strategies for both central and peripheral nervous system injury from cancer therapies is a major unmet medical need. Clinical trials designed to test pharmacotherapeutic interventions (including anti-dementia drugs or cognitive stimulants) for cognitive symptoms after conventional chemotherapy have produced conflicting results. In the case of acute neurotoxic adverse events from immunotherapies, reversal of T cell expansion together with drugs targeting specific pro-inflammatory interleukins have shown beneficial effects in selected patients. Large clinical trials to test novel strategies and pharmacotherapeutic interventions for acute or delayed neurotoxicity are ongoing. Informed by data derived from clinical trials and preclinical models, promising treatment strategies are on the horizon.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rashid, T. et al. Mortality from leading cancers in districts of England from 2002 to 2019: a population-based, spatiotemporal study. Lancet Oncol. 25, 86–98 (2024).
Howlader, N. et al. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 383, 640–649 (2020).
Shapiro, C. L. Cancer survivorship. N. Engl. J. Med. 379, 2438–2450 (2018).
Robison, L. L. & Hudson, M. M. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat. Rev. Cancer 14, 61–70 (2014).
Janelsins, M. C. et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J. Clin. Oncol. 35, 506–514 (2017).
Phillips, N. S. et al. Late-onset cognitive impairment and modifiable risk factors in adult childhood cancer survivors. JAMA Netw. Open 6, e2316077 (2023).
Sanchez, V. A. et al. Patient-reported functional impairment due to hearing loss and tinnitus after cisplatin-based chemotherapy. J. Clin. Oncol. 41, 2211–2226 (2023).
Shah, A. et al. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 89, 636–641 (2018).
Kandula, T. et al. Chemotherapy-induced peripheral neuropathy in long-term survivors of childhood cancer: clinical, neurophysiological, functional, and patient-reported outcomes. JAMA Neurol. 75, 980–988 (2018).
Tay, C. G. et al. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr. Blood Cancer 64, e26471 (2017).
Andries, A. et al. Polyneuropathy in adolescent childhood cancer survivors: the PACCS study. Pediatr. Neurol. 140, 9–17 (2023).
Ezendam, N. P. et al. Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: results from the population-based PROFILES registry. Gynecol. Oncol. 135, 510–517 (2014).
Bao, T. et al. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 159, 327–333 (2016).
Karschnia, P. et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood 133, 2212–2221 (2019).
Karschnia, P. et al. Neurologic toxicities following adoptive immunotherapy with BCMA-directed CAR T cells. Blood 142, 1243–1248 (2023). A clinical case series describing the presentation and management of neurotoxicities mediated by BCMA-directed CAR-T cells in patients with multiple myeloma.
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Kantarjian, H. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847 (2017).
Moreau, P. et al. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 387, 495–505 (2022).
Wefel, J. S., Saleeba, A. K., Buzdar, A. U. & Meyers, C. A. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 116, 3348–3356 (2010).
Whittaker, A. L., George, R. P. & O’Malley, L. Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: a systematic review and meta-analysis. Sci. Rep. 12, 2135 (2022).
Rapp, S. R. et al. Phase III randomized, placebo-controlled clinical trial of donepezil for treatment of cognitive impairment in breast cancer survivors after adjuvant chemotherapy (WF-97116). J. Clin. Oncol. 42, 2546–2557 (2024). A phase III clinical trial failing to show superiority of donepezil over placebo in improving memory or other cognitive functions in survivors of breast cancer.
Boone, M., Roussel, M., Chauffert, B., Le Gars, D. & Godefroy, O. Prevalence and profile of cognitive impairment in adult glioma: a sensitivity analysis. J. Neurooncol. 129, 123–130 (2016).
De Roeck, L. et al. Cognitive outcomes after multimodal treatment in adult glioma patients: a meta-analysis. Neuro Oncol. 25, 1395–1414 (2023).
Dietrich, J., Han, R., Yang, Y., Mayer-Pröschel, M. & Noble, M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 5, 22 (2006). First comprehensive preclinical study to suggest that neuroglial progenitor cells of the adult CNS and in particular oligodendroglial cells are highly vulnerable to the effects of systemically administered chemotherapeutic agents at clinically relevant dosing, including alkylating agents and anti-metabolites.
Kesler, S. R. & Blayney, D. W. Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol. 2, 185–192 (2016).
Correa, D. D., Kryza-Lacombe, M., Baser, R. E., Beal, K. & DeAngelis, L. M. Cognitive effects of donepezil therapy in patients with brain tumors: a pilot study. J. Neurooncol. 127, 313–319 (2016).
Conklin, H. M. et al. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J. Clin. Oncol. 28, 4465–4472 (2010). A prospective controlled trial showing that methylphenidate improves attention and behaviour in survivors of childhood cancer over placebo.
Gehring, K. et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J. Neurooncol. 107, 165–174 (2012).
Karschnia, P. et al. Association of MTHFR polymorphisms with leukoencephalopathy risk in patients with primary CNS lymphoma treated with methotrexate-based regimens. Neurology 101, e1741–e1746 (2023).
Mañas-Padilla, M. C. et al. Temozolomide treatment inhibits spontaneous motivation for exploring a complex object in mice: a potential role of adult hippocampal neurogenesis in “curiosity”. J. Comp. Neurol. 531, 548–560 (2023).
Egeland, M. et al. Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Transl. Psychiatry 7, e1101 (2017).
Licht, T. et al. VEGF preconditioning leads to stem cell remodeling and attenuates age-related decay of adult hippocampal neurogenesis. Proc. Natl Acad. Sci. USA 113, E7828–e7836 (2016).
Gibson, E. M. et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176, 43–55.e13 (2019). A preclinical study showing that the anti-metabolite methotrexate causes cognitive change via injury to astrocytes, microglia and oligodendroglia.
Williamson, M. R. et al. Subventricular zone cytogenesis provides trophic support for neural repair in a mouse model of stroke. Nat. Commun. 14, 6341 (2023).
Dubois, M. et al. Chemotherapy-induced long-term alteration of executive functions and hippocampal cell proliferation: role of glucose as adjuvant. Neuropharmacology 79, 234–248 (2014).
Park, H. S. et al. Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology 133, 451–461 (2018).
Wardill, H. R. et al. Irinotecan-induced gastrointestinal dysfunction and pain are mediated by common TLR4-dependent mechanisms. Mol. Cancer Ther. 15, 1376–1386 (2016).
Patai, R. et al. Persisting blood-brain barrier disruption following cisplatin treatment in a mouse model of chemotherapy-associated cognitive impairment. Geroscience 47, 3835–3847 (2025).
Ahire, C. et al. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell 22, e13832 (2023).
Demby, T., Gross, P. S., Mandelblatt, J., Huang, J. K. & Rebeck, G. W. The chemotherapeutic agent doxorubicin induces brain senescence, with modulation by APOE genotype. Exp. Neurol. 371, 114609 (2024).
van der Willik, K. D. et al. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: a cohort study. Breast Cancer Res. 20, 135 (2018).
Allen, B. D. et al. Attenuation of neuroinflammation reverses adriamycin-induced cognitive impairments. Acta Neuropathol. Commun. 7, 186 (2019).
Groves, T. R. et al. 5-Fluorouracil chemotherapy upregulates cytokines and alters hippocampal dendritic complexity in aged mice. Behav. Brain Res. 316, 215–224 (2017).
Schroyen, G. et al. Neuroinflammation and its association with cognition, neuronal markers and peripheral inflammation after chemotherapy for breast cancer. Cancers 13, 4198 (2021).
Briones, T. L. & Woods, J. Dysregulation in myelination mediated by persistent neuroinflammation: possible mechanisms in chemotherapy-related cognitive impairment. Brain Behav. Immun. 35, 23–32 (2014).
Daniel, E. et al. Cortical thinning in chemotherapy-treated older long-term breast cancer survivors. Brain Imaging Behav. 17, 66–76 (2023).
Karunamuni, R. et al. Dose-dependent cortical thinning after partial brain irradiation in high-grade glioma. Int. J. Radiat. Oncol. Biol. Phys. 94, 297–304 (2016).
Douw, L. et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 8, 810–818 (2009). A prospective cohort study in patients with low-grade gliomas showing progressive decline in cognition and attention after CNS-directed radiotherapy.
Gardner, M. M. et al. Brain volume loss after cranial irradiation: a controlled comparison study between photon vs proton radiotherapy for WHO grade 2-3 gliomas. J. Neurooncol. 171, 351–363 (2025).
Connor, M. et al. Dose-dependent white matter damage after brain radiotherapy. Radiother. Oncol. 121, 209–216 (2016).
Oyefiade, A. et al. Abnormalities of structural brain connectivity in pediatric brain tumor survivors. Neurooncol. Adv. 4, vdac064 (2022).
Kovács, Á. et al. Changes in functional MRI signals after 3D based radiotherapy of glioblastoma multiforme. J. Neurooncol. 125, 157–166 (2015).
Lee, R. X. & Tang, F. R. Radiation-induced neuropathological changes in the oligodendrocyte lineage with relevant clinical manifestations and therapeutic strategies. Int. J. Radiat. Biol. 98, 1519–1531 (2022).
Monje, M. L., Mizumatsu, S., Fike, J. R. & Palmer, T. D. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 8, 955–962 (2002). A preclinical study demonstrating that CNS-directed radiotherapy ablates hippocampal neurogenesis by altering the function of neuronal progenitor cells.
Suman, S. et al. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging 5, 607–622 (2013).
Feng, X. et al. Colony-stimulating factor 1 receptor blockade prevents fractionated whole-brain irradiation-induced memory deficits. J. Neuroinflamm. 13, 215 (2016).
Dietrich, J. et al. Bone marrow drives central nervous system regeneration after radiation injury. J. Clin. Invest. 128, 281–293 (2018). A preclinical study highlighting that bone marrow-derived and G-CSF-responsive myeloid cells are able to effectively home to the injured brain after radiotherapy and are crucial for brain repair and regeneration.
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Nakkazi, A., Forster, D., Whitfield, G. A., Dyer, D. P. & Dickie, B. R. A systematic review of normal tissue neurovascular unit damage following brain irradiation-factors affecting damage severity and timing of effects. Neurooncol. Adv. 6, vdae098 (2024).
Wick, W., Hertenstein, A. & Platten, M. Neurological sequelae of cancer immunotherapies and targeted therapies. Lancet Oncol. 17, e529–e541 (2016).
Winter, S. F., Vaios, E. J. & Dietrich, J. Central nervous system injury from novel cancer immunotherapies. Curr. Opin. Neurol. 33, 723–735 (2020).
Widakowich, C., de Castro, G. Jr., de Azambuja, E., Dinh, P. & Awada, A. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist 12, 1443–1455 (2007).
Carden, C. P., Larkin, J. M. & Rosenthal, M. A. What is the risk of intracranial bleeding during anti-VEGF therapy? Neuro Oncol. 10, 624–630 (2008).
Bennett, C. L. et al. Progressive multifocal leukoencephalopathy in patients treated with rituximab: a 20-year review from the Southern network on adverse reactions. Lancet Haematol. 8, e593–e604 (2021).
Solomon, B. J. et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 19, 1654–1667 (2018).
Salvestrini, V. et al. Safety profile of trastuzumab-emtansine (T-DM1) with concurrent radiation therapy: a systematic review and meta-analysis. Radiother. Oncol. 186, 109805 (2023).
Bartsch, R. et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat. Med. 28, 1840–1847 (2022).
Raison, C. L., Demetrashvili, M., Capuron, L. & Miller, A. H. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs 19, 105–123 (2005).
Farooq, M. Z. et al. Association of immune checkpoint inhibitors with neurologic adverse events: a systematic review and meta-analysis. JAMA Netw. Open 5, e227722 (2022).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 25, 625–638 (2019). A widely adopted consensus grading system for cytokine release syndrome and neurotoxicity associated with immune effector cell therapies.
Karschnia, P. et al. Clinicopathologic findings in fatal neurotoxicity after adoptive immunotherapy with CD19-directed CAR T-cells. Hemasphere 5, e533 (2021).
Bachy, E. et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat. Med. 28, 2145–2154 (2022). A matched comparison study between CAR-T cell products, suggesting a higher efficacy but also a higher toxicity of axi-cel compared with tisa-cel for the treatment of B cell lymphoma.
Gust, J. et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017). A translational study that shows that endothelial dysfunction and increased blood –brain barrier permeability are hallmarks of ICANS following CD19-directed CAR-T cell therapy.
Mahdi, J. et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 29, 803–810 (2023). This article proposes TIAN as a novel and specific type of neurotoxicity, which is uniquely relevant to patients with brain tumours treated with immunotherapies, and presents a grading system for its assessment.
Monje, M. et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature 637, 708–715 (2025).
Santomasso, B. D. et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 8, 958–971 (2018).
Berger, S. C. et al. Molecular monitoring of T-cell kinetics and migration in severe neurotoxicity after real-world CD19-specific chimeric antigen receptor T cell therapy. Haematologica 108, 444–456 (2023).
Stoecklein, S. et al. Functional connectivity MRI provides an imaging correlate for chimeric antigen receptor T-cell-associated neurotoxicity. Neurooncol. Adv. 5, vdad135 (2023).
Karschnia, P. et al. Neurotoxicity and management of primary and secondary central nervous system lymphoma after adoptive immunotherapy with CD19-directed chimeric antigen receptor T-cells. Neuro Oncol. 25, 2239–2249 (2023).
Cohen, A. D. et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 12, 32 (2022).
Gudera, J. A., Baehring, J. M. & Karschnia, P. Parkinsonism following chimeric antigen receptor T cell therapy. JAMA Neurol. 81, 1223–1224 (2024).
Ellithi, M. et al. Neurotoxicity and rare adverse events in BCMA-directed CAR T cell therapy: a comprehensive analysis of real-world FAERS data. Transpl. Cell Ther. 31, 71.e71–71.e14 (2025).
Percik, R., Larkin, J. & Morganstein, D. L. Endocrinopathies induced by immune checkpoint inhibitors: the need for clear endocrine diagnosis. Lancet Oncol. 22, 905–907 (2021).
Eggermont, A. M. et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 16, 522–530 (2015).
Safa, H. et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J. Immunother. Cancer 7, 319 (2019).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142.e117 (2020).
Timmins, H. C. et al. Taxane-induced peripheral neuropathy: differences in patient report and objective assessment. Support. Care Cancer 28, 4459–4466 (2020).
Chen, X. et al. Electrophysiological features of taxane-induced polyneuropathy in patients with breast cancer. J. Clin. Neurophysiol. 30, 199–203 (2013).
Cavaletti, G., Alberti, P. & Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity in cancer survivors: an underdiagnosed clinical entity? Am. Soc. Clin. Oncol. Educ. Book 35, e553-60 (2015).
Lavoie Smith, E. M. et al. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 questionnaire. Qual. Life Res. 22, 2787–2799 (2013).
Le-Rademacher, J. et al. Patient-reported (EORTC QLQ-CIPN20) versus physician-reported (CTCAE) quantification of oxaliplatin- and paclitaxel/carboplatin-induced peripheral neuropathy in NCCTG/Alliance clinical trials. Support. Care Cancer 25, 3537–3544 (2017).
Knoerl, R. et al. Measurement properties of brief neuropathy screening items in cancer patients receiving taxanes, platinums, or proteasome inhibitors. J. Patient Rep. Outcomes 5, 101 (2021).
Li, T. et al. Validity of patient-reported outcome measures in evaluating nerve damage following chemotherapy. JAMA Netw. Open 7, e2424139 (2024).
Seretny, M. et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155, 2461–2470 (2014). A systematic review and meta-analysis that estimates the prevalence of CIPN at various time points after chemotherapy and qualifies risk factors associated with its formation.
Glendenning, J. L. et al. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer 116, 2322–2331 (2010).
Molassiotis, A. et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 19, 132 (2019).
Attal, N. et al. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain 144, 245–252 (2009).
Johnson, D. B. et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J. Immunother. Cancer 7, 134 (2019).
Duong, S. L., Barbiero, F. J., Nowak, R. J. & Baehring, J. M. Neurotoxicities associated with immune checkpoint inhibitor therapy. J. Neurooncol. 152, 265–277 (2021).
Tezuka, T. et al. Dysautonomia associated with immune checkpoint inhibitors. J. Neurol. 270, 3413–3423 (2023).
Dubey, D. et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology 93, e1093–e1103 (2019).
Pan, P. C. & Haggiagi, A. Neurologic immune-related adverse events associated with immune checkpoint inhibition. Curr. Oncol. Rep. 21, 108 (2019).
Rossi, S. et al. Peripheral nervous system adverse events associated with immune checkpoint inhibitors. J. Neurol. 270, 2975–2986 (2023).
Vilaseca, A. et al. Neurological autoimmunity in melanoma patients: a comparison between those exposed and non-exposed to immune checkpoint inhibitors. J. Neurol. 271, 3279–3290 (2024).
Ruggiero, R. et al. Do peripheral neuropathies differ among immune checkpoint inhibitors? Reports from the European post-marketing surveillance database in the past 10 years. Front. Immunol. 14, 1134436 (2023).
Vogrig, A. et al. Cranial nerve disorders associated with immune checkpoint inhibitors. Neurology 96, e866–e875 (2021).
Yin, Q. et al. Immune-related adverse events of immune checkpoint inhibitors: a review. Front. Immunol. 14, 1167975 (2023).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728 (2018).
Belin, C. et al. Description of neurotoxicity in a series of patients treated with CAR T-cell therapy. Sci. Rep. 10, 18997 (2020).
Graham, C. E. et al. Non-ICANS neurological complications after CAR T-cell therapies: recommendations from the EBMT practice harmonisation and guidelines committee. Lancet Oncol. 26, e203–e213 (2025).
Chen, Y., Ren, X., Dai, Y. & Wang, Y. Pharmacovigilance study of the association between peripheral neuropathy and antibody-drug conjugates using the FDA adverse event reporting system. Sci. Rep. 14, 21386 (2024).
Zhu, Y., Liu, K., Wang, K. & Zhu, H. Treatment-related adverse events of antibody-drug conjugates in clinical trials: a systematic review and meta-analysis. Cancer 129, 283–295 (2023).
Masters, J. C., Nickens, D. J., Xuan, D., Shazer, R. L. & Amantea, M. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Invest. New Drugs 36, 121–135 (2018).
Viatchenko-Karpinski, V., Ling, J. & Gu, J. G. Down-regulation of Kv4.3 channels and a-type K+ currents in V2 trigeminal ganglion neurons of rats following oxaliplatin treatment. Mol. Pain. 14, 1744806917750995 (2018).
Fujita, S., Hirota, T., Sakiyama, R., Baba, M. & Ieiri, I. Identification of drug transporters contributing to oxaliplatin-induced peripheral neuropathy. J. Neurochem. 148, 373–385 (2019).
Xiao, W. H., Zheng, H. & Bennett, G. J. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 203, 194–206 (2012).
Matsui, T., Nakata, T., Kurohmaru, M. & Kobayashi, Y. Neurochemical characterization of mouse dorsal root ganglion neurons expressing organic cation transporter 2. Neuroreport 31, 274–280 (2020).
Liu, J. J. et al. Neuronal expression of copper transporter 1 in rat dorsal root ganglia: association with platinum neurotoxicity. Cancer Chemother. Pharmacol. 64, 847–856 (2009).
Imai, S. et al. Taxanes and platinum derivatives impair Schwann cells via distinct mechanisms. Sci. Rep. 7, 5947 (2017).
Yang, Y. et al. Nrf2 inhibits oxaliplatin-induced peripheral neuropathy via protection of mitochondrial function. Free Radic. Biol. Med. 120, 13–24 (2018).
Xu, D. et al. Participation of pro-inflammatory cytokines in neuropathic pain evoked by chemotherapeutic oxaliplatin via central GABAergic pathway. Mol. Pain. 14, 1744806918783535 (2018).
Huang, Z. Z. et al. Cerebrospinal fluid oxaliplatin contributes to the acute pain induced by systemic administration of oxaliplatin. Anesthesiology 124, 1109–1121 (2016).
Kawashiri, T. et al. L type Ca²+ channel blockers prevent oxaliplatin-induced cold hyperalgesia and TRPM8 overexpression in rats. Mol. Pain. 8, 7 (2012).
Deuis, J. R. et al. An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral. Pain 154, 1749–1757 (2013).
Pereira, A. F. et al. Neurotoxic effect of oxaliplatin: comparison with its oxalate-free analogue cis-[PtII(1R,2R-DACH)(3-acetoxy-1,1-cyclobutanedicarboxylato)] (LLC-1402) in mice. Toxicol. Appl. Pharmacol. 340, 77–84 (2018).
Persohn, E. et al. Morphological and morphometric analysis of paclitaxel and docetaxel-induced peripheral neuropathy in rats. Eur. J. Cancer 41, 1460–1466 (2005).
Kober, K. M. et al. Expression of mitochondrial dysfunction-related genes and pathways in paclitaxel-induced peripheral neuropathy in breast cancer survivors. Mol. Pain. 14, 1744806918816462 (2018).
Siau, C. & Bennett, G. J. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth. Analg. 102, 1485–1490 (2006).
Zhang, H. & Dougherty, P. M. Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology 120, 1463–1475 (2014).
Peters, C. M. et al. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp. Neurol. 203, 42–54 (2007).
Pease-Raissi, S. E. et al. Paclitaxel reduces axonal Bclw to initiate IP(3)R1-dependent axon degeneration. Neuron 96, 373–386.e376 (2017).
Topp, K. S., Tanner, K. D. & Levine, J. D. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J. Comp. Neurol. 424, 563–576 (2000).
Starobova, H. et al. Vincristine-induced peripheral neuropathy is driven by canonical NLRP3 activation and IL-1β release. J. Exp. Med. 218, e20201452 (2021).
Gomez-Deza, J., Slavutsky, A. L., Nebiyou, M. & Le Pichon, C. E. Local production of reactive oxygen species drives vincristine-induced axon degeneration. Cell Death Dis. 14, 807 (2023).
Casafont, I., Berciano, M. T. & Lafarga, M. Bortezomib induces the formation of nuclear poly(A) RNA granules enriched in Sam68 and PABPN1 in sensory ganglia neurons. Neurotox. Res. 17, 167–178 (2010).
Meregalli, C. et al. Evaluation of tubulin polymerization and chronic inhibition of proteasome as citotoxicity mechanisms in bortezomib-induced peripheral neuropathy. Cell Cycle 13, 612–621 (2014).
Carozzi, V. A. et al. Bortezomib-induced painful peripheral neuropathy: an electrophysiological, behavioral, morphological and mechanistic study in the mouse. PLoS ONE 8, e72995 (2013).
Pei, X. Y., Dai, Y. & Grant, S. The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia 17, 2036–2045 (2003).
Snavely, A. R. et al. Bortezomib-induced neurotoxicity in human neurons is the consequence of nicotinamide adenine dinucleotide depletion. Dis. Model. Mech. 15, dmm049358 (2022).
Wani, T. H. et al. The dihydroxy metabolite of the teratogen thalidomide causes oxidative DNA damage. Chem. Res. Toxicol. 30, 1622–1628 (2017).
Gerdts, J., Brace, E. J., Sasaki, Y., DiAntonio, A. & Milbrandt, J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348, 453–457 (2015).
Geisler, S. et al. Vincristine and bortezomib use distinct upstream mechanisms to activate a common SARM1-dependent axon degeneration program. JCI Insight 4, e129920 (2019).
Cetinkaya-Fisgin, A. et al. Cisplatin induced neurotoxicity is mediated by Sarm1 and calpain activation. Sci. Rep. 10, 21889 (2020).
Bosanac, T. et al. Pharmacological SARM1 inhibition protects axon structure and function in paclitaxel-induced peripheral neuropathy. Brain 144, 3226–3238 (2021). Building upon the earlier identification of SARM1 as a mediator of axonal degeneration, this work demonstrates that inhibition of SARM1 NADase may recover or protect nerve function during CIPN.
Liu, Y., Peng, L., Seto, E., Huang, S. & Qiu, Y. Modulation of histone deacetylase 6 (HDAC6) nuclear import and tubulin deacetylase activity through acetylation. J. Biol. Chem. 287, 29168–29174 (2012).
d’Ydewalle, C. et al. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat. Med. 17, 968–974 (2011).
Hubbert, C. et al. HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 (2002).
Chen, S., Owens, G. C., Makarenkova, H. & Edelman, D. B. HDAC6 regulates mitochondrial transport in hippocampal neurons. PLoS ONE 5, e10848 (2010).
Krukowski, K. et al. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain 158, 1126–1137 (2017). This study demonstrates that HDAC6 inhibitors show promise in reversing cisplatin-induced peripheral neuropathy likely through improved mitochondrial transport and bioenergetics.
Stockstill, K. et al. Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. J. Exp. Med. 215, 1301–1313 (2018).
Janes, K. et al. The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1. J. Biol. Chem. 289, 21082–21097 (2014).
Liu, D. et al. Inhibition of TRPA1 and IL-6 signal alleviates neuropathic pain following chemotherapeutic bortezomib. Physiol. Res. 68, 845–855 (2019).
Best, R. L. et al. Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): mechanistic insights into MMAE ADC peripheral neuropathy. Toxicol. Appl. Pharmacol. 421, 115534 (2021).
Ghosh, N., Chan, K. K., Jivanelli, B. & Bass, A. R. Autoantibodies in patients with immune-related adverse events from checkpoint inhibitors: a systematic literature review. J. Clin. Rheumatol. 28, e498–e505 (2022).
Leandro-García, L. J. et al. Genome-wide association study identifies ephrin type A receptors implicated in paclitaxel induced peripheral sensory neuropathy. J. Med. Genet. 50, 599–605 (2013).
Mahmoudpour, S. H. et al. Chemotherapy-induced peripheral neuropathy: evidence from genome-wide association studies and replication within multiple myeloma patients. BMC Cancer 18, 820 (2018).
Hooshmand, K. et al. Polygenic risk of paclitaxel-induced peripheral neuropathy: a genome-wide association study. J. Transl. Med. 20, 564 (2022).
Chua, K. C. et al. Genomewide meta-analysis validates a role for S1PR1 in microtubule targeting agent-induced sensory peripheral neuropathy. Clin. Pharmacol. Ther. 108, 625–634 (2020).
Dolan, M. E. et al. Clinical and genome-wide analysis of cisplatin-induced peripheral neuropathy in survivors of adult-onset cancer. Clin. Cancer Res. 23, 5757–5768 (2017).
Palugulla, S., Thakkar, D. N., Kayal, S., Narayan, S. K. & Dkhar, S. A. Association of voltage-gated sodium channel genetic polymorphisms with oxaliplatin-induced chronic peripheral neuropathy in South indian cancer patients. Asian Pac. J. Cancer Prev. 18, 3157–3165 (2017).
Harrison, R. A., Rao, V. & Kesler, S. R. The association of genetic polymorphisms with neuroconnectivity in breast cancer patients. Sci. Rep. 11, 6169 (2021).
Park, S. B. et al. Clinical and genetic predictors of paclitaxel neurotoxicity based on patient- versus clinician-reported incidence and severity of neurotoxicity in the ICON7 trial. Ann. Oncol. 28, 2733–2740 (2017).
Hershman, D. L. et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest oncology group clinical trials. J. Clin. Oncol. 34, 3014–3022 (2016). Evaluation of 1,401 patients receiving taxane-based therapy from 23 phase II or III trials demonstrating increased risk for CIPN development with paclitaxel compared with docetaxel, in participants receiving combination therapy with both taxanes and platinum agents, and in patients with increased age or a history of diabetes.
Karavasilis, V. et al. Safety and tolerability of anthracycline-containing adjuvant chemotherapy in elderly high-risk breast cancer patients. Clin. Breast Cancer 16, 291–298.e293 (2016).
Lemanska, A. et al. The association of clinical and patient factors with chemotherapy-induced peripheral neuropathy (CIPN) in colorectal cancer: secondary analysis of the SCOT trial. ESMO Open 8, 102063 (2023).
Molassiotis, A. et al. Risk factors for chemotherapy-induced peripheral neuropathy in patients receiving taxane- and platinum-based chemotherapy. Brain Behav. 9, e01312 (2019).
Sánchez-Barroso, L. et al. Concomitant medications and risk of chemotherapy-induced peripheral neuropathy. Oncologist 24, e784–e792 (2019).
Gu, J. et al. Diabetes mellitus as a risk factor for chemotherapy-induced peripheral neuropathy: a meta-analysis. Support. Care Cancer 29, 7461–7469 (2021).
Schneider, B. J. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 39, 4073–4126 (2021). Evidence-based guidelines from ASCO regarding the management of ir-nAEs including continued therapy for most grade 1 toxicity, holding treatment until recovery for most grade 2 adverse events, corticosteroid use for grade 3 toxicity and permanent discontinuation for grade 4 adverse events.
Karschnia, P., Parsons, M. W. & Dietrich, J. Pharmacologic management of cognitive impairment induced by cancer therapy. Lancet Oncol. 20, e92–e102 (2019).
Vinnakota, J. M. et al. Targeting TGFβ-activated kinase-1 activation in microglia reduces CAR T immune effector cell-associated neurotoxicity syndrome. Nat. Cancer 5, 1227–1249 (2024).
Cavaletti, G. et al. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann. Oncol. 15, 1439–1442 (2004).
De Santis, S. et al. Patients treated with antitumor drugs displaying neurological deficits are characterized by a low circulating level of nerve growth factor. Clin. Cancer Res. 6, 90–95 (2000).
Azoulay, D. et al. Bortezomib-induced peripheral neuropathy is related to altered levels of brain-derived neurotrophic factor in the peripheral blood of patients with multiple myeloma. Br. J. Haematol. 164, 454–456 (2014).
Disanto, G. et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 81, 857–870 (2017).
Gafson, A. R. et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain 143, 1975–1998 (2020).
Meda, F. J. et al. Neurofilament light oligomers in neurodegenerative diseases: quantification by homogeneous immunoassay in cerebrospinal fluid. BMJ Neurol. Open 5, e000395 (2023).
Mortensen, C. et al. Neurofilament light chain as a biomarker of axonal damage in sensory neurons and paclitaxel-induced peripheral neuropathy in patients with ovarian cancer. Pain 164, 1502–1511 (2023).
Meregalli, C. et al. Neurofilament light chain: a specific serum biomarker of axonal damage severity in rat models of Chemotherapy-induced peripheral neurotoxicity. Arch. Toxicol. 94, 2517–2522 (2020).
Huehnchen, P. et al. Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy. JCI Insight 7, e154395 (2022).
Karteri, S. et al. Prospectively assessing serum neurofilament light chain levels as a biomarker of paclitaxel-induced peripheral neurotoxicity in breast cancer patients. J. Peripher. Nerv. Syst. 27, 166–174 (2022). This prospective analysis of 59 patients with breast cancer scheduled to receive a paclitaxel-based regimen demonstrates longitudinal increases in serum NfL levels throughout treatment and with CIPN development, arguing for a potential prognostic and disease-monitoring role for NfL.
Kim, S. H. et al. Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci. Rep. 10, 7995 (2020).
Cebulla, N. et al. Neurofilament light chain levels indicate acute axonal damage under bortezomib treatment. J. Neurol. 270, 2997–3007 (2023).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT03997981 (2024).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT04633655 (2025).
Seretny, M. et al. Neuroimaging reveals a potential brain-based pre-existing mechanism that confers vulnerability to development of chronic painful chemotherapy-induced peripheral neuropathy. Br. J. Anaesth. 130, 83–93 (2023).
Lycan, T. W. et al. Neuromuscular ultrasound for taxane peripheral neuropathy in breast cancer. Muscle Nerve 61, 587–594 (2020).
Matsuoka, A. et al. Quantitative assessment of chemotherapy-induced peripheral neurotoxicity using a point-of-care nerve conduction device. Cancer Sci. 107, 1453–1457 (2016).
Timmins, H. C. et al. Electrophysiological and phenotypic profiles of taxane-induced neuropathy. Clin. Neurophysiol. 131, 1979–1985 (2020).
Krøigård, T., Metaxas, A., Wirenfeldt, M. & Finsen, B. Protective effect of ibuprofen in a rat model of chronic oxaliplatin-induced peripheral neuropathy. Exp. Brain Res. 237, 2645–2651 (2019).
Cho, K. H. et al. Comparison of clinical symptoms and neurophysiological findings in patients with chemotherapy induced peripheral neuropathy. Front. Neurol. 13, 838302 (2022).
Dalla Torre, C. et al. Lenalidomide long-term neurotoxicity: clinical and neurophysiologic prospective study. Neurology 87, 1161–1166 (2016).
Voltz, R. D., Posner, J. B., Dalmau, J. & Graus, F. Paraneoplastic encephalomyelitis: an update of the effects of the anti-Hu immune response on the nervous system and tumour. J. Neurol. Neurosurg. Psychiatry 63, 133–136 (1997).
Conklin, H. M. et al. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: a randomized, double-blind, cross-over trial. J. Pediatr. Psychol. 32, 1127–1139 (2007).
Mulhern, R. K. et al. Short-term efficacy of methylphenidate: a randomized, double-blind, placebo-controlled trial among survivors of childhood cancer. J. Clin. Oncol. 22, 4795–4803 (2004).
Mar Fan, H. G. et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support. Care Cancer 16, 577–583 (2008).
Lower, E. E. et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J. Pain. Symptom Manag. 38, 650–662 (2009).
Escalante, C. P. et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 20, 8–14 (2014).
Kohli, S. et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer 115, 2605–2616 (2009).
Shaw, E. G. et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J. Clin. Oncol. 24, 1415–1420 (2006).
Castellino, S. M. et al. Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: a pilot study. Pediatr. Blood Cancer 59, 540–547 (2012).
Rapp, S. R. et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J. Clin. Oncol. 33, 1653–1659 (2015). A placebo-controlled phase III trial showing only modest improvement in some cognitive domains following treatment with donepezil in survivors of irradiated brain tumours.
Lawrence, J. A. et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J. Cancer Surviv. 10, 176–184 (2016).
Attia, A. et al. Phase II study of Ginkgo biloba in irradiated brain tumor patients: effect on cognitive function, quality of life, and mood. J. Neurooncol. 109, 357–363 (2012).
Schagen, S. B., Tsvetkov, A. S., Compter, A. & Wefel, J. S. Cognitive adverse effects of chemotherapy and immunotherapy: are interventions within reach? Nat. Rev. Neurol. 18, 173–185 (2022).
Roth, P. et al. Neurological complications of cancer immunotherapy. Cancer Treat. Rev. 97, 102189 (2021).
Haanen, J. et al. Management of toxicities from immunotherapy: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33, 1217–1238 (2022).
Jain, M. D., Smith, M. & Shah, N. N. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood 141, 2430–2442 (2023).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Park, J. H. et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat. Med. 29, 1710–1717 (2023).
Frigault, M. J. et al. A phase II trial of anakinra for the prevention of CAR-T cell mediated neurotoxicity. Blood 138, 2814 (2021).
Bailey, S. R. et al. Blockade or deletion of IFNγ reduces macrophage activation without compromising CAR T-cell function in hematologic malignancies. Blood Cancer Discov. 3, 136–153 (2022).
Chung, M. et al. Immune checkpoint inhibitor induced anti-glutamic acid decarboxylase 65 (Anti-GAD 65) limbic encephalitis responsive to intravenous immunoglobulin and plasma exchange. J. Neurol. 267, 1023–1025 (2020).
Graham, C. E. et al. Chemotherapy-induced reversal of ciltacabtagene autoleucel-associated movement and neurocognitive toxicity. Blood 142, 1248–1252 (2023).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 (2011).
Loprinzi, C. L. et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J. Clin. Oncol. 38, 3325–3348 (2020). Evidence-based guidelines from the ASCO regarding the prevention and treatment of CIPN, highlighting the lack of established strategies for both and discussing agents with established, limited or no data to support their use.
Smith, E. M. et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 309, 1359–1367 (2013).
Bao, T. et al. Effect of acupuncture vs sham procedure on chemotherapy-induced peripheral neuropathy symptoms: a randomized clinical trial. JAMA Netw. Open 3, e200681 (2020).
Childs, D. S. et al. Randomized trial of scrambler therapy for chemotherapy-induced peripheral neuropathy: crossover analysis. J. Pain Symptom Manag. 61, 1247–1253 (2021).
Greenlee, H. et al. Randomized sham-controlled pilot trial of weekly electro-acupuncture for the prevention of taxane-induced peripheral neuropathy in women with early stage breast cancer. Breast Cancer Res. Treat. 156, 453–464 (2016).
Wen, C. H. et al. Effect of intrathecal NIS-lncRNA antisense oligonucleotides on neuropathic pain caused by nerve trauma, chemotherapy, or diabetes mellitus. Br. J. Anaesth. 130, 202–216 (2023).
Huang, H., Tay, S. H., Ng, W., Ng, S. Y. & Soong, T. W. Targeting novel human transient receptor potential ankyrin 1 splice variation with splice-switching antisense oligonucleotides. Pain 162, 2097–2109 (2021).
Marcotti, A. et al. TRPA1 modulation by sigma-1 receptor prevents oxaliplatin-induced painful peripheral neuropathy. Brain 146, 475–491 (2023).
Bruna, J. et al. Efficacy of a novel sigma-1 receptor antagonist for oxaliplatin-induced neuropathy: a randomized, double-blind, placebo-controlled phase IIa clinical trial. Neurotherapeutics 15, 178–189 (2018).
Van Helleputte, L. et al. Inhibition of histone deacetylase 6 (HDAC6) protects against vincristine-induced peripheral neuropathies and inhibits tumor growth. Neurobiol. Dis. 111, 59–69 (2018).
Gondi, V. et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J. Clin. Oncol. 32, 3810–3816 (2014).
Brown, P. D. et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J. Clin. Oncol. 38, 1019–1029 (2020).
Brown, P. D. et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 15, 1429–1437 (2013).
Robbins, M. E. et al. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int. J. Radiat. Oncol. Biol. Phys. 73, 499–505 (2009).
Kim, J. H. et al. Modification of radiation injury by ramipril, inhibitor of angiotensin-converting enzyme, on optic neuropathy in the rat. Radiat. Res. 161, 137–142 (2004).
Khasraw, M., Ashley, D., Wheeler, G. & Berk, M. Using lithium as a neuroprotective agent in patients with cancer. BMC Med. 10, 131 (2012).
Ha, J. et al. Pioglitazone use and reduced risk of dementia in patients with diabetes mellitus with a history of ischemic stroke. Neurology 100, e1799–e1811 (2023).
Barton, D. L. et al. The use of Ginkgo biloba for the prevention of chemotherapy-related cognitive dysfunction in women receiving adjuvant treatment for breast cancer, N00C9. Support. Care Cancer 21, 1185–1192 (2013).
Massa, E., Madeddu, C., Lusso, M. R., Gramignano, G. & Mantovani, G. Evaluation of the effectiveness of treatment with erythropoietin on anemia, cognitive functioning and functions studied by comprehensive geriatric assessment in elderly cancer patients with anemia related to cancer chemotherapy. Crit. Rev. Oncol. Hematol. 57, 175–182 (2006).
O’Shaughnessy, J. A. et al. Feasibility of quantifying the effects of epoetin alfa therapy on cognitive function in women with breast cancer undergoing adjuvant or neoadjuvant chemotherapy. Clin. Breast Cancer 5, 439–446 (2005).
Fan, H. G. et al. The influence of erythropoietin on cognitive function in women following chemotherapy for breast cancer. Psychooncology 18, 156–161 (2009).
Lyons, L., ElBeltagy, M., Bennett, G. & Wigmore, P. Fluoxetine counteracts the cognitive and cellular effects of 5-fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery. PLoS ONE 7, e30010 (2012).
Chang, P. K., Khatchadourian, A., McKinney, R. A. & Maysinger, D. Docosahexaenoic acid (DHA): a modulator of microglia activity and dendritic spine morphology. J. Neuroinflamm. 12, 34 (2015).
Akaike, A., Takada-Takatori, Y., Kume, T. & Izumi, Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J. Mol. Neurosci. 40, 211–216 (2010).
Myers, G. D., Verneris, M. R., Goy, A. & Maziarz, R. T. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J. Immunother. Cancer 9, e002056 (2021).
Amidi, Y. et al. Forecasting immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor t-cell therapy. J. Immunother. Cancer 10, e005459 (2022).
Rubin, D. B. et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 77, 1536–1542 (2020).
Santomasso, B. D., Gust, J. & Perna, F. How I treat unique and difficult-to-manage cases of CAR T-cell therapy-associated neurotoxicity. Blood 141, 2443–2451 (2023).
Ursu, R. et al. Long-term neurological safety in B-cell lymphoma patients treated with anti-CD19 CAR T-cell therapy. Neurology 99, 511–515 (2022).
Hershman, D. L. et al. Two-year trends of taxane-induced neuropathy in women enrolled in a randomized trial of acetyl-L-carnitine (SWOG S0715). J. Natl Cancer Inst. 110, 669–676 (2018).
Kruse, F. L., Bille, M. B., Lendorf, M. E., Vaabengaard, S. & Birk, S. Coasting related to taxane-induced peripheral neuropathy in patients with breast cancer: a systematic review. Acta Oncol. 64, 78–86 (2025).
Cao, A. et al. Effect of exercise on chemotherapy-induced peripheral neuropathy among patients treated for ovarian cancer: a secondary analysis of a randomized clinical trial. JAMA Netw. Open 6, e2326463 (2023).
Hughes, R. O. et al. Small molecule SARM1 inhibitors recapitulate the SARM1-/- phenotype and allow recovery of a metastable pool of axons fated to degenerate. Cell Rep. 34, 108588 (2021).
Bratkowski, M. et al. Uncompetitive, adduct-forming SARM1 inhibitors are neuroprotective in preclinical models of nerve injury and disease. Neuron 110, 3711–3726.e3716 (2022).
Frachet, S. et al. Renin-angiotensin-system inhibitors for the prevention of chemotherapy-induced peripheral neuropathy: OncoToxSRA, a preliminary cohort study. J. Clin. Med. 11, 2939 (2022).
Kim, E., Hwang, S. H., Kim, H. K., Abdi, S. & Kim, H. K. Losartan, an angiotensin II type 1 receptor antagonist, alleviates mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain by inhibiting inflammatory cytokines in the dorsal root ganglia. Mol. Neurobiol. 56, 7408–7419 (2019).
Khasabova, I. A. et al. Pioglitazone, a PPARγ agonist, reduces cisplatin-evoked neuropathic pain by protecting against oxidative stress. Pain 160, 688–701 (2019).
Brandolini, L. et al. DF2726A, a new IL-8 signalling inhibitor, is able to counteract chemotherapy-induced neuropathic pain. Sci. Rep. 9, 11729 (2019).
Inyang, K. E. et al. Alleviation of paclitaxel-induced mechanical hypersensitivity and hyperalgesic priming with AMPK activators in male and female mice. Neurobiol. Pain. 6, 100037 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT05790538 (2025).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT06389721 (2025).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.D. is a consultant for and on the Advisory Board of: Amgen, Novartis, Janssen. He also receives research support from Ono Therapeutics and Novartis, and royalties from Wolters Kluwer. P.K. and T.A.N. declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Guido Cavaletti, Stefanie Geisler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://www.clinicaltrials.gov
Glossary
- Acute inflammatory demyelinating polyradiculoneuropathy
-
(AIDP). Autoimmune disorder with rapid onset of weakness and sensory loss due to inflammatory damage to the myelin sheaths of peripheral nerves.
- Allodynia
-
A condition that causes pain in response to normally non-painful stimuli (cold allodynia: pain in response to normally non-painful cold temperatures). Mechanical allodynia is a specific type of allodynia prompted by mechanical stimuli such as light touch.
- Aphasia
-
Disruption of language function due to injury to the brain. Expressive aphasia specifically is the loss of the ability to produce language while comprehension generally remains intact.
- Corticospinal tracts
-
Large pathways of axons that control voluntary movement-related information from the cerebral cortex in the brain to the spinal cord (also known as pyramidal tracts).
- Dendritic spine
-
Specialized protrusion on neuron dendrites that often receives a single excitatory input.
- Dysaesthesia
-
Abnormal sense of touch.
- Dysautonomia
-
Malfunction of the autonomic nervous system.
- Dysgeusia
-
Distortion of the sense of taste.
- Dysgraphia
-
Impaired writing by hand.
- Electroencephalogram
-
(EEG). An electrophysiological study that measures brain activity.
- Ex vacuo hydrocephalus
-
Compensatory enlargement of the cerebrospinal fluid spaces caused by brain volume loss.
- Executive function
-
The ability to regulate goal-directed thoughts and behaviours.
- Fibre tracking techniques
-
MRI technique to estimate the axonal (white matter) organization of the brain.
- Guillain–Barré syndrome
-
Autoimmune polyneuropathy with numbness and muscle weakness.
- Hyperalgesia
-
Exaggerated response to normally painful stimulus.
- Hypoaesthesia
-
Decreased sensation, synonymous with ‘numbness’.
- Hypophysitis
-
Inflammation of the pituitary gland.
- Myalgias
-
Painful sensations evolving from muscle tissue.
- Myasthenia gravis
-
Autoimmune disorder characterized by antibodies that block receptors at the neuromuscular junction.
- Neurodegeneration
-
Progressive loss of structure or function of neurons.
- Neuronopathy
-
A broad term that encompasses neuron damage or degeneration.
- Neurovascular unit
-
Multicellular unit composed of neurons, glia cells, endothelial cells, perivascular cells and smooth muscle cells, with structural and functional relevance for blood flow regulation, the blood–brain barrier, metabolism and regeneration.
- Nociceptor
-
Specialized sensory nerve ending that acts as a receptor for painful or potentially noxious stimuli.
- Orthostatic hypotension
-
Drop in blood pressure when standing up from a sitting or lying position.
- Paraneoplastic ganglionopathy
-
Cancer-associated autoimmune disorder with antibodies directed against neurons in peripheral ganglia, mainly dorsal root ganglia neurons.
- Periaqueductal grey matter
-
A region of the midbrain that integrates autonomic and behavioural responses.
- Peripheral neuropathy
-
Damage to peripheral nerves involved in sensory, motor or autonomic function.
- Polyneuropathy
-
Pathology in which multiple peripheral nerves are injured.
- Processing speed
-
Ability to identify, discriminate, integrate and respond to information.
- Robin–Virchow spaces
-
Perivascular fluid-filled cavities that surround perforating vessels in the brain parenchyma.
- Scrambler therapy
-
Non-invasive pain-modifying technique that uses transcutaneous electrical stimulation of pain fibres with the intention of reorganizing maladaptive signalling pathways.
- Sensory ataxia
-
Loss of coordination caused by lack of sensory input.
- Somnolence
-
State of drowsiness with strong desire to fall asleep.
- Sural nerve
-
A cutaneous sensory nerve serving the posterior lower leg. The dorsal sural nerve in particular is the terminal branch of the sensory sural nerve serving the lateral foot and little toe.
- Wallerian degeneration
-
Distal nerve fibre degeneration due to proximal nerve injury that results in disintegration of the axon and its myelin sheath.
- White matter
-
Subcortical brain and spinal cord tissue, composed of nerve fibres, their myelin sheaths and glial cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karschnia, P., Nelson, T.A. & Dietrich, J. Mechanisms and treatment of cancer therapy-induced peripheral and central neurotoxicity. Nat Rev Cancer (2025). https://doi.org/10.1038/s41568-025-00863-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s41568-025-00863-2