Abstract
Coronary CT angiography is widely implemented, with an estimated 2.2 million procedures in patients with stable chest pain every year in Europe alone. In parallel, artificial intelligence and machine learning are poised to transform coronary atherosclerotic plaque evaluation by improving reliability and speed. However, little is known about how to use coronary atherosclerosis imaging biomarkers to individualize recommendations for medical treatment. This Consensus Statement from the Quantitative Cardiovascular Imaging (QCI) Study Group outlines key recommendations derived from a three-step Delphi process that took place after the third international QCI Study Group meeting in September 2024. Experts from various fields of cardiovascular imaging agreed on the use of age-adjusted and gender-adjusted percentile curves, based on coronary plaque data from the DISCHARGE and SCOT-HEART trials. Two key issues were addressed: the need to harness the reliability and precision of artificial intelligence and machine learning tools and to tailor treatment on the basis of individualized plaque analysis. The QCI Study Group recommends that the presence of any atherosclerotic plaque should lead to a recommendation of pharmacological treatment, whereas the 70th percentile of total plaque volume warrants high-intensity treatment. The aim of these recommendations is to lay the groundwork for future trials and to unlock the potential of coronary CT angiography to improve patient outcomes globally.
Similar content being viewed by others
Introduction
Coronary CT angiography (CCTA) has emerged as a first-line imaging modality for patients with suspected coronary artery disease (CAD), with up to 2.2 million CCTA procedures conducted annually in Europe alone1,2,3,4. Performing CCTA as the first line of investigation in patients with an intermediate probability of stable CAD5,6 is supported by pivotal randomized, controlled cardiovascular outcome trials, such as the SCOT-HEART3 and DISCHARGE7,8 trials. These studies demonstrated the superiority of CCTA over standard care and invasive coronary angiography in reducing major adverse cardiovascular events (MACEs) and the non-inferiority of CCTA versus invasive coronary angiography in reducing major procedure-related complications. In the SCOT-HEART trial7, the addition of CCTA to standard care reduced the incidence of the primary outcome measure (MACE; coronary heart disease-related death and non-fatal myocardial infarction) over the initial 5-year study period as well as the extended follow-up period of up to 10 years9. These findings were primarily attributed to improved diagnostic precision and optimized preventative therapies10 and are consistent with evidence from the surgical literature linking the prognostic effect of invasive treatment with accurate risk prediction11,12. Similarly, the DISCHARGE trial8,13 showed that CCTA, as an initial diagnostic strategy in patients with stable chest pain and an intermediate pre-test probability of CAD, led to an incidence of MACE (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke; the primary outcome) that was similar to that with invasive coronary angiography (HR 0.70, 95% CI 0.46–1.07). In terms of secondary outcomes, the use of CCTA was associated with a lower incidence of major procedure-related complications (HR 0.26, 95% CI 0.13–0.55), especially in women (HR 0.14, 95% CI 0.04–0.46)14 and in patients aged <65 years (OR 0.10, 95% CI 0.02–0.36)15.
This evidence from clinical trials has led to the global adoption of CCTA and subsequently evolving reimbursement policies. Beyond the detection of obstructive CAD, CCTA enables the quantification of coronary atherosclerosis6,16 offering crucial insights into atherosclerotic plaque burden. However, the reliability of this quantification remains variable17 and is the ‘Achilles’ heel’ of successful clinical implementation of CCTA, despite the increasing clinical use of the procedure18. Advances in artificial intelligence (AI) and machine learning (ML) now enable detailed quantification and characterization of atherosclerotic plaque with increased speed and reliability19. However, challenges to the translation of these technological advances into clinical practice remain. Clear, evidence-based guidance specifying clinical treatment recommendations for each CCTA finding is needed, particularly for patients with stable chest pain and an intermediate probability of CAD, in whom the clinical value of CCTA has been proven. This expert Consensus Statement, formulated through a Delphi process, addresses these challenges and provides evidence-based recommendations for incorporating CCTA and AI tools into cardiovascular care. By linking innovations in imaging technologies with tailored therapeutic strategies, we aim to optimize outcomes and reduce the global burden of atherosclerotic CAD.
The potential of AI-supported tools
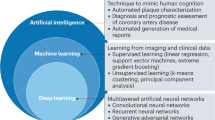
AI-supported tools, which often incorporate machine learning techniques, are poised to transform medical image analysis, offering opportunities to improve patient care and accelerate scientific discovery20,21,22. AI-supported tools to evaluate atherosclerotic plaque are reliable and fast, enabling clinicians and researchers to improve efficiency and precision23. Most automated tools and services for atherosclerotic plaque analysis first identify and label the coronary branches on CCTA24, segment the coronary lumen and outer vessel border and, finally, subclassify atherosclerotic plaque, typically based on measured attenuation values25 or advanced deep learning-supported methods for atherosclerotic plaque characterization26. Despite the technical challenges of imaging small, moving structures using CCTA, high accuracy and agreement between AI-supported tools for atherosclerotic plaque quantification and intravascular ultrasonography (IVUS) have been demonstrated27,28. To date, however, no large-scale, head-to-head comparisons between atherosclerotic plaque analysis tools and services have been performed.
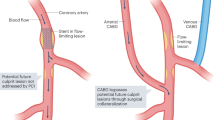
In principle, AI-supported analysis of coronary atherosclerosis on CCTA allows age-adjusted and gender-adjusted percentile curves to be generated for atherosclerosis imaging biomarkers, such as coronary total plaque volume (TPV)29. This approach offers potential for individualized risk stratification and imaging-based treatment. One particular application is to refine thresholds for the initiation of lipid-lowering and anti-atherosclerotic agents in patients with stable chest pain and suspected CAD but no class I indications for statin therapy. Human analysis of CCTA supported by AI (‘bionic radiologist’) could lead to greater reliability and more cost-effective imaging of atherosclerosis than can be achieved by radiologist evaluation alone30. In addition, identification of adverse characteristics of the atherosclerotic plaque enables individualized management strategies31. Interestingly, response to therapy can be measured by monitoring changes in the atherosclerotic plaque during follow-up (Fig. 1). This approach is being used in various large-scale, ongoing research projects, but it is not yet included in clinical practice guidelines. Advances in AI-supported tools have further implications for research. The ability to analyse large data sets of images in a fairly short timeframe facilitates pathophysiological, pharmacological and technical discoveries and could improve our understanding of treatment effects32. Non-invasive measurement of changes in coronary atherosclerosis also has the potential to expand the role of CCTA in clinical research owing to high agreement between AI-supported tools for atherosclerotic plaque quantification and IVUS33,34. The applications, limitations and reliability of AI-supported tools for atherosclerosis imaging are discussed in detail later in this Consensus Statement.
Adherence to recommended statin therapy results in stabilization of atherosclerotic plaque, characterized by an increase in calcified plaque volume and a decrease in non-calcified plaque volume, thereby reducing the risk of atherosclerotic cardiovascular disease events. a, Coronary CT angiography of a man aged 71 years, with atypical angina at baseline (left). Guideline-recommended lipid-lowering therapies resulted in stabilization of the atherosclerotic plaque in the right coronary artery (RCA) at 6-year follow-up (right). b, Coronary CT angiography of a man aged 59 years, with de novo angina at baseline (left). Dismissing recommendations for lipid-lowering treatment, despite a diagnosis of atherosclerosis, led to the progression of primarily, non-calcified atherosclerotic plaques in the left circumflex artery (LCX) at 12-year follow-up (right), consequently increasing the risk of atherosclerotic cardiovascular disease events.
Paradigm shift
Prevention and treatment of coronary atherosclerosis encompass several strategies, including ‘treat to target’ (LDL cholesterol (LDL-C) reduction) as a stand-alone method or, preferably, as part of a comprehensive risk-factor-based strategy35,36. A ‘fire-and-forget’ model, consisting of intervention and minimal follow-up, also remains prevalent in clinical practice worldwide. Although these conventional approaches to prevention and treatment have contributed to the global reduction in age-standardized mortality from CAD, their limitations are highlighted by the rising prevalence of coronary atherosclerosis and persistent regional disparities37,38.
The treat-to-target model, although effective in achieving guideline-recommended LDL-C levels, often fails to address residual cardiovascular risk39. A key limitation of this approach is that atherosclerotic plaque burden and morphology are not considered40, both of which are crucial for accurately personalizing cardiovascular risk assessment41. Atheroma burden, identified using advanced techniques for imaging the coronary artery, is not consistently correlated with LDL-C concentration and can remain undetected in the current framework of management strategies32. Similarly, risk-factor-based strategies often oversimplify the heterogeneity and vulnerability of both patients and atherosclerotic plaques, overlooking the interaction of coexisting pathophysiological mechanisms. Although theoretically comprehensive, these approaches might not identify high-risk individuals, who have minimal traditional risk factors but have coronary atherosclerosis, while overtreating others who have risk factors but no coronary atherosclerosis on CCTA42,43. Clinical risk factor estimates are known to be poor predictors of atherosclerotic burden, whereas CCTA-based quantification of atherosclerotic plaque has been shown to have superior predictive value for long-term outcomes44.
The fire-and-forget approach, which was historically proposed as a population-based management strategy and is mostly used in primary prevention, can also lead to overtreatment or undertreatment, potentially increasing adverse effects or leaving atherosclerotic plaque unaddressed45. Additionally, approaches involving fixed-dose therapy, without subsequent patient monitoring or inadequate treatment of comorbidities, fail to adapt to changing patient profiles or the dynamic natural history of atherosclerosis. To overcome these limitations, we propose a paradigm shift, emphasizing the integration of individualized treatment recommendations informed by CCTA-derived, AI-supported evaluation of atherosclerotic plaque (Fig. 2). This approach uses percentile-based risk stratification and patient-tailored treatment, combining the strengths of current strategies while mitigating their weaknesses.
Conventional treatment strategies for coronary artery disease include treat to target (adjusting therapy to achieve specific goals), risk-factor-based treatment (focusing on managing overall cardiovascular risk by addressing multiple risk factors) and fire-and-forget (prescribing fixed doses of drugs without monitoring specific targets). These strategies often overlook the complexity of pathology, ignoring the benefits of individualized therapy. Artificial intelligence (AI)-supported evaluation of atherosclerotic plaque could improve patient management by revealing the extent and nature of disease-regulating coronary atherosclerosis in each patient and by informing individualized treatment recommendations based on age-adjusted and gender-adjusted coronary total plaque volume (TPV) percentiles. The percentile curves depicted are for illustrative purposes only and do not represent real data. On the basis of the Delphi consensus process conducted after the third meeting of the Quantitative Cardiovascular Imaging Study Group, the initiation of lipid-lowering medication is recommended if the presence of any coronary atherosclerotic plaque is detected, and treatment escalation to high-intensity regimens (Table 1) is advised when the 70th percentile TPV threshold is reached on coronary CT angiography.
Method for generating consensus recommendations
Developing explicit treatment recommendations based on population percentiles of AI-supported distribution of atherosclerotic plaque volume requires experts from numerous fields. Therefore, we assembled the Quantitative Cardiovascular Imaging (QCI) Study Group of 35 experts, comprising 12 cardiologists, 11 radiologists, 5 computer scientists, 3 biomedical engineers and scientists, 2 general practitioners, 1 radiographer and 1 epidemiologist. The expert talks were held during the third QCI Study Group consensus meeting at Charité — Universitätsmedizin Berlin, Germany, on 6 September 2024. Similar to our previous consensus processes6,20,46, we used the Delphi method to generate and ask participants a set of 12 questions (Supplementary Material 1) in a total of three rounds.
The questionnaire was designed to derive expert opinion on the feasibility and best way of translating atherosclerotic plaque imaging on CCTA to medical treatment recommendations. We assessed four key points: the reliability of atherosclerotic plaque measures, the meaningfulness of atherosclerotic plaque phenotypes for treatment initiation and escalation, the translation of atherosclerotic plaque analysis to explicit medical treatment recommendations and risk modulators for the escalation of treatment recommendations. We included various types of question, including binary questions, ranking questions and quantitative questions (Supplementary Material 2). A 5-point Likert scale was primarily used for the quantitative questions, but when comparisons across more than five categories were required, a 9-point scale was used to capture finer distinctions in expert opinions.
The first Delphi round took place online 2 weeks after the third QCI Study Group consensus meeting. Participants received a personalized and anonymous link to the questionnaire via the ‘Welphi’ web application. The second and third Delphi rounds each started 1 week after the previous round. The process was designed to facilitate the exchange and convergence of expert opinions to gain a collective understanding of the subject discussed and, ideally, to reach a consensus in a streamlined and consistent manner. To achieve this goal, in the second and third rounds of questions, participants were shown their answers from the previous round in the online tool and could revise them when deemed appropriate. In addition, in the second and third rounds, anonymized interim responses given by all experts in the previous round of questions were shown as boxplots and bar plots. Related information was provided for multiple questions in the questionnaires, including current literature and both published and unpublished evidence derived from analyses of data from the SCOT-HEART and DISCHARGE trials29 (Supplementary Material 3). The consensus recommendations of the QCI Study Group are summarized in Box 1.
In many of the trials discussed in this article, participants self-reported as ‘male’, ‘female’ or ‘other’ based on a questionnaire, without further clarification on biological sex. Given that this information was collected as self-reported identity rather than a biological classification, we have chosen to use the term ‘gender’ rather than ‘sex’, for consistency with the original data.
Biomarkers and clinical parameters for individualized treatment recommendations
Coronary atherosclerotic plaque
In clinical practice, CCTA is commonly used to differentiate between calcified and non-calcified plaque (NCP) components. Each atherosclerotic plaque phenotype has different clinical indications, making them uniquely suited for defining treatment recommendations (Box 2). Available evidence suggests that atherosclerosis begins with endothelial dysfunction, leading to increased permeability of the endothelium to LDL. The subsequent inflammatory response attracts macrophages that infiltrate the intima and phagocytize oxidized LDL to form foam cells, which amplify inflammation and stimulate the proliferation of smooth muscle cells. Atherosclerotic plaque progression continues with the development of a lipid-rich necrotic core and fibrous cap. Some atherosclerotic plaques calcify, with large calcium deposits contributing to stability, whereas others remain vulnerable owing to a thin fibrous cap and ongoing inflammation47,48.
Calcified plaque
Coronary artery calcium (CAC) scoring is widely used for risk stratification, particularly for primary prevention in patients who are asymptomatic. CAC has also been shown to have prognostic relevance beyond risk-factor-based assessment in patients who are symptomatic49. The prognostic utility of CAC scoring and calcified plaque volume (CPV), assessed by CCTA, have been demonstrated in many observational studies49,50,51,52,53,54,55,56,57. In addition, the EISNER study58 demonstrated that shared decision-making using CAC scoring positively influenced adherence to lifestyle changes and medical treatment recommendations in a cohort of 2,137 patients who are asymptomatic with a low pre-test probability. Notably, the high negative predictive value of CAC scoring can rule out extensive atherosclerotic CAD, leveraged with easily performed, low-risk and low-cost imaging.
Despite its prognostic relevance, the use of CAC score or CPV to make treatment recommendations is limited by several factors. First, although several studies have demonstrated a strong association between CAC score and MACE, a direct causal relationship has not been demonstrated. Vulnerable atherosclerotic plaques prone to rupture primarily consist of inflammatory NCP32, whereas the proportion of densely calcified atherosclerotic plaque on a per-patient and per-lesion basis is inversely related to the risk of atherosclerotic CAD events59. Second, CAC score and CPV are strongly correlated with age, and the use of these measures for prognostication of atherosclerotic CAD events can overestimate risk, especially in elderly individuals. Conversely, focusing on CPV (or CAC score) as a predictor can lead to an underestimation of risk in those with low CPV and high NCP volume (NCPV), especially in younger individuals (Fig. 3) with MACE (25% of whom had a CAC score of 0 on imaging before the event in the Multi-Ethnic Study of Atherosclerosis)60,61. Third, after atherosclerotic plaque stabilization with lipid-lowering therapies, CAC score and CPV increase, whereas the risk of MACE decreases62. As a consequence of the widespread prescription of lipid-lowering medication, the correlation between CAC score or CPV and MACE is vague63. Therefore, CAC score or CPV is not the preferred marker for the longitudinal evaluation of treatment efficacy. Collectively, the data suggest that, although proven to be prognostic at the general population level, CAC score and CPV have limited suitability for determining individual treatment recommendations. Nevertheless, the number of studies with a head-to-head comparison between the use of CPV and NCPV or total plaque burden for prognostication is limited, and further randomized studies are needed.
Coronary arteries with non-calcified plaque (NCP; blue) emphasize the presence of clinically relevant atherosclerotic plaques that are undetected by coronary artery calcium (CAC) scoring alone. a, Left anterior descending artery (LAD) of a man aged 39 years, with typical angina and a CAC score of 0. b, LAD of a man aged 48 years, with atypical angina and a CAC score of 0. c, Right coronary artery (RCA) of an asymptomatic woman aged 52 years, with a CAC score of 0. The absence of calcified plaques underscores the importance of incorporating measurement of NCP volume (NCPV) into treatment recommendations, complementing traditional calcium-based assessments, to ensure comprehensive management. The NCPV is quantified for the segment shown, whereas CAC scoring is quantified over the entire cardiovascular tree.
Non-calcified plaque
NCP is an important component of coronary atherosclerotic plaque, which cannot be visualized on non-contrast CT. On CCTA, NCP can be identified and quantified as the NCPV or normalized to vessel or segment volume or area. NCP incorporates a range of attenuation values comprising various subtypes, including fibrous, fibrofatty and low-attenuation NCP. Atherosclerotic plaques in the early stages of development are usually non-calcified; they could represent metabolically active CAD, particularly in the presence of low-attenuation plaque, or could signify older, ‘burnt-out’ fibrotic disease. Owing to this pathophysiology, younger patients tend to have a greater volume of NCP than calcified atherosclerotic plaque. Over time, NCP can progress and calcify, thereby indicating response to treatment through an increase in CPV and a decrease in NCPV or remain stable. NCPV is a valuable prognostic marker, associated with an increased risk of MACEs on both visual and quantitative assessments32,64. In a retrospective analysis of the SCOT-HEART trial32 of 1,769 patients with stable chest pain, low-attenuation NCP burden was the strongest predictor of fatal or non-fatal myocardial infarction after 5 years of follow-up (adjusted HR 1.60, 95% CI 1.10–2.34 per doubling of atherosclerotic plaque burden). In a post hoc assessment of 422 patients in the RAPID-CTCA trial65, TPV (HR 25.4, 95% CI 3.44–188.0), NCPV (HR 26.4, 95% CI 3.58–196.0) and low-attenuation atherosclerotic plaque (HR 7.80, 95% CI 2.33–26.0) were the strongest predictors of future non-fatal myocardial infarction and all-cause death. In addition, NCPV can be used in the assessment of new medical therapies as a surrogate imaging end point that occurs years before MACEs66. For example, the primary outcome analysis of the EVAPORATE trial37 showed a reduction in low-attenuation NCPV after 18 months of treatment with icosapent ethyl in 68 patients with elevated triglyceride levels, and a retrospective substudy of the REPRIEVE trial33,67 showed that pitavastatin therapy led to a reduction in NCPV in 611 patients with HIV and no known cardiovascular disease (CVD).
Despite the advantages of measuring NCPV in coronary imaging, not all patients have NCP. In the Miami Heart study cohort (n = 2,459), almost 50% of participants with atherosclerotic plaques were observed to have exclusively calcified plaques, underscoring the heterogeneity of plaque composition in populations68. The role of NCPV is, therefore, unclear. The QCI Study Group reached consensus on the use of NCPV per se; however, no consensus was achieved on its specific role in treatment recommendations, whether as a risk modulator (57% of votes), as a complementary factor alongside TPV (40% of votes) or to use TPV only (3% of votes) (Supplementary Material 1). If proven to be an actionable imaging biomarker, NCPV could help to guide patient management in the future and might be the marker best suited to monitoring response to treatment.
Total plaque
CCTA effectively identifies and stratifies the future risk of myocardial infarction by providing detailed evidence of CAD. The absence of atherosclerotic disease is associated with extremely low rates of MACEs (less than one myocardial infarction per 1,000 patient-years)69. Historically, risk stratification for CAD has been focused on the presence of obstructive disease, with both the number and severity of stenoses carrying prognostic value. However, most myocardial infarctions are caused by non-obstructive disease, partly due to the higher prevalence of non-obstructive disease in the general population. Several CT-based clinical trials have demonstrated the dominance of non-obstructive disease in the occurrence of subsequent myocardial infarctions, as well as the fact that most people who have a myocardial infarction will have no evidence of ischaemia on functional stress testing70,71. These findings have led to the hypothesis that TPV is the most important metric for risk stratification. Indeed, the extent of stenotic disease is more important than simply the presence of disease and is a very powerful predictor of risk69. TPV combines CPV and NCPV and, using AI-supported analysis, is the most reliable imaging biomarker for clinical practice25,72,73. The QCI Study Group deemed TPV to be the most meaningful parameter for recommendations to initiate or escalate medical treatment (Fig. 4). Moreover, TPV can be reliably assessed using AI tools and has prognostic value for MACEs in clinical trial populations74. However, similar to CPV, TPV is not suitable for monitoring drug response, because this measure does not differentiate between changes in NCPV and CPV, which is necessary for risk assessment.
Key recommendations from the 35 experts (the Quantitative Cardiovascular Imaging Study Group) completing the three-round Delphi process are shown with regard to individualized medical treatment based on artificial intelligence (AI)-supported quantification of atherosclerotic plaque. a, Questions on the suitability and preferred measures of coronary atherosclerotic plaque for guiding treatment recommendations. b, Results of the vote on the appropriate age-adjusted and gender-adjusted percentile threshold of total plaque volume (TPV) for initiating high-intensity lipid-lowering treatment. The expert group reached consensus (82%) on escalating to high-intensity regimens when TPV is above the 70th percentile. c, Reliability and meaningfulness of AI-supported quantification of TPV, non-calcified plaque volume (NCPV) and calcified plaque volume (CPV) for medical treatment recommendations on a 5-point Likert scale (1–2 = inappropriate, 3 = uncertain, 4–5 = appropriate). d, Appropriateness of medical treatment escalation in the presence of risk factors or high-risk plaque features on a 9-point Likert scale (1–3 = inappropriate, 4–6 = uncertain, 7–9 = appropriate). No atherosclerotic plaque measure or risk modulator was deemed inappropriate. The values in panels c and d are medians. aNumerical cut-off values of atherosclerotic plaque volumes. BMI, body mass index; CAD, coronary artery disease; IVUS, intravascular ultrasonography.
High-risk plaque features
Among the various semiquantitative characteristics of atherosclerotic plaque that have been studied, four have demonstrated prognostic relevance and are collectively referred to as ‘high-risk plaque features’6,25,75: low-attenuation plaque, napkin-ring sign, positive remodelling and spotty calcification18,76. In the SCOT-HEART trial32, low-attenuation plaque burden was the strongest predictor of the future risk of myocardial infarction. Furthermore, a meta-analysis in patients with stable CAD identified the napkin-ring sign as the strongest high-risk plaque feature for predicting MACEs (HR 5.06, 95% CI 3.23–7.94)76. Several studies have shown that patients with acute coronary syndrome have a higher remodelling index than patients with stable CAD77,78,79. Although atherosclerotic plaques with extensive calcification typically remain clinically silent, spotty calcifications are associated with accelerated disease progression and culprit lesions in acute coronary syndrome80,81. Of the high-risk plaque features defined on CCTA, low-attenuation plaque and positive remodelling have emerged as the most important features for the assessment of cardiovascular risk82.
CT attenuation measurements in atherosclerotic plaques are influenced by factors such as intraluminal contrast concentration, tube voltage, slice thickness and reconstruction filters83,84,85,86. Moreover, high-risk plaque features are largely reported as visual measures in daily practice87,88. Differences in image quality, reader experience and software can affect the consistency of atherosclerotic plaque characterization89. This situation results in moderate interobserver agreement for the identification of high-risk plaque features, with κ values ranging from 0.56 to 0.69 in research settings90,91. In addition, different virtual monoenergetic images from photon-counting CT alter attenuation values and, therefore, corresponding atherosclerotic plaque characteristics, further contributing to reduced reproducibility92.
Although high-risk plaque features show strong overall predictive performance, they are not well suited to determining treatment recommendations, for three reasons. First, their positive predictive value is low and most patients with such features will not have events. Second, high-risk plaque features are not present in all patients with atherosclerotic plaque and, therefore, patients without such features who might benefit from treatment would be missed. Third, the reliability of assessing high-risk plaque features is limited. However, high-risk plaque features could inform the adjustment of existing treatment, such as escalating dose intensity. Further investigation and improvements are needed to improve reproducibility and interobserver agreement in assessing high-risk plaque features93.
Additional risk modulators
Cardiovascular risk factors, such as smoking, obesity and a family history of CVD, have been well defined for many years and can be directly linked to an increase in the rate of CVD progression. Shifting the focus of treatment from LDL-C to plaque-based recommendations does not diminish the importance of risk factors. Their integration as factors that modulate treatment intensity is crucial to the individualization of patient management.
Non-modifiable and modifiable risk factors
Assessment of cardiovascular risk using validated scoring systems, such as the Systematic Coronary Risk Evaluation (SCORE), SCORE2 and atherosclerotic CVD (ASCVD) 2013 Risk Calculator, is a fundamental component of the clinical decision trees recommended by major international guidelines to inform the use of preventative therapies94,95. In general, the higher the risk, the greater the benefit from risk factor modification, including pharmacological interventions. Although some cardiovascular risk factors, such as age and genetic predisposition, are non-modifiable, modifying dyslipidaemia, hypertension, diabetes mellitus and smoking can drastically improve long-term prognosis. In the primary analysis of the prospective SPRINT trial91 involving 9,361 patients with hypertension but no diabetes, intensive blood pressure lowering to <120 mmHg was associated with a reduced risk of MACEs (primary myocardial infarction, other acute coronary syndrome, stroke, heart failure or death from cardiovascular causes) compared with standard therapy (HR 0.73, 95% CI 0.60–0.90). Similarly, the primary analysis of the ADVANCE trial96 showed that intensive glycaemic control reduced MACEs (cardiovascular causes, non-fatal myocardial infarction or non-fatal stroke) compared with standard control (HR 0.86, 95% CI 0.77–0.97) in 11,140 patients with type 2 diabetes.
However, some clinical risk factors are not well captured by conventional risk algorithms, including obesity, physical inactivity, metabolic syndrome, chronic kidney disease, immune-mediated inflammatory diseases, psychosocial stress, psychiatric disorders, social deprivation and frailty97,98,99. Moreover, a high proportion of asymptomatic individuals undergoing CCTA in population-based screening studies, such as the MIAMI Heart Study64 and SCAPIS96 cohorts, had subclinical atherosclerosis, despite seemingly low 10-year event risk calculations. In addition, the optimal treatment of patients with a borderline indication for statins is often unclear, and most individuals who present with acute myocardial infarction do not have a history of angina or other symptoms that could herald their underlying condition. Therefore, a need exists for more refined risk stratification than can be achieved through the assessment of clinical risk factors alone. In the SCOT-HEART trial100, the use of CCTA led to greater diagnostic certainty and risk stratification, with increased use of procedural and pharmacological interventions, compared with standard care despite similar 10-year cardiovascular risk scores between groups. Crucially, this finding was independent of the presentation of symptoms, with the greatest benefit seen in patients without angina owing to coronary heart disease100. These data suggest that CCTA could be a powerful clinical adjunct for assessing total cardiovascular risk in asymptomatic individuals with risk factors. This hypothesis is the subject of the ongoing, randomized SCOT-HEART 2 study101, in which the investigators will assess whether CCTA-guided care leads to improvements in management and outcomes in patients with stable angina and suspected CAD after a 10-year follow-up period.
Surrounding adipose tissue
CCTA-derived metrics of fat surrounding the coronary arteries and heart represent promising markers of inflammation, a key mechanism in atherosclerosis102. On non-contrast CT scans, standardized at 120 kVp for calcium scoring, increased volume of epicardial adipose tissue is linked to an increased risk of cardiovascular events, offering predictive value beyond the CAC score103. Although previously constrained by lengthy segmentation processes, advances in AI-supported software now enable automated evaluation of epicardial adipose tissue104. Moreover, on CCTA, pericoronary adipose tissue (PCAT) attenuation can be quantified105. PCAT is currently incorporated into proprietary measurements and risk scores, which may add prognostic value106. In the ORFAN study106 cohort of 40,091 patients with a clinical indication for CCTA, the PCAT attenuation score was independently associated with cardiovascular mortality (HR 29.8, 95% CI 13.9–63.9) and MACEs (myocardial infarction, new heart failure and cardiac death; HR 12.6, 95% CI 8.5–18.6) irrespective of CAD severity, although this PCAT score contributed only marginally to the area under the curve. Moreover, the difference in PCAT attenuation values between healthy and stenosed arteries is subtle105, and PCAT attenuation measurements are affected by tube voltage, image reconstruction kernel and iterative reconstruction techniques, which can result in attenuation differences far greater than those between healthy and diseased vessels107,108. Further validation of epicardial adipose tissue and PCAT for clinical decision-making in a prospective, randomized, controlled, cardiovascular outcomes trial is required to determine the clinical value for individualized management102.
AI in atherosclerosis imaging
Applications and limitations
AI and ML are transforming the landscape of atherosclerotic cardiovascular risk assessment: first, through the direct application of deep learning algorithms to image data for automated quantification of imaging biomarkers, such as atherosclerotic plaque24, and second, by combining clinical and AI-supported imaging metrics for individualized outcome prediction31. Applications for deep learning models in cardiac CT are diverse and include CAC quantification on non-contrast CT109,110,111, quantification of CAD Reporting and Data System score, identification of atherosclerotic plaque type and atherosclerotic plaque quantification on CCTA (Fig. 5). Fully automated, deep learning methods for atherosclerotic plaque and vessel lumen segmentation promise to provide accelerated quantification, saving the reader time19.
Each panel shows a multiplanar reformation of the right coronary artery along three axial cross-sections with overlayed segmentation of calcified plaque, non-calcified plaque and low-attenuation plaque. The positions of the cross-sections are denoted by white arrows. Quantification of atherosclerotic coronary plaque modestly improved event prediction compared with atherosclerotic cardiovascular disease risk score alone53. Analysis was performed using eight different commercially available systems approved by the FDA25. a, Reference. b, AutoPlaque version 3.0 (Cedars–Sinai Medical Center, USA). c, syngo.via Frontier Coronary Analysis Prototype version 1.0.3 (Siemens Healthineers). d, Vitrea Sure Plaque Analysis version 7.16 (Canon Medical Systems). e, PlaqueIQ (Elucid Bioimaging). f, Artrya Salix Coronary Plaque (RUO) version 1.0 (Artrya). g, Aquarius iNtuition version 4.10 (TeraRecon). h, Cleerly LABS version 2.0 (Cleerly). i, QAngio CT Research Edition version 3.2.14.1 (Medis Medical Imaging). Heartflow was unable to provide the required analysis for inclusion in the publication. The orientation in part a was supplied as a visual reference to all vendors.
In a retrospective analysis of 1,611 patients from the SCOT-HEART trial, a deep learning-supported method for atherosclerotic plaque quantification on CCTA was shown to be concordant with IVUS and also predictive of myocardial infraction19. The deep learning-based TPV threshold was associated with an increased risk of myocardial infarction (HR 5.36, 95% CI 1.70–16.86) after adjustment for the presence of deep learning-based obstructive stenosis (HR 2.49, 95% CI 1.07–5.50) and the ASSIGN clinical risk score (HR 1.01, 95% CI 0.99–1.04)15. In the ISCHEMIA trial72, CCTA data were available from 3,711 participants with myocardial ischaemia. AI-based TPV was associated with cardiovascular death or myocardial infarction (HR 1.56, 95% CI 1.25–1.97 per interquartile range increase (559 mm3)), with atherosclerotic plaque volume and composition metrics modestly improving event prediction compared with ASCVD risk score alone72. Owing to the inclusion of patients who had previously undergone percutaneous coronary intervention and the limited image quality in this study, further randomized controlled trials are needed to support these findings.
The main challenge associated with AI tools is the limited availability of the large, well-curated and diverse data sets required for training and generalizability112. A wide range of scanners and scan protocols should be included in training data to improve performance. As with other diagnostic approaches, deep learning-supported methods must be evaluated before integration with the corresponding invasive reference standards, such as IVUS for atherosclerotic plaque quantification, and also externally with fully independent data25. High image quality (and the absence of artefacts) is central to quantitative assessment of atherosclerotic plaque and, together with vessel size, determines whether quantitative analysis of atherosclerotic plaque should be performed for that vessel segment25.
Reliability
AI-supported CCTA quantification of atherosclerotic plaque has been compared with assessment by human readers and using IVUS. The reliability of AI tools between scans performed at short intervals was also evaluated. Agreement between AI tools and expert human readers is excellent (intraclass correlation coefficient (ICC) 0.96)19. However, wide limits of agreement between humans and AI (–114 mm3 to 126 mm3) have been reported, although this range is narrower than between two human readers (–157 mm3 to 203 mm3)19. Agreement with experts is best for TPV (ICC 0.96) and worst for low-density NCP (ICC 0.81). AI-supported quantification of atherosclerotic plaque also shows excellent agreement with IVUS for TPV (r = 0.91), NCPV (r = 0.87) and CPV (r = 0.91), but poor agreement for low-density NCP (r = 0.28)27. Interscan reliability is also good and, again, better for TPV, NCPV and CPV (r = 0.93–0.98) than for low-density NCP (r = 0.74)65,113.
The reliability of AI tools is not without limitations. Although population-level agreement is good, limits of agreement between scan and rescan are wide (±50% for TPV, NCPV and CPV and ±100% for low-density NCP55,96). These findings mean that, for individual patient follow-up, it is not possible to determine whether shifts in atherosclerotic plaque volume of up to 50% represent response to therapy, disease progression or simply interscan variability. This effect is further compounded by intersoftware variability. An evaluation of various software packages on a single vessel demonstrated that the TPV (normalized to vessel volume) ranged from 58% to 88% across software vendors, NCPV ranged from 75% to 99% and the proportion of low-density NCP varied from 0.3% to 35% of the TPV25. This variability has important clinical implications for patients whose scores fall near the thresholds of risk categories and who could be reclassified solely on the basis of the software used for their scans on different days.
Individualized recommendations for medical treatment
Metrics
Population-based CCTA studies, such as the Multi-Ethnic Study of Atherosclerosis66 and the subsequent Miami Heart Study68,114, have greatly advanced our knowledge of atherosclerotic plaque prevalence and its association with cardiovascular events. The 2018 multisociety clinical guideline on the management of blood cholesterol recommends using CAC scores to guide decisions on statin therapy for adults aged 40–75 years without diabetes, LDL-C concentration of 70–189 mg/dl (1.81–4.89 mmol/l) and a 10-year ASCVD risk of 7.5–20% when the need for statins is unclear36. If the CAC score is 0, statin therapy can generally be delayed, except for patients who smoke or those with diabetes or a strong family history of premature heart disease36.
CCTA is a first-line test in patients with chest pain and can visualize both the coronary artery lumen and atherosclerotic plaque (calcified and non-calcified), including the high-risk, low-attenuation component of NCP. In the SCOT-HEART trial7, women presenting with stable chest pain had lower atherosclerotic plaque volumes of all subtypes than men, although quantitative low-attenuation plaque burden was as strong a predictor of subsequent myocardial infarction in both women and men115. As a consequence, fixed cut-off values for atherosclerotic plaque volumes have been proposed for risk stratification73,116,117,118. However, atherosclerotic plaque distribution is known to vary according to age and gender and, therefore, requires a wide range of cut-off values. Age-specific and gender-specific percentile thresholds have been proposed in two separate studies with different AI software applications for atherosclerotic plaque analysis29,119. In a 2024 study29, per-patient age-specific and gender-specific atherosclerotic plaque distributions were shown to be strongly predictive of myocardial infarction, with the highest risk seen in patients with coronary plaque volumes above the 75th percentile (HR 2.65, 95% CI 1.47–4.78). Therefore, percentiles adjusted for age and gender could provide context to better interpret risk assessment from atherosclerotic plaque imaging and allow practical, individualized clinical treatment recommendations. In contrast to large deviations in proposed cut-off values73,116,117, two multicentre studies on different patient cohorts showed similarities between the age-adjusted and gender-adjusted percentile curves29,119 (Fig. 6).
A treatment paradigm based on population distribution percentiles was deemed by the QCI Study Group to be the most suitable approach for medical treatment recommendations, with the 70th percentile being the threshold for recommending high-intensity treatment and the presence of any atherosclerotic plaque for standard-dose medical treatment (Fig. 4). Although the percentile distributions from both studies follow similar trajectories, very divergent patient groups (in terms of ethnicities or risk profiles) could have greater differences in atherosclerotic plaque distributions. Further research is needed on whether treatment recommendations derived from average atherosclerotic plaque distribution can be effectively translated from one end of the spectrum of risk to the other.
Medical therapies and dose intensity
The aim of pharmacological management of chronic coronary syndrome is to prevent MACEs and to alleviate ischaemic symptoms. Central to this management strategy is the optimization of cardiovascular risk factors. Lifestyle interventions, such as diet modification and exercise, serve as basic measures, but are often insufficient to achieve the stringent LDL-C targets recommended for high-risk patients with chronic coronary syndrome. Effective blood pressure control in the general population and precise glucose level management in patients with diabetes are essential to reduce vascular complications and associated risk. The antihypertensive agents olmesartan and amlodipine have been shown to have beneficial effects on atherosclerotic plaque progression120,121. Medications indicated for diabetes, such as sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists, are also thought to have beneficial effects on CAD progression and could have a role in future treatment strategies122.
Statins are the cornerstone of pharmacological lipid-lowering strategies, with a central role in reducing atherogenic burden and the occurrence of MACEs through LDL-C reduction, and secondary benefits such as atherosclerotic plaque stabilization and modulation of inflammation. The 2023 American123 and 2024 European124 guidelines emphasize the importance of LDL-C lowering and advocate for precise targets based on individual risk profiles. Both guidelines reinforce the treat-to-target paradigm, specifying both percentage-based LDL-C reductions and absolute thresholds for patients with chronic coronary syndrome. Meta-analyses of data from patients with stable CAD or acute coronary syndrome, in trials comparing statins versus no statin or low-dose versus high-dose statin theapy125,126, have revealed that standard-intensity statin therapy effectively reduces MACEs (coronary death, myocardial infarction, coronary revascularization and ischaemic stroke), but is inferior to high-intensity statin therapy125. The recommendations from the QCI Study Group for standard-intensity and high-intensity statin regimens are summarized in Table 1. These recommendations on statin doses should be supplemented with adequate guideline-adherent lifestyle changes, antihypertensive therapy and antiplatelet therapy, if indicated.
Although statins are the first-line lipid-lowering therapy, other medications, such as cholesterol-absorption inhibitors (ezetimibe) and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, are potential adjunctive treatments for patients who are at very high risk or unable to achieve the required LDL-C goals, despite the maximum-tolerated statin dose. PCSK9 inhibitors, such as alirocumab and evolocumab, markedly lower LDL-C levels by up to 60% when used in combination with statins127,128,129,130. Other lipid-lowering agents available or currently under investigation, such as bempedoic acid, monoclonal antibodies (canakinumab)124 and small interfering RNA-based agents (inclisiran), hold promise for the future individualization of CAD management, although data are not yet available from CCTA-based clinical studies evaluating their effects on atherosclerotic plaque.
Percentile-based treatment recommendations
The presence of atherosclerotic plaque, even if non-obstructive, detected by CCTA is associated with increased cardiovascular risk. The majority of the QCI Study Group members voted for a ‘treat any plaque’ strategy (Fig. 4), involving standard-intensity medical treatment after the detection of any atherosclerotic plaque, irrespective of size or composition. This strategy was found to be beneficial for patients in the prospective, randomized, controlled SCOT-HEART3 and DISCHARGE7,8 trials. As recommended in international guidelines, dose escalation might be warranted for stronger lipid-lowering and anti-atherosclerotic effects. However, many individuals with elevated LDL-C levels, but no signs of atherosclerosis, will not have a cardiovascular event43,131, suggesting that a treatment paradigm based on atherosclerotic plaque quantification rather than surrogate markers could improve patient outcome122. The QCI Study Group defined the TPV threshold for treatment escalation to high-intensity treatment as the age-adjusted and gender-adjusted 70th percentile (Fig. 4).
Clinical risk factors and other atherosclerotic plaque features also have a pivotal role in risk modulation. Smoking, a positive family history of CVD, the presence of low-attenuation plaque and positive remodelling were deemed to be the most important factors in the decision to escalate towards high-intensity treatment (Fig. 4). By contrast, very small fibrotic and calcified plaques almost never cause cardiovascular events and, consequently, allow for the de-escalation of medical treatment recommendations.
Limitations and future directions
Although consensus was reached on treatment percentiles, the Delphi process did not produce a unanimous position on the treatment of any atherosclerotic plaque. Standard-intensity medical treatment addresses the underlying pathophysiology of atherosclerosis, reduces the risk of cardiovascular events and stabilizes existing atherosclerotic plaques. However, with advances in scanner technology and increased scan availability, detecting small atherosclerotic plaques in patients with stable chest pain, or even healthy individuals, with low cardiovascular risk will become more common. Therefore, defining an optimal treatment threshold might be warranted in the future. The integration of NCP also proved to be a divisive topic, and no consensus could be reached. Although agreement was reached that NCP should not be disregarded, its role in individualized treatment recommendations and monitoring of treatment response is yet to be determined on the basis of data from future randomized trials.
AI in plaque assessment
Research shows that fully automated AI methods for the analysis of atherosclerotic plaque in conventional, single-energy CT perform at the level of inter-observer agreement with fast inferences19,26,132. Further advances in the robustness and resilience of these methods have the potential to bring the analysis to clinical use. Crucially, AI-supported analysis of CT imaging depends on high image quality, and a novel treatment paradigm based on AI-supported quantification of TPV requires accurate detection of atherosclerotic plaque. Owing to limited spatial resolution of CCTA, atherosclerotic plaque quantification is recommended only in vessels with a diameter >2 mm (refs. 25,93). In addition, the presence of artefacts and noise can impede detailed delineation of plaque contours. For example, blooming of calcified components can affect the accurate characterization of adjacent low-attenuation plaque16. Variations in scanner systems and acquisition protocols affect atherosclerotic plaque characterization, presenting challenges for the accurate quantification of atherosclerotic plaques near to intensity-based cut-off points. Efforts to standardize CT acquisition and analysis are crucial to mitigate such differences25,132.
Advances in CT image reconstruction133 and photon-counting CT technology are expected to substantially advance the imaging of atherosclerotic plaque in the near future, owing to increased spatial resolution134 and spectral capabilities135. Nevertheless, the application of AI-supported analysis of these novel CT images will be associated with new challenges, mainly concerning optimal image reconstruction and the exploitation of multienergy level imaging136. Further development of existing AI methods for use with (spectral) photon-counting CT could involve the handling of large and high-dimensional data. In the next few years, advances in AI technologies could unlock the potential for the improved analysis of atherosclerotic plaque anatomy and easier identification of vulnerable plaque, facilitating individualized treatment recommendations.
Clinical implications
The increased use of AI-supported coronary atherosclerosis imaging will generate unprecedented volumes of data. The challenge will be to integrate these data into meaningful information for effective guidance of patient management. We should resist the temptation to generate hypotheses based on the data but instead focus on testing hypotheses that are firmly grounded in our understanding of CAD pathophysiology. By integrating quantitative assessments of coronary plaque burden, composition and progression, advanced algorithms for patient management have the potential to deliver truly individualized treatment, considering crucial factors such as ethnic variability, comorbidities and concurrent therapies.
Given the expanding options for medical therapy, with the growing armamentarium of lipid-lowering and anti-inflammatory therapies, a need clearly exists to individualize treatment intensity. The relationship among disease burden, inflammatory activity and atherosclerotic plaque progression must guide these advances, because they are the key determinants of cardiovascular risk. AI-supported imaging, incorporating age-adjusted and gender-adjusted TPV percentiles, could provide a foundation to refine treatment thresholds. Inflammatory markers and coronary imaging risk modifiers could inform the treatment intensity required to halt disease progression. Finally, atherosclerotic plaque characteristics will add nuanced adjustments to optimize treatment. The effectiveness of these innovative approaches must be rigorously validated through well-designed, prospective, randomized, controlled clinical trials. This requirement presents a major challenge, given the many options for tailored management and the need for individual effectiveness research. The recommendations contained in this Consensus Statement could provide a foundation for these trials.
Conclusions
AI-supported tools to evaluate atherosclerotic plaque offer the potential to refine the precision of risk stratification and improve clinical decision-making by providing reliable and accurate assessments of atherosclerotic plaque burden and morphology. The QCI Study Group deemed the age-adjusted and gender-adjusted percentiles of TPV to be the most meaningful parameter for the individualized initiation and escalation of medical treatment. TPV is a major risk predictor for MACEs and outperforms other atherosclerotic plaque subtype metrics, such as CPV or NCPV, as well as providing modest independent discrimination in addition to cardiovascular risk factors72. Treatment escalation to high-intensity regimens is recommended for patients with TPV above the 70th percentile. Clinical risk factors and high-risk plaque features can also inform decisions on treatment escalation.
The implementation of individualized treatment recommendations, guided by AI-supported assessment of coronary atherosclerotic plaque, represents a substantial paradigm shift in cardiovascular risk management. Although these consensus recommendations provide a practical framework for integrating AI-supported evaluation of atherosclerotic plaque into routine clinical practice, further large-scale, randomized, controlled cardiovascular outcome trials integrating novel anti-atherosclerotic agents are necessary to validate these recommendations. This evolving approach highlights the growing importance of advanced imaging of coronary atherosclerosis biomarkers and AI-driven analysis as companion diagnostics in transforming cardiovascular care, moving from generalized risk scoring to individualized treatment pathways based on coronary atherosclerotic plaque morphology and should be tested in trials of CT versus CT with AI.
References
Weir-McCall, J. R. et al. National trends in coronary artery disease imaging: associations with health care outcomes and costs. JACC Cardiovasc. Imaging 16, 659–671 (2023).
Schmidt, M. et al. The Western Denmark Heart Registry: its influence on cardiovascular patient care. J. Am. Coll. Cardiol. 71, 1259–1272 (2018).
Langenbach, M. C. et al. German Radiological Society and the Professional Association of German Radiologists Position Paper on coronary computed tomography: clinical evidence and quality of patient care in chronic coronary syndrome. Rofo 195, 115–134 (2023).
van den Boogert, T. P. W. et al. The impact and challenges of implementing CTCA according to the 2019 ESC guidelines on chronic coronary syndromes: a survey and projection of CTCA services in the Netherlands. Insights Imaging 12, 186 (2021).
Dewey, M. et al. Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial. BMJ 355, i5441 (2016).
Mézquita, A. J. V. et al. Clinical quantitative coronary artery stenosis and coronary atherosclerosis imaging: a Consensus Statement from the Quantitative Cardiovascular Imaging Study Group. Nat. Rev. Cardiol. 20, 696–714 (2023).
The SCOT-HEART Investigators. Coronary CT angiography and 5-year risk of myocardial infarction. N. Engl. J. Med. 379, 924–933 (2018). The SCOT-HEART and DISCHARGE trials were key studies that changed reimbursement paradigms in Europe and beyond. Analysis of data from both trials was used to inform the selection of participants to choose optimal treatment thresholds.
The DISCHARGE Trial Group. CT or invasive coronary angiography in stable chest pain. N. Engl. J. Med. 386, 1591–1602 (2022).
Williams, M. C. et al. Coronary CT angiography-guided management of patients with stable chest pain: 10-year outcomes from the SCOT-HEART randomised controlled trial in Scotland. Lancet 405, 329–337 (2025).
Adamson, P. D. & Newby, D. E. The SCOT-HEART trial. What we observed and what we learned. J. Cardiovasc. Comput. Tomogr. 13, 54–58 (2019).
Doenst, T. et al. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J. Am. Coll. Cardiol. 73, 964–976 (2019).
Doenst, T., Bonow, R. O., Bhatt, D. L., Falk, V. & Gaudino, M. Improving terminology to describe coronary artery procedures: JACC review topic of the week. J. Am. Coll. Cardiol. 78, 180–188 (2021).
Napp, A. E. et al. Computed tomography versus invasive coronary angiography: design and methods of the pragmatic randomised multicentre DISCHARGE trial. Eur. Radiol. 27, 2957–2968 (2017).
The DISCHARGE Trial Group. Comparative effectiveness of initial computed tomography and invasive coronary angiography in women and men with stable chest pain and suspected coronary artery disease: multicentre randomised trial. BMJ 379, e071133 (2022).
The DISCHARGE Trial Group. Age and computed tomography and invasive coronary angiography in stable chest pain: a prespecified secondary analysis of the DISCHARGE randomized clinical trial. JAMA Cardiol. 9, 346–356 (2024).
Williams, M. C., Earls, J. P. & Hecht, H. Quantitative assessment of atherosclerotic plaque, recent progress and current limitations. J. Cardiovasc. Comput. Tomogr. 16, 124–137 (2022).
Trost, J. et al. CCTA should be the new diagnostic gateway for evaluating intermediate-risk stable angina patients. JACC Adv. 1, 100116 (2022).
Maurovich-Horvat, P., Ferencik, M., Voros, S., Merkely, B. & Hoffmann, U. Comprehensive plaque assessment by coronary CT angiography. Nat. Rev. Cardiol. 11, 390–402 (2014).
Lin, A. et al. Deep learning-enabled coronary CT angiography for plaque and stenosis quantification and cardiac risk prediction: an international multicentre study. Lancet Digit. Health 4, e256–e265 (2022). This externally validated, deep learning system demonstrated that AI-supported analysis of atherosclerotic plaque on coronary CT angiography agrees closely with expert readers and intravascular ultrasonography and could have prognostic value for predicting future myocardial infarction.
Föllmer, B. et al. Roadmap on the use of artificial intelligence for imaging of vulnerable atherosclerotic plaque in coronary arteries. Nat. Rev. Cardiol. 21, 51–64 (2024).
Varoquaux, G. & Cheplygina, V. Machine learning for medical imaging: methodological failures and recommendations for the future. npj Digit. Med. 5, 48 (2022).
Lin, A. et al. Artificial intelligence in cardiovascular imaging for risk stratification in coronary artery disease. Radiol. Cardiothorac. Imaging 3, e200512 (2021).
van Herten, R. L. M., Lagogiannis, I., Leiner, T. & Išgum, I. The role of artificial intelligence in coronary CT angiography. Neth. Heart J. 32, 417–425 (2024).
Nannini, G. et al. A fully automated deep learning approach for coronary artery segmentation and comprehensive characterization. APL Bioeng. 8, 016103 (2024).
Nieman, K. et al. Standards for quantitative assessments by coronary computed tomography angiography (CCTA): an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J. Cardiovasc. Comput. Tomogr. 18, 429–443 (2024). This SCCT consensus document emphasizes the standardization of AI-supported analysis of atherosclerotic plaque in coronary artery disease, thereby serving as the counterpart to the proposed individualization.
Herten, V. et al. Automatic coronary artery plaque quantification and CAD-RADS prediction using mesh priors. IEEE Trans. Med. Imaging 43, 1272–1283 (2024).
Narula, J. et al. Prospective deep learning-based quantitative assessment of coronary plaque by computed tomography angiography compared with intravascular ultrasound: the REVEALPLAQUE study. Eur. Heart J. Cardiovasc. Imaging 25, 1287–1295 (2024).
Jie, P. et al. Diagnostic value of artificial intelligence-assisted CTA for the assessment of atherosclerosis plaque: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11, 1398963 (2024).
Miller, R. J. H. et al. Patient-specific myocardial infarction risk thresholds from AI-enabled coronary plaque analysis. Circ. Cardiovasc. Imaging 17, e016958 (2024). This article demonstrated that age-adjusted and gender-adjusted percentile curves of atherosclerotic plaque volumes are predictive of cardiovascular events.
Dewey, M. The future of radiology: adding value to clinical care. Lancet 392, 472–473 (2018).
Nurmohamed, N. S. et al. AI-guided quantitative plaque staging predicts long-term cardiovascular outcomes in patients at risk for atherosclerotic CVD. JACC Cardiovasc. Imaging 17, 269–280 (2024).
Williams, M. C. et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish Computed Tomography of the HEART). Circulation 141, 1452–1462 (2020).
Budoff, M. J. et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur. Heart J. 41, 3925–3932 (2020).
Nieman, K. et al. Multislice computed tomography angiography for noninvasive assessment of the 18-month performance of a novel radiolucent bioresorbable vascular scaffolding device: the ABSORB trial (a clinical evaluation of the bioabsorbable everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). J. Am. Coll. Cardiol. 62, 1813–1814 (2013).
Corrigendum to: 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 41, 4255 (2020).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J. Am. Coll. Cardiol. 73, e285–e350 (2019).
Martin, S. S. et al. 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation 149, e347–e913 (2024).
Di Cesare, M. et al. World Heart Report 2023: confronting the world’s number one killer. World Heart Federation https://world-heart-federation.org/wp-content/uploads/World-Heart-Report-2023.pdf (2023).
Akosah, K. O., Schaper, A., Cogbill, C. & Schoenfeld, P. Preventing myocardial infarction in the young adult in the first place: how do the National Cholesterol Education Panel III guidelines perform? J. Am. Coll. Cardiol. 41, 1475–1479 (2003).
Bautista, L. E. & Rueda-Ochoa, O. L. Methodological challenges in studies of the role of blood lipids variability in the incidence of cardiovascular disease. Lipids Health Dis. 20, 51 (2021).
Zaman, S. et al. The Lancet Commission on rethinking coronary artery disease: moving from ischaemia to atheroma. Lancet 405, 1264–1312 (2025).
Bittner, D. O. et al. Influence of cardiovascular risk factors on the prevalence of coronary atherosclerosis in patients with angiographically normal coronary arteries. Acad. Radiol. 24, 580–586 (2017).
Mortensen, M. B. et al. Low-density lipoprotein cholesterol is predominantly associated with atherosclerotic cardiovascular disease events in patients with evidence of coronary atherosclerosis: the Western Denmark Heart Registry. Circulation 147, 1053–1063 (2023).
Reynolds, H. R. et al. Outcomes in the ISCHEMIA trial based on coronary artery disease and ischemia severity. Circulation 144, 1024–1038 (2021).
Karpe, F. & Holman, R. Fire-and-forget in prevention of coronary heart disease. Lancet 360, 1984 (2002).
Dewey, M. et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia. Nat. Rev. Cardiol. 17, 427–450 (2020).
Stary, H. C. et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92, 1355–1374 (1995).
Jebari-Benslaiman, S. et al. Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 23, 47 (2022).
Biavati, F. et al. Coronary artery calcium score predicts major adverse cardiovascular events in stable chest pain. Radiology 310, e231557 (2024).
Pinto-Sietsma, S. J., Velthuis, B. K., Nurmohamed, N. S., Vliegenthart, R. & Martens, F. Computed tomography and coronary artery calcium score for screening of coronary artery disease and cardiovascular risk management in asymptomatic individuals. Neth. Heart J. 32, 371–377 (2024).
Valenti, V. et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc. Imaging 8, 900–909 (2015).
Blaha, M. J. et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 133, 849–858 (2016).
Sandesara, P. B. et al. Clinical significance of zero coronary artery calcium in individuals with LDL cholesterol ≥190 mg/dl: the multi-ethnic study of atherosclerosis. Atherosclerosis 292, 224–229 (2020).
van der Aalst, C. M. et al. Screening for cardiovascular disease risk using traditional risk factor assessment or coronary artery calcium scoring: the ROBINSCA trial. Eur. Heart J. Cardiovasc. Imaging 21, 1216–1224 (2020).
Mitchell, J. D. et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J. Am. Coll. Cardiol. 72, 3233–3242 (2018).
Miedema, M. D. et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ. Cardiovasc. Qual. Outcomes 7, 453–460 (2014).
Indraratna, P. et al. Aspirin and statin therapy for nonobstructive coronary artery disease: five-year outcomes from the CONFIRM registry. Radiol. Cardiothorac. Imaging 4, e210225 (2022).
Rozanski, A. et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J. Am. Coll. Cardiol. 57, 1622–1632 (2011).
van Rosendael, A. R. et al. Association of high-density calcified 1 K plaque with risk of acute coronary syndrome. JAMA Cardiol. 5, 282–290 (2020).
Hussain, A., Ballantyne, C. M. & Nambi, V. Zero coronary artery calcium score: desirable, but enough? Circulation 142, 917–919 (2020).
Budoff, M. J. et al. Prognostic value of coronary artery calcium in the PROMISE study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 136, 1993–2005 (2017).
Di Giovanni, G., Kataoka, Y., Bubb, K., Nelson, A. J. & Nicholls, S. J. Impact of lipid lowering on coronary atherosclerosis moving from the lumen to the artery wall. Atherosclerosis 367, 8–14 (2023).
Houslay, E. S. et al. Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart 92, 1207–1212 (2006).
Nadjiri, J. et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J. Cardiovasc. Comput. Tomogr. 10, 97–104 (2016).
Meah, M. N. et al. Plaque burden and 1-year outcomes in acute chest pain: results from the multicenter RAPID-CTCA trial. JACC Cardiovasc. Imaging 15, 1916–1925 (2022).
van Rosendael, A. R. et al. Association of statin treatment with progression of coronary atherosclerotic plaque composition. JAMA Cardiol. 6, 1257–1266 (2021).
Lu, M. T. et al. Effects of pitavastatin on coronary artery disease and inflammatory biomarkers in HIV: mechanistic substudy of the REPRIEVE randomized clinical trial. JAMA Cardiol. 9, 323–334 (2024).
Nasir, K. et al. Coronary atherosclerosis in an asymptomatic U.S. population: Miami Heart Study at Baptist Health South Florida. JACC Cardiovasc. Imaging 15, 1604–1618 (2022).
Fuchs, A. et al. Subclinical coronary atherosclerosis and risk for myocardial infarction in a Danish cohort: a prospective observational cohort study. Ann. Intern. Med. 176, 433–442 (2023).
Hoffmann, U. et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 135, 2320–2332 (2017).
Singh, T. et al. Exercise electrocardiography and computed tomography coronary angiography for patients with suspected stable angina pectoris: a post hoc analysis of the randomized SCOT-HEART trial. JAMA Cardiol. 5, 920–928 (2020).
Nurmohamed, N. S. et al. Atherosclerosis quantification and cardiovascular risk: the ISCHEMIA trial. Eur. Heart J. 45, 3735–3747 (2024). Results from the ISCHEMIA trial demonstrated that total plaque volume has incremental value for patient management compared with conventional visual coronary CT angiography.
Deseive, S. et al. Quantified coronary total plaque volume from computed tomography angiography provides superior 10-year risk stratification. Eur. Heart J. Cardiovasc. Imaging 22, 314–321 (2021).
Bell, J. S. et al. Plaque quantification from coronary computed tomography angiography in predicting cardiovascular events: a systematic review and meta-analysis. J. Cardiovasc. Comput. Tomogr. https://doi.org/10.1016/j.jcct.2025.05.003 (2025).
Vergallo, R. et al. Vulnerable or high-risk plaque: a JACC: Cardiovascular Imaging Position Statement. JACC Cardiovasc. Imaging 18, 709–740 (2025).
Nerlekar, N. et al. Computed tomographic coronary angiography-derived plaque characteristics predict major adverse cardiovascular events: a systematic review and meta-analysis. Circ. Cardiovasc. Imaging 11, e006973 (2018).
Pflederer, T. et al. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis 211, 437–444 (2010).
Hoffmann, U. et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J. Am. Coll. Cardiol. 47, 1655–1662 (2006).
Kitagawa, T. et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc. Imaging 2, 153–160 (2009).
Kataoka, Y. et al. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: insights from serial intravascular ultrasound. J. Am. Coll. Cardiol. 59, 1592–1597 (2012).
Ehara, S. et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 110, 3424–3429 (2004).
Motoyama, S. et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 54, 49–57 (2009).
Cademartiri, F. et al. Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur. Radiol. 15, 1426–1431 (2005).
Achenbach, S. et al. Influence of slice thickness and reconstruction kernel on the computed tomographic attenuation of coronary atherosclerotic plaque. J. Cardiovasc. Comput. Tomogr. 4, 110–115 (2010).
Ferencik, M. et al. Arterial wall imaging: evaluation with 16-section multidetector CT in blood vessel phantoms and ex vivo coronary arteries. Radiology 240, 708–716 (2006).
Suzuki, S. et al. Accuracy of attenuation measurement of vascular wall in vitro on computed tomography angiography: effect of wall thickness, density of contrast medium, and measurement point. Invest. Radiol. 41, 510–515 (2006).
Otsuka, K. et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc. Imaging 6, 448–457 (2013).
Ferencik, M. et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. 3, 144–152 (2018).
Maroules, C. D. et al. Coronary Artery Disease Reporting and Data System (CAD-RADS(TM)): inter-observer agreement for assessment categories and modifiers. J. Cardiovasc. Comput. Tomogr. 12, 125–130 (2018).
Ferencik, M. et al. A computed tomography-based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. Am. J. Cardiol. 110, 183–189 (2012).
Puchner, S. B. et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 64, 684–692 (2014).
Vattay, B. et al. Impact of virtual monoenergetic levels on coronary plaque volume components using photon-counting computed tomography. Eur. Radiol. 33, 8528–8539 (2023).
Shaw, L. J. et al. Society of Cardiovascular Computed Tomography/North American Society of Cardiovascular Imaging — Expert Consensus Document on Coronary CT Imaging of Atherosclerotic Plaque. J. Cardiovasc. Comput. Tomogr. 15, 93–109 (2021).
Visseren, F. L. J. et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 42, 3227–3337 (2021).
Arnett, D. K. et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task force on Clinical Practice Guidelines. Circulation 140, e596–e646 (2019).
ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008).
Talha, I., Elkhoudri, N. & Hilali, A. Major limitations of cardiovascular risk scores. Cardiovasc. Ther. 2024, 4133365 (2024).
Wormser, D. et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 377, 1085–1095 (2011).
Damen, J. A. et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ 353, i2416 (2016).
Adamson, P. D. et al. Guiding therapy by coronary CT angiography improves outcomes in patients with stable chest pain. J. Am. Coll. Cardiol. 74, 2058–2070 (2019).
McDermott, M. et al. Rationale and design of SCOT-HEART 2 trial. JACC Cardiovasc. Imaging 17, 1101–1112 (2024).
Grodecki, K. et al. Phenotyping atherosclerotic plaque and perivascular adipose tissue: signalling pathways and clinical biomarkers in atherosclerosis. Nat. Rev. Cardiol. 22, 443–455 (2025).
Spearman, J. V. et al. Prognostic value of epicardial fat volume measurements by computed tomography: a systematic review of the literature. Eur. Radiol. 25, 3372–3381 (2015).
Miller, R. J. H. et al. AI-derived epicardial fat measurements improve cardiovascular risk prediction from myocardial perfusion imaging. npj Digit. Med. 7, 24 (2024).
Ma, R. et al. Evaluation of pericoronary adipose tissue attenuation on CT. Br. J. Radiol. 96, 20220885 (2023).
Chan, K. et al. Inflammatory risk and cardiovascular events in patients without obstructive coronary artery disease: the ORFAN multicentre, longitudinal cohort study. Lancet 403, 2606–2618 (2024).
Ma, R. et al. Towards reference values of pericoronary adipose tissue attenuation: impact of coronary artery and tube voltage in coronary computed tomography angiography. Eur. Radiol. 30, 6838–6846 (2020).
Lisi, C. et al. The pericoronary adipose tissue attenuation in CT strongly depends on kernels and iterative reconstructions. Eur. Radiol. 35, 2866–2876 (2025).
Pieszko, K. et al. Deep learning of coronary calcium scores from PET/CT attenuation maps accurately predicts adverse cardiovascular events. JACC Cardiovasc. Imaging 16, 675–687 (2023).
van Velzen, S. G. M. et al. Deep learning for automatic calcium scoring in CT: validation using multiple cardiac CT and chest CT protocols. Radiology 295, 66–79 (2020).
Föllmer, B. et al. Automated segment-level coronary artery calcium scoring on non-contrast CT: a multi-task deep-learning approach. Insights Imaging 15, 250 (2024).
Lekadir, K. et al. FUTURE-AI: international consensus guideline for trustworthy and deployable artificial intelligence in healthcare. BMJ 388, e081554 (2025).
Iraqi, N. et al. Interscan reproducibility of computed tomography derived coronary plaque volume measurements. J. Cardiovasc. Comput. Tomogr. 18, 583–592 (2024).
Blaha Michael, J. & DeFilippis Andrew, P. Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. 77, 3195–3216 (2021).
Williams, M. C. et al. Sex-specific computed tomography coronary plaque characterization and risk of myocardial infarction. JACC Cardiovasc. Imaging 14, 1804–1814 (2021).
van Diemen, P. A. et al. Prognostic value of RCA pericoronary adipose tissue CT-attenuation beyond high-risk plaques, plaque volume, and ischemia. JACC Cardiovasc. Imaging 14, 1598–1610 (2021).
Dundas, J. et al. Interaction of AI-enabled quantitative coronary plaque volumes on coronary CT angiography, FFRCT, and clinical outcomes: a retrospective analysis of the ADVANCE registry. Circ. Cardiovasc. Imaging 17, e016143 (2024).
Freeman, A. M. et al. Integrating coronary atherosclerosis burden and progression with coronary artery disease risk factors to guide therapeutic decision making. Am. J. Med. 136, 260–269.e7 (2023).
Tzimas, G. et al. Age- and sex-specific nomographic CT quantitative plaque data from a large international cohort. JACC Cardiovasc. Imaging 17, 165–175 (2024).
Hirohata, A. et al. Four-year clinical outcomes of the OLIVUS-Ex (impact of olmesartan on progression of coronary atherosclerosis: evaluation by intravascular ultrasound) extension trial. Atherosclerosis 220, 134–138 (2012).
Nissen, S. E. et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 291, 1071–1080 (2004).
Dawson, L. P., Lum, M., Nerleker, N., Nicholls, S. J. & Layland, J. Coronary atherosclerotic plaque regression: JACC state-of-the-art review. J. Am. Coll. Cardiol. 79, 66–82 (2022).
Virani, S. S. et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 148, e9–e119 (2023).
Vrints, C. et al. 2024 ESC guidelines for the management of chronic coronary syndromes: developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 45, 3415–3537 (2024).
Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681 (2010).
Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 385, 1397–1405 (2015).
Bohula, E. A. et al. Inflammatory and cholesterol risk in the FOURIER trial. Circulation 138, 131–140 (2018).
Byrne, P. et al. Evaluating the association between low-density lipoprotein cholesterol reduction and relative and absolute effects of statin treatment: a systematic review and meta-analysis. JAMA Intern. Med. 182, 474–481 (2022).
Nurmohamed, N. S., Navar, A. M. & Kastelein, J. J. P. New and emerging therapies for reduction of LDL-cholesterol and apolipoprotein B: JACC Focus Seminar 1/4. J. Am. Coll. Cardiol. 77, 1564–1575 (2021).
Pérez de Isla, L. et al. Alirocumab and coronary atherosclerosis in asymptomatic patients with familial hypercholesterolemia: the ARCHITECT study. Circulation 147, 1436–1443 (2023).
Welty, F. K. et al. Regression of coronary fatty plaque and risk of cardiac events according to blood pressure status: data from a randomized trial of eicosapentaenoic acid and docosahexaenoic acid in patients with coronary artery disease. J. Am. Heart Assoc. 12, e030071 (2023).
Williams, M. C. et al. Artificial intelligence and machine learning for cardiovascular computed tomography (CCT): a white paper of the Society of Cardiovascular Computed Tomography (SCCT). J. Cardiovasc. Comput. Tomogr. 18, 519–532 (2024).
Koetzier, L. R. et al. Deep learning image reconstruction for CT: technical principles and clinical prospects. Radiology 306, e221257 (2023).
Mergen, V., Eberhard, M., Manka, R., Euler, A. & Alkadhi, H. First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography. Front. Cardiovasc. Med. 9, 981012 (2022).
Dunning, C. A. S. et al. Classification of high-risk coronary plaques using radiomic analysis of multi-energy photon-counting-detector computed tomography (PCD-CT) images. Proc. SPIE Int. Soc. Opt. Eng. 12465, 124652T (2023).
Healy, J. et al. Ex-vivo atherosclerotic plaque characterization using spectral photon-counting CT: comparing material quantification to histology. Atherosclerosis 378, 117160 (2023).
Acknowledgements
The authors thank the German Research Foundation (DFG) for funding the third Quantitative Cardiovascular Imaging meeting and this Consensus Statement on the quantitative assessment of coronary artery stenosis and atherosclerosis (DE 1361/32-1). This work was also supported by the DFG through its graduate programme on quantitative biomedical imaging (BIOQIC, GRK 2260/1, DFG project number 289347353), the DFG Priority Programme Radiomics (DFG project number 402688427) for the investigation of coronary plaque and coronary flow (DE 1361/19-1: DFG project number 428222922 and DE 1361/20-1: DFG project number 428223139 in SPP 2177/1) and the GUIDE-IT project on data sharing of medical imaging trials (DE 1361/24-1, DFG project number 495697118).
Author information
Authors and Affiliations
Contributions
K.S., A.-M.S., M.C.W., A.-C.S., H.B., D.D., D.E.N. and M.D. researched data for the article. K.S., A.-M.S., M.C.W., P.M.-H., B.F., F.B., D.D., D.E.N. and M.D. discussed the content of the article. K.S., A.-M.S., M.C.W., V.S.V., A.A.G., K.N., P.M.-H., J.M.T., R.V., J.W.-M., M.M., B.F., F.B., N.G., I.I., A.A.-Z., H.A., E.D.N., N.S.N., F.M.A.C.M., D.D., D.E.N. and M.D. wrote the manuscript. All the authors reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.C.W. has given talks for Canon Medical Systems, GE Healthcare, Novartis and Siemens Healthineers and performed consultancy for Canon Medical Systems and FEops. A.A.G. reports research grant support from GE Healthcare, the Iten-Kohaut Foundation and Promedica Stiftung, has given talks for GE Healthcare and has performed consultancy for Artrya Ltd. J.M.T is supported by the Cambridge BHF Centre of Research Excellence (RE/24/130011) and the Wellcome Trust (211100/Z/18/Z) and has received research grants from AstraZeneca, the British Heart Foundation and General Electric Healthcare. R.V. is supported by institutional research grants from Siemens Healthineers and has received honoraria for lectures/moderatorship from Bayer Healthcare and Siemens Healthineers. I.I. receives institutional research grants from the Dutch Research Council with the participation of Abbott Vascular, Philips Healthcare and Pie Medical Imaging, and institutional research grants from Esaote, Health Holland with participation of Braun and Infraredex, Horizon Europe, the EU Innovative Health Initiative with the participation of Philips Healthcare and Pie Medical Imaging. A.A.-Z. receives grant support from Canon Medical Systems. H.A. receives institutional grants from Bayer, Canon, Guerbet and Siemens and receives royalties from Springer Nature for a textbook on cardiac CT. H.A. received speaker honoraria from Siemens. R.M. receives speaker fees from Bayer, Bristol Meyers Squib, Philips and Siemens and research grant support from the Swissheart Foundation and the USZ Foundation. E.D.N. is on the Scientific Advisory Board of Caristo and is the immediate Past President of the Society of Cardiovascular Computed Tomography (SCCT; the opinions expressed in this article are the author’s own and do not represent the view of the SCCT). N.S.N. reports grants from the Dutch Heart Foundation (Dekker 03-007-2023-0068), European Atherosclerosis Society (2023), research funding/speaker fees from Cleerly, Daiichi Sankyo and Novartis and is co-founder of Lipid Tools. M.D. was the publications chair of the European Society of Radiology (ESR) from 2022 to 2025 (the opinions expressed in this article are the author’s own and do not represent the view of the ESR). He is also the editor of Cardiac CT (Springer Nature) and has institutional master research agreements with Canon, General Electric, Philips and Siemens, the arrangements of which are managed by Charité — Universitätsmedizin Berlin. He also holds a joint approved patent on dynamic perfusion analysis using fractal analysis (EPO 2022 EP3350773A1 and USPTO 2021 10,991,109). The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Meinrad Beer, Leslee Shaw and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schulze, K., Stantien, AM., Williams, M.C. et al. Coronary CT angiography evaluation with artificial intelligence for individualized medical treatment of atherosclerosis: a Consensus Statement from the QCI Study Group. Nat Rev Cardiol (2025). https://doi.org/10.1038/s41569-025-01191-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41569-025-01191-6