Abstract
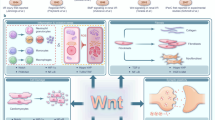
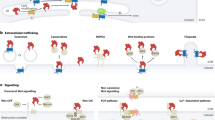
WNT signalling comprises a diverse spectrum of receptor-mediated pathways activated by a large family of WNT ligands and influencing fundamental biological processes. WNT signalling includes the β-catenin canonical pathway and the non-canonical pathways, namely the planar cell polarity and the calcium-dependent pathways. Advances over the past decade have linked non-canonical WNT signalling with key mechanisms of atherosclerosis, including oxidative stress, endothelial dysfunction, macrophage activation and vascular smooth muscle cell phenotype regulation. In addition, non-canonical WNT signalling is involved in crucial aspects of myocardial biology, from fibrosis to hypertrophy and oxidative stress. Importantly, non-canonical WNT signalling activation has complex effects in adipose tissue in the context of obesity, thereby potentially linking metabolic and vascular diseases. Tissue-specific targeting of non-canonical WNT signalling might be associated with substantial risks of off-target tumorigenesis, challenging its therapeutic potential. However, novel technologies, such as monoclonal antibodies, recombinant decoy receptors, tissue-specific gene silencing with small interfering RNAs and gene editing with CRISPR–Cas9, might enable more efficient therapeutic targeting of WNT signalling in the cardiovascular system. In this Review, we summarize the components of non-canonical WNT signalling, their links with the main mechanisms of atherosclerosis, heart failure and arrhythmias, and the rationale for targeting individual components of non-canonical WNT signalling for the treatment of cardiovascular disease.
Key points
-
Cardiovascular disease is a major cause of morbidity and mortality worldwide, prompting the need for a better understanding of the underlying pathogenic mechanisms.
-
Non-canonical WNT signalling involves an evolutionarily conserved and ubiquitous range of pathways affecting fundamental processes such as inflammation, metabolism, cell motility, oxidative stress and homeostasis.
-
Non-canonical WNT signalling is a promising target in vascular disease, influencing vascular oxidative stress, endothelial dysfunction, inflammation, vascular smooth muscle cell phenotypes and cellular insulin resistance, all of which can affect atherosclerosis progression and plaque stability.
-
Non-canonical WNT signalling has putative links to cardiac disease by influencing myocardial oxidative stress, inflammation, repair capacity, energetics and remodelling, including fibrotic or adipose infiltration of the myocardium, all of which can generate the substrate for contractile dysfunction and arrhythmogenic potential.
-
Non-canonical WNT ligands are secreted by adipose tissue and are upregulated in obesity, acting as an endocrine and paracrine link between obesity and cardiovascular complications via adipose tissue–cardiovascular system crosstalk.
-
Non-canonical WNT signalling can be therapeutically targeted at multiple levels and could involve several state-of-the-art technologies; however, more research in this area is required.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Manemann, S. M. et al. Recent trends in cardiovascular disease deaths: a state specific perspective. BMC Public Health 21, 1031 (2021).
Hansson, K. The heart of immunology: immune mechanisms in cardiovascular medicine. Cardiovasc. Res. 117, e166–e168 (2021).
Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 117, 2525–2536 (2021).
Fredman, G. & MacNamara, K. C. Atherosclerosis is a major human killer and non-resolving inflammation is a prime suspect. Cardiovasc. Res. 117, 2563–2574 (2021).
Akoumianakis, I. & Antoniades, C. The interplay between adipose tissue and the cardiovascular system: is fat always bad? Cardiovasc. Res. 113, 999–1008 (2017).
Akoumianakis, I. & Antoniades, C. Impaired vascular redox signaling in the vascular complications of obesity and diabetes mellitus. Antioxid. Redox Signal. 30, 333–353 (2019).
Wang, J. C. & Bennett, M. Aging and atherosclerosis. Circ. Res. 111, 245–259 (2012).
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 2045–2051 (2012).
Xu, L. et al. NOX1 mediates metabolic heart disease in mice and is upregulated in monocytes of humans with diastolic dysfunction. Cardiovasc. Res. https://doi.org/10.1093/cvr/cvab349 (2021).
Tsutsui, H., Kinugawa, S. & Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 301, H2181–H2190 (2011).
Travers, J. G., Kamal, F. A., Robbins, J., Yutzey, K. E. & Blaxall, B. C. Cardiac fibrosis: the fibroblast awakens. Circ. Res. 118, 1021–1040 (2016).
Kazbanov, I. V., Ten Tusscher, K. H. W. J. & Panfilov, A. V. Effects of heterogeneous diffuse fibrosis on arrhythmia dynamics and mechanism. Sci. Rep. 6, 20835 (2016).
Logan, C. Y. & Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 (2004).
Akoumianakis, I. et al. Adipose tissue–derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci. Transl Med. 11, eaav5055 (2019).
Laudes, M. Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J. Mol. Endocrinol. 46, R65–R72 (2011).
Sethi, J. K. & Vidal-puig, A. Wnt signalling and the control of cellular metabolism. Biochem. J. 427, 1–17 (2015).
Zimmerman, Z. F., Moon, R. T. & Chien, A. J. Targeting wnt pathways in disease. Cold Spring Harb. Perspect. Biol. 4, a008086 (2012).
Dijksterhuis, J. P., Petersen, J. & Schulte, G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br. J. Pharmacol. 171, 1195–1209 (2014).
Choi, E. Y. et al. Wnt5a and Wnt11 as acute respiratory distress syndrome biomarkers for severe acute respiratory syndrome coronavirus 2 patients. Eur. Respir. J. 56, 2001531 (2020).
Christman, M. A. et al. Wnt5a is expressed in murine and human atherosclerotic lesions. Am. J. Physiol. Heart Circ. Physiol. 294, H2864–H2870 (2008).
Kim, J. et al. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J. Immunol. 185, 1274–1282 (2010).
Bhatt, P. M. & Malgor, R. Wnt5a: a player in the pathogenesis of atherosclerosis and other inflammatory disorders. Atherosclerosis 237, 155–162 (2014).
Klaus, A. & Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 8, 387–398 (2008).
Wiese, K. E., Nusse, R. & van Amerongen, R. Wnt signalling: conquering complexity. Development 145, dev165902 (2018).
Foulquier, S. et al. WNT signaling in cardiac and vascular disease. Pharmacol. Rev. 70, 68–141 (2018).
Mikels, A. J. & Nusse, R. Wnts as ligands: processing, secretion and reception. Oncogene 25, 7461–7468 (2006).
Wada, N. et al. Selective modulation of Wnt ligands and their receptors in adipose tissue by chronic hyperadiponectinemia. PLoS ONE 8, e67712 (2013).
Marinou, K., Christodoulides, C., Antoniades, C. & Koutsilieris, M. Wnt signaling in cardiovascular physiology. Trends Endocrinol. Metab. 23, 628–636 (2012).
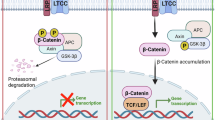
Clevers, H. & Nusse, R. Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 (2012).
Wook-Jin, C. & Bothwell, A. L. M. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 39, 830–847 (2018).
Baksh, D., Boland, G. M. & Tuan, R. S. Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J. Cell. Biochem. 101, 1109–1124 (2007).
James, R. G., Conrad, W. H. & Moon, R. T. β-Catenin-independent Wnt pathways: signals, core proteins, and effectors. Methods Mol. Biol. 468, 131–144 (2008).
Farb, M. G. et al. WNT5A-JNK regulation of vascular insulin resistance in human obesity. Vasc. Med. 21, 489–496 (2016).
Semenov, M. V., Habas, R., MacDonald, B. T. & He, X. SnapShot: noncanonical wnt signaling pathways. Cell 131, 1378 (2007).
Zhao, Y. et al. Wnt5a promotes inflammatory responses via nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. J. Biol. Chem. 289, 21028–21039 (2014).
Slusarski, D. C., Corces, V. G. & Moon, R. T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390, 410–413 (1997).
Crabtree, G. R. & Olson, E. N. NFAT signaling: choreographing the social lives of cells. Cell 109, S67–S79 (2002).
Vitezslav Bryja, E. R. A. et al. The extracellular domain of lrp5/6 inhibits noncanonical wnt signaling in vivo. Mol. Biol. Cell 20, 924–936 (2009).
van Amerongen, R., Fuerer, C., Mizutani, M. & Nusse, R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev. Biol. 369, 101–114 (2012).
Fu, H.-D. et al. Wnt5a mediated canonical Wnt signaling pathway activation in orthodontic tooth movement: possible role in the tension force-induced bone formation. J. Mol. Histol. 47, 455–466 (2016).
Mill, C. et al. Wnt5a-induced Wnt1-inducible secreted protein-1 suppresses vascular smooth muscle cell apoptosis induced by oxidative stress. Arterioscler. Thromb. Vasc. Biol. 34, 2449–2456 (2014).
Mani, A. et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315, 1278–1282 (2007).
Lu, Y. C. et al. Circulating secreted frizzled-related protein 5 (Sfrp5) and wingless-type MMTV integration site family member 5a (Wnt5a) levels in patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 29, 551–556 (2013).
Albanese, I. et al. Role of noncanonical wnt signaling pathway in human aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 37, 543–552 (2017).
Albanese, I., Khan, K., Barratt, B., Al-Kindi, H. & Schwertani, A. Atherosclerotic calcification: Wnt is the hint. J. Am. Heart Assoc. 7, e007356 (2018).
Badimon, L. & Borrell-Pages, M. Wnt signaling in the vessel wall. Curr. Opin. Hematol. 24, 230–239 (2017).
Förstermann, U. & Sessa, W. C. Nitric oxide synthases: regulation and function. Eur. Heart J. 33, 829–837 (2012).
Dubiella, U. et al. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl Acad. Sci. USA 110, 8744–8749 (2013).
Cook-Mills, J. M. et al. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem. J. 378, 539–547 (2004).
Fontayne, A., Dang, P. M.-C., Gougerot-Pocidalo, M.-A. & El Benna, J. Phosphorylation of p47phox sites by PKC α, βΙΙ, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41, 7743–7750 (2002).
Reis, M. & Liebner, S. Wnt signaling in the vasculature. Exp. Cell Res. 319, 1317–1323 (2013).
Bretón-Romero, R. et al. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler. Thromb. Vasc. Biol. 36, 561–569 (2016).
Shiraishi, H. et al. cGMP inhibits GTP cyclohydrolase I activity and biosynthesis of tetrahydrobiopterin in human umbilical vein endothelial cells. J. Pharmacol. Sci. 93, 265–271 (2003).
Takahashi, S. & Mendelsohn, M. E. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt. J. Biol. Chem. 278, 30821–30827 (2003).
Murthy, S. et al. Endothelial CaMKII as a regulator of eNOS activity and NO-mediated vasoreactivity. PLoS ONE 12, e0186311 (2017).
Gómez-Orte, E., Sáenz-Narciso, B., Moreno, S. & Cabello, J. Multiple functions of the noncanonical Wnt pathway. Trends Genet. 29, 545–553 (2013).
Masckauchán, T. N. H. et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol. Biol. Cell 17, 5163–5172 (2006).
Siman-Tov, R. et al. Circulating Wnt ligands activate the wnt signaling pathway in mature erythrocytes. Arterioscler. Thromb. Vasc. Biol. 41, E243–E264 (2021).
Hulin-Curtis, S., Williams, H., Wadey, K. S., Sala-Newby, G. B. & George, S. J. Targeting Wnt/β-catenin activated cells with dominant-negative N-cadherin to reduce neointima formation. Mol. Ther. Methods Clin. Dev. 5, 191–199 (2017).
Brown, B. A. et al. Aging differentially modulates the Wnt pro-survival signalling pathways in vascular smooth muscle cells. Aging Cell 18, e12844 (2019).
Srivastava, R. et al. Impaired LRP6-TCF7L2 activity enhances smooth muscle cell plasticity and causes coronary artery disease. Cell Rep. 13, 746–759 (2015).
Tsaousi, A., Mill, C. & George, S. J. The Wnt pathways in vascular disease. Curr. Opin. Lipidol. 22, 350–357 (2011).
San José, G. et al. Insulin-induced NADPH oxidase activation promotes proliferation and matrix metalloproteinase activation in monocytes/macrophages. Free Radic. Biol. Med. 46, 1058–1067 (2009).
Kong, Y.-Z. et al. Macrophage migration inhibitory factor induces MMP-9 expression: implications for destabilization of human atherosclerotic plaques. Atherosclerosis 178, 207–215 (2005).
Fiotti, N. et al. MMP-9 microsatellite polymorphism and susceptibility to carotid arteries atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26, 1330–1336 (2006).
Fiotti, N. et al. MMP-9 microsatellite polymorphism: association with the progression of intima-media thickening and constrictive remodeling of carotid atherosclerotic plaques. Atherosclerosis 182, 287–292 (2005).
Pandey, S. & Chandravati Wnt signaling cascade in restenosis: a potential therapeutic target of public health relevance in a North American cohort of Nebraska State. Mol. Biol. Rep. 41, 4549–4554 (2014).
Woldt, E. et al. The nuclear hormone receptor PPARγ counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat. Commun. 3, 1077 (2012).
Xin, H., Xin, F., Zhou, S. & Guan, S. The Wnt5a/Ror2 pathway is associated with determination of the differentiation fate of bone marrow mesenchymal stem cells in vascular calcification. Int. J. Mol. Med. 31, 583–588 (2013).
Cheng, S.-L. et al. Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR-/- mice by restraining noncanonical Wnt signals. Circ. Res. 117, 142–156 (2015).
Qin, L. et al. The novel role and underlying mechanism of Wnt5a in regulating cellular cholesterol accumulation. Clin. Exp. Pharmacol. Physiol. 41, 671–678 (2014).
Wang, J. et al. WNT11-conditioned medium promotes angiogenesis through the activation of non-canonical WNT-PKC-JNK signaling pathway. Genes 11, 1277 (2020).
Stefater, J. A. et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 474, 511–515 (2011).
Pashirzad, M. et al. Role of Wnt5a in the pathogenesis of inflammatory diseases. J. Cell. Physiol. 232, 1611–1616 (2017).
Tian, F., Mauro, T. M. & Li, Z. The pathological role of Wnt5a in psoriasis and psoriatic arthritis. J. Cell. Mol. Med. 23, 5876–5883 (2019).
Fiechter, R. H. et al. IL-12p40/IL-23p40 blockade with ustekinumab decreases the synovial inflammatory infiltrate through modulation of multiple signaling pathways including MAPK-ERK and Wnt. Front. Immunol. 12, 611656 (2021).
Wang, Y. et al. The role of Ca2+/NFAT in dysfunction and inflammation of human coronary endothelial cells induced by sera from patients with Kawasaki disease. Sci. Rep. 10, 4706 (2020).
Morishita, Y. et al. Wnt11 gene therapy with adeno-associated virus 9 improves recovery from myocardial infarction by modulating the inflammatory response. Sci. Rep. 6, 21705 (2016).
Chaussabel, D. et al. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102, 672–681 (2003).
Nau, G. J. et al. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl Acad. Sci. USA 99, 1503–1508 (2002).
Lehtonen, A. et al. Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J. Leukoc. Biol. 82, 710–720 (2007).
Shao, Y. et al. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget 7, 67674–67684 (2016).
Ackers, I. et al. Blocking Wnt5a signaling decreases CD36 expression and foam cell formation in atherosclerosis. Cardiovasc. Pathol. 34, 1–8 (2018).
Pereira, C., Schaer, D. J., Bachli, E. B., Kurrer, M. O. & Schoedon, G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler. Thromb. Vasc. Biol. 28, 504–510 (2008).
Feng, Y. et al. The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J. Biol. Chem. 293, 19290–19302 (2018).
Naskar, D. et al. Wnt5a–Rac1–NF-κB homeostatic circuitry sustains innate immune functions in macrophages. J. Immunol. 192, 4386–4397 (2014).
Barbero, G. et al. An autocrine Wnt5a loop promotes NF-κB pathway activation and cytokine/chemokine secretion in melanoma. Cells 8, 1060 (2019).
Zhang, C.-J. et al. Wnt5a/Ror2 pathway contributes to the regulation of cholesterol homeostasis and inflammatory response in atherosclerosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1865, 158547 (2020).
Ouchi, N. et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329, 454–457 (2010).
Fuster, J. J. et al. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes 64, 1235–1248 (2015).
Ghila, L. et al. Chronically elevated exogenous glucose elicits antipodal effects on the proteome signature of differentiating human IPSC-derived pancreatic progenitors. Int. J. Mol. Sci. 22, 3698 (2021).
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Hotamisligil, G. S. et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271, 665–668 (1996).
da Costa, R. M. et al. TNF-α induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc. Diabetol. 15, 119 (2016).
Hotamisligil, G. Mechanisms of TNF-α-induced insulin resistance. Exp. Clin. Endocrinol. Diabetes 107, 119–125 (2009).
Shoelson, S. E., Lee, J. & Yuan, M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int. J. Obes. 27, S49–S52 (2003).
Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 11, 363–371 (2015).
Oikonomou, E. K. & Antoniades, C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 16, 83–99 (2019).
Van Gaal, L. F. Mechanisms linking obesity with cardiovascular disease. Nature 444, 875–880 (2006).
Camarena, V. et al. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr. Metab. Cardiovasc. Dis. 27, 739–750 (2017).
Antonopoulos, A. S. et al. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/Adiponectin Signalling. Circ. Res. 118, 842–855 (2016).
Iacobellis, G. & Baroni, M. G. Cardiovascular risk reduction throughout GLP-1 receptor agonist and SGLT2 inhibitor modulation of epicardial fat. J. Endocrinol. Invest. 45, 489–495 (2021).
Malavazos, A. E., Goldberger, J. J. & Iacobellis, G. Does epicardial fat contribute to COVID-19 myocardial inflammation? Eur. Heart J. 41, 2333–2333 (2020).
Villasante Fricke, A. C. & Iacobellis, G. Epicardial adipose tissue: clinical biomarker of cardio-metabolic risk. Int. J. Mol. Sci. 20, 5989 (2019).
Akoumianakis, I., Tarun, A. & Antoniades, C. Perivascular adipose tissue as a regulator of vascular disease pathogenesis: identifying novel therapeutic targets. Br. J. Pharmacol. 174, 3411–3424 (2016).
Antonopoulos, A. S. et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl Med. 9, eaal2658 (2017).
Oikonomou, E. K. et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: integration in clinical care as a prognostic medical device. Cardiovasc. Res. 117, 2677–2689 (2021).
Oikonomou, E. K. et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 392, 929–939 (2018).
Kotanidis, C. P. & Antoniades, C. Perivascular fat imaging by computed tomography (CT): a virtual guide. Br. J. Pharmacol. 178, 4270–4290 (2021).
Wang, B. et al. Sfrp5/Wnt5a and leptin/adiponectin levels in the serum and the periarterial adipose tissue of patients with peripheral arterial occlusive disease. Clin. Biochem. 87, 46–51 (2021).
Catalán, V. et al. Activation of noncanonical wnt signaling through WNT5A in visceral adipose tissue of obese subjects is related to inflammation. J. Clin. Endocrinol. Metab. 99, E1407-17 (2014).
Ackers, I. & Malgor, R. Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diabetes Vasc. Dis. Res. 15, 3–13 (2018).
Fuster, J. J., Ouchi, N., Gokce, N. & Walsh, K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 118, 1786–1807 (2016).
Christodoulides, C., Lagathu, C., Sethi, J. K. & Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 20, 16–24 (2009).
Tse, G. Mechanisms of cardiac arrhythmias. J. Arrhythmia 32, 75–81 (2016).
Voorhees, A. P. & Han, H. C. Biomechanics of cardiac function. Compr. Physiol. 5, 1623–1644 (2015).
Azevedo, P. S., Polegato, B. F., Minicucci, M. F., Paiva, S. A. R. & Zornoff, L. A. M. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arquivos Bras. Cardiol. 106, 62–69 (2016).
Lopez, R. et al. Impaired myocardial energetics causes mechanical dysfunction in decompensated failing hearts. Function 1, zqaa018 (2020).
Doenst, T., Nguyen, T. D. & Abel, E. D. Cardiac metabolism in heart failure: implications beyond atp production. Circ. Res. 113, 709–724 (2013).
D’Oria, R. et al. The role of oxidative stress in cardiac disease: from physiological response to injury factor. Oxid. Med. Cell. Longev. 2020, 1–29 (2020).
Abraityte, A. et al. Wnt5a is elevated in heart failure and affects cardiac fibroblast function. J. Mol. Med. 95, 767–777 (2017).
Abraityte, A. et al. Wnt5a is associated with right ventricular dysfunction and adverse outcome in dilated cardiomyopathy. Sci. Rep. 7, 3490 (2017).
Dumotier, B. M. A straightforward guide to the basic science behind arrhythmogenesis. Heart 100, 1907–1915 (2014).
Austin, K. M. et al. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat. Rev. Cardiol. 16, 519–537 (2019).
Dawson, K., Aflaki, M. & Nattel, S. Role of the Wnt-Frizzled system in cardiac pathophysiology: a rapidly developing, poorly understood area with enormous potential. J. Physiol. 591, 1409–1432 (2013).
Lv, X. et al. Overexpression of miR-27b-3p targeting Wnt3a regulates the signaling pathway of Wnt/β-catenin and attenuates atrial fibrosis in rats with atrial fibrillation. Oxid. Med. Cell. Longev. 2019, 5703764 (2019).
Parrotta, E. I. et al. Deciphering the role of wnt and rho signaling pathway in IPSC-derived ARVC cardiomyocytes by in silico mathematical modeling. Int. J. Mol. Sci. 22, 2004 (2021).
Guo, F., Yi, X., Li, M., Fu, J. & Li, S. Snail1 is positively correlated with atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease. Exp. Ther. Med. 14, 4231–4237 (2017).
Dissanayake, S. K. et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J. Biol. Chem. 282, 17259–17271 (2007).
Beljaars, L., Daliri, S., Dijkhuizen, C., Poelstra, K. & Gosens, R. WNT-5A regulates TGF-β-related activities in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G219–G227 (2017).
Kato, T. et al. Endothelial–mesenchymal transition in human atrial fibrillation. J. Cardiol. 69, 706–711 (2017).
Hinderer, S. & Schenke-Layland, K. Cardiac fibrosis–a short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 146, 77–82 (2019).
Działo, E. et al. WNT3a and WNT5a transported by exosomes activate WNT signaling pathways in human cardiac fibroblasts. Int. J. Mol. Sci. 20, 1436 (2019).
Hagenmueller, M. et al. Dapper-1 is essential for Wnt5a induced cardiomyocyte hypertrophy by regulating the Wnt/PCP pathway. FEBS Lett. 588, 2230–2237 (2014).
Wang, Y. et al. Wnt5a-mediated neutrophil recruitment has an obligatory role in pressure overload-induced cardiac dysfunction. Circulation 140, 487–499 (2019).
Anumonwo, J. M. B. & Herron, T. Fatty infiltration of the myocardium and arrhythmogenesis: potential cellular and molecular mechanisms. Front. Physiol. 9, 2 (2018).
Lorenzon, A. et al. Wnt/β-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget 8, 60640–60655 (2017).
Abou Ziki, M. D. & Mani, A. The interplay of canonical and noncanonical Wnt signaling in metabolic syndrome. Nutr. Res. 70, 18–25 (2019).
Wang, J. et al. Vibration and β-hydroxy-β-methylbutyrate treatment suppresses intramuscular fat infiltration and adipogenic differentiation in sarcopenic mice. J. Cachexia Sarcopenia Muscle 11, 564–577 (2020).
Wang, S. et al. Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a. FASEB J. 29, 3436–3445 (2015).
Tuomainen, T. & Tavi, P. The role of cardiac energy metabolism in cardiac hypertrophy and failure. Exp. Cell Res. 360, 12–18 (2017).
Lopaschuk, G. D., Karwi, Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. Circ. Res. 128, 1487–1513 (2021).
Wang, X. et al. SGLT2 inhibitors break the vicious circle between heart failure and insulin resistance: targeting energy metabolism. Heart Fail. Rev. 27, 961–980 (2022).
Moorer, M. C. & Riddle, R. C. Regulation of osteoblast metabolism by Wnt signaling. Endocrinol. Metab. 33, 318–330 (2018).
Mo, Y. et al. The role of Wnt signaling pathway in tumor metabolic reprogramming. J. Cancer 10, 3789–3797 (2019).
Serrat, R. et al. The non-canonical Wnt/PKC pathway regulates mitochondrial dynamics through degradation of the arm-like domain-containing protein Alex3. PLoS ONE 8, e67773 (2013).
Arrázola, M. S., Silva-Alvarez, C. & Inestrosa, N. C. How the Wnt signaling pathway protects from neurodegeneration: the mitochondrial scenario. Front. Cell. Neurosci. 9, 1–13 (2015).
Li, H.-X., Lin, J., Jiang, B. & Yang, X.-J. Wnt11 preserves mitochondrial membrane potential and protects cardiomyocytes against hypoxia through paracrine signaling. J. Cell. Biochem. 121, 1144–1155 (2020).
Zhu, L. et al. Upregulation of non-canonical Wnt ligands and oxidative glucose metabolism in NASH induced by methionine-choline deficient diet. Trends Cell Mol. Biol. 13, 47–56 (2018).
Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 17, 773–789 (2020).
van der Pol, A., van Gilst, W. H., Voors, A. A. & van der Meer, P. Treating oxidative stress in heart failure: past, present and future. Eur. J. Heart Fail. 21, 425–435 (2019).
Kurian, G. A., Rajagopal, R., Vedantham, S. & Rajesh, M. The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Oxid. Med. Cell. Longev. 2016, 1656450 (2016).
Iwata, K. et al. Up-regulation of NOX1/NADPH oxidase following drug-induced myocardial injury promotes cardiac dysfunction and fibrosis. Free Radic. Biol. Med. 120, 277–288 (2018).
Scolletta, S. & Biagioli, B. Energetic myocardial metabolism and oxidative stress: let’s make them our friends in the fight against heart failure. Biomed. Pharmacother. 64, 203–207 (2010).
Chen, B. et al. Co-expression of Akt1 and Wnt11 promotes the proliferation and cardiac differentiation of mesenchymal stem cells and attenuates hypoxia/reoxygenation-induced cardiomyocyte apoptosis. Biomed. Pharmacother. 108, 508–514 (2018).
Nakamura, K. et al. Secreted frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J. Biol. Chem. 291, 2566–2575 (2016).
Matsushima, S., Tsutsui, H. & Sadoshima, J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc. Med. 24, 202–205 (2014).
Akoumianakis, I. et al. Insulin-induced vascular redox dysregulation in human atherosclerosis is ameliorated by dipeptidyl peptidase 4 inhibition. Sci. Transl Med. 12, eaav8824 (2020).
Zhang, P. CaMKII: the molecular villain that aggravates cardiovascular disease. Exp. Ther. Med. 13, 815–820 (2017).
Liu, J. & Lin, A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 15, 36–42 (2005).
Blagodatski, A., Poteryaev, D. & Katanaev, V. L. Targeting the Wnt pathways for therapies. Mol. Cell. Ther. 2, 28 (2014).
Nusse, R. & Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Naik, P., Marwah, M., Venkataraman, M., Rajawat, G. S. & Nagarsenker, M. Drug Delivery Approaches and Nanosystems. Volume 2: Drug Targeting Aspects of Nanotechnology (Apple Academic, 2017).
Zhang, F., Wen, Y. & Guo, X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum. Mol. Genet. 23, R40–R46 (2014).
Crooke, S. T. Molecular mechanisms of antisense oligonucleotides. Nucleic Acid. Ther. 27, 70–77 (2017).
Reichert, J. Monoclonal antibodies as innovative therapeutics. Curr. Pharm. Biotechnol. 9, 423–430 (2008).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Petros, R. A. & DeSimone, J. M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 9, 615–627 (2010).
Luo, Y.-L. et al. Macrophage-specific in vivo gene editing using cationic lipid-assisted polymeric nanoparticles. ACS Nano 12, 994–1005 (2018).
Schulte, G. & Bryja, V. WNT signalling: mechanisms and therapeutic opportunities. Br. J. Pharmacol. 174, 4543–4546 (2017).
Li, J. et al. Deficiency of Rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 59, 2033–2042 (2010).
Porte, B., Marguerit, G., Thomasseau, S., Paquet, C. & Hugon, J. Dose-dependent neuroprotective effect of the JNK inhibitor Brimapitide in 5xFAD transgenic mice. Brain Res. 1727, 146587 (2020).
Greenberg, S. et al. Evaluation of the JNK inhibitor, CC-90001, in a phase 1b pulmonary fibrosis trial. Eur. Respir. J. 50, OA474 (2017).
Ebenebe, O., Heather, A. & Erickson, J. The role of CaMKII in atherosclerotic plaque calcification in the ApoE-null mouse. Heart Lung Circ. 26, S65 (2017).
He, Q., Cheng, J. & Wang, Y. Chronic CaMKII inhibition reverses cardiac function and cardiac reserve in HF mice. Life Sci. 219, 122–128 (2019).
Douglas, G. et al. A key role for the novel coronary artery disease gene JCAD in atherosclerosis via shear stress mechanotransduction. Cardiovasc. Res. 116, 1863–1874 (2020).
Weber, C. & Noels, H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 17, 1410–1422 (2011).
Pirillo, A., Bonacina, F., Norata, G. D. & Catapano, A. L. The interplay of lipids, lipoproteins, and immunity in atherosclerosis. Curr. Atheroscler. Rep. 20, 12 (2018).
Silvestre-Roig, C. et al. Atherosclerotic plaque destabilization. Circ. Res. 114, 214–226 (2014).
Hansson, G. K. Inflammation, atherosclerosis and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Acknowledgements
C.A. acknowledges support from the British Heart Foundation (CH/F/21/90009, TG/19/2/34831 and RG/F/21/110040), theOxford BHF Centre of Research Excellence (RE/18/3/34214) and the Oxford NIHR Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
I.A. and M.P. researched data for the article. I.A. and C.A. contributed to the discussion of content, and I.A. wrote the manuscript. All the authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.A. is founder, shareholder and director of Caristo Diagnostics, a CT image analysis company. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akoumianakis, I., Polkinghorne, M. & Antoniades, C. Non-canonical WNT signalling in cardiovascular disease: mechanisms and therapeutic implications. Nat Rev Cardiol 19, 783–797 (2022). https://doi.org/10.1038/s41569-022-00718-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-022-00718-5
This article is cited by
-
Wnt11 mediates fibroblast–smooth muscle cell interaction to promote neurogenic bladder fibrosis in rats
Communications Biology (2026)
-
Nuclear β-catenin: molecular regulation, challenges and therapeutic opportunities
Cell Communication and Signaling (2025)
-
Engineered immune-driven theranostics for clinical cardiology
Military Medical Research (2025)
-
Fzd2 orchestrates canonical and non-canonical Wnt signaling to regulate lung development and fibrosis
Cell Communication and Signaling (2025)
-
WNT signaling in cancer: molecular mechanisms and potential therapies
Molecular Biomedicine (2025)