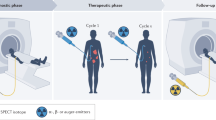
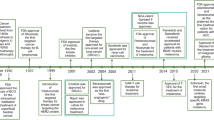
Abstract
Targeted radionuclide therapy (TRT) is a cutting-edge treatment approach in oncology that combines the molecular precision of targeted agents with the effect of radiotherapy to selectively deliver cytotoxic radiation to cancer cells. Research efforts from the past few decades have led to a diverse molecular landscape of TRT and have provided lessons for further rational development of targeted radiopharmaceuticals and expansion of the clinical applications of this treatment modality. In this Review, we discuss TRT in the context of therapeutic approaches currently available in oncology, describe the broad range of established and emerging targets for TRT including innovative approaches to exploit vulnerabilities presented by the tumour microenvironment, and address the challenges for clinical translation and molecular optimization. By bridging technological innovation and preclinical discoveries with real-world clinical implementation, ongoing research on TRT is seeking to provide effective and safe treatment options for patients across a variety of cancer types and treatment settings. Overall, we emphasize the transformative potential of TRT and highlight how a comprehensive understanding of what constitutes an optimal target can redefine clinical practice, fostering the evolution of TRT as a highly individualized and adaptable therapeutic option that improves outcomes across a broad range of cancer types.
Key points
-
Targeted radionuclide therapy (TRT) is a systemic therapeutic modality that uses ligands to tumour-associated targets to deliver radioactive payloads, leveraging molecular recognition to cause selective radiation-induced cancer cell damage while minimizing the toxicities in non-malignant tissues.
-
Increased radionuclide accessibility and advances in vector design have unlocked the potential of TRTs against a broad spectrum of tumour-associated targets, spanning pan-cancer markers that leverage tumour and microenvironmental vulnerabilities to targets confined to specific cancers.
-
Owing to advances in profiling technologies, artificial intelligence-powered tools and drug repurposing, the identification and validation of novel TRT targets is progressing at unprecedented speed.
-
Robust clinical translation requires trials that emulate real-world complexities and dynamically adapt treatments to shifting target expression, with continuous monitoring to overcome heterogeneity and resistance, while minimizing the toxicities that typically arise with conventional cancer therapies.
-
Innovations in multimerized, multitargeted, covalent-bond ligation and pretargeting strategies can improve tumour targeting while overcoming off-target and on-target off-tumour effects, and penetration barriers, expanding the potential of TRT across complex cancer landscapes.
-
Incorporating TRT early in the course of treatment and strategically selecting combination partners, such as chemotherapy, immunotherapy and targeted therapies, can help to maximize therapeutic efficacy, address resistance and enable broader oncology applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bodei, L., Herrmann, K., Schöder, H., Scott, A. M. & Lewis, J. S. Radiotheranostics in oncology: current challenges and emerging opportunities. Nat. Rev. Clin. Oncol. 19, 534–550 (2022).
Sgouros, G., Bodei, L., McDevitt, M. R. & Nedrow, J. R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug. Discov. 19, 589–608 (2020).
Strosberg, J. et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017).
Sartor, O. et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 385, 1091–1103 (2021).
Kratochwil, C. et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 57, 1941–1944 (2016).
Delpassand, E. S. et al. Targeted α-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: first-in-humans dose-escalation clinical trial. J. Nucl. Med. 63, 1326–1333 (2022).
Pouget, J. P. & Constanzo, J. Revisiting the radiobiology of targeted alpha therapy. Front. Med. 8, 692436 (2021).
Marcu, L., Bezak, E. & Allen, B. J. Global comparison of targeted alpha vs targeted beta therapy for cancer: in vitro, in vivo and clinical trials. Crit. Rev. Oncol. Hematol. 123, 7–20 (2018).
O’Donoghue, J. A., Bardiès, M. & Wheldon, T. E. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J. Nucl. Med. 36, 1902–1909 (1995).
Aghevlian, S., Boyle, A. J. & Reilly, R. M. Radioimmunotherapy of cancer with high linear energy transfer (LET) radiation delivered by radionuclides emitting α-particles or Auger electrons. Adv. Drug. Deliv. Rev. 109, 102–118 (2017).
Rogoza, O., Megnis, K., Kudrjavceva, M., Gerina-Berzina, A. & Rovite, V. Role of somatostatin signalling in neuroendocrine tumours. Int. J. Mol. Sci. 23, 1447 (2022).
Bakht, M. K. & Beltran, H. Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer. Nat. Rev. Urol. 22, 26–45 (2025).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Bhusari, P. et al. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int. J. Cancer 140, 938–947 (2017).
Pougoue Ketchemen, J. et al. Complete remissions of HER2-positive trastuzumab-resistant xenografts using a potent [225Ac]Ac-labeled anti-HER2 antibody–drug radioconjugate. Clin. Cancer Res. 31, 685–696 (2025).
Privé, B. M. et al. Fibroblast activation protein-targeted radionuclide therapy: background, opportunities, and challenges of first (pre)clinical studies. Eur. J. Nucl. Med. Mol. Imaging 50, 1906–1918 (2023).
Strosberg, J. et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-dotatate in the phase III NETTER-1 trial. J. Clin. Oncol. 36, 2578–2584 (2018).
Fizazi, K. et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 24, 597–610 (2023).
Baltussen, J. C. et al. Chemotherapy-related toxic effects and quality of life and physical functioning in older patients. JAMA Netw. Open. 6, e2339116 (2023).
Hofman, M. S. et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 397, 797–804 (2021).
Hofman, M. S. et al. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol. 25, 99–107 (2024).
Morris, M. J. et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): a phase 3, randomised, controlled trial. Lancet 404, 1227–1239 (2024).
Feuerecker, B. et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur. Urol. 79, 343–350 (2021).
Sathekge, M. M. et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): a multicentre, retrospective study. Lancet Oncol. 25, 175–183 (2024).
Zeng, Z. C. et al. Comparison between radioimmunotherapy and external beam radiation therapy for patients with hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 29, 1657–1668 (2002).
Partelli, S. et al. Neoadjuvant 177Lu-DOTATATE for non-functioning pancreatic neuroendocrine tumours (NEOLUPANET): multicentre phase II study. Br. J. Surg. 111, znae178 (2024).
van Vliet, E. I. et al. Neoadjuvant treatment of nonfunctioning pancreatic neuroendocrine tumors with [177Lu-DOTA0,Tyr3]octreotate. J. Nucl. Med. 56, 1647–1653 (2015).
Camidge, D. R., Pao, W. & Sequist, L. V. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat. Rev. Clin. Oncol. 11, 473–481 (2014).
Fu, H. et al. Fibroblast activation protein-targeted radioligand therapy with 177Lu-EB-FAPI for metastatic radioiodine-refractory thyroid cancer: first-in-human, dose-escalation study. Clin. Cancer Res. 29, 4740–4750 (2023).
Kryza, D. et al. A multicentric, single arm, open-label, phase I/II study evaluating PSMA targeted radionuclide therapy in adult patients with metastatic clear cell renal cancer (PRadR). BMC Cancer 24, 163 (2024).
Escorcia, F. E. et al. ImmunoPET predicts response to Met-targeted radioligand therapy in models of pancreatic cancer resistant to Met kinase inhibitors. Theranostics 10, 151–165 (2020).
Raymond, E. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 501–513 (2011).
Chan, J. A. et al. Phase 3 trial of cabozantinib to treat advanced neuroendocrine tumors. N. Engl. J. Med. 392, 653–665 (2025).
Tsuchikama, K., Anami, Y., Ha, S. Y. Y. & Yamazaki, C. M. Exploring the next generation of antibody–drug conjugates. Nat. Rev. Clin. Oncol. 21, 203–223 (2024).
Chi, K. N. et al. Safety analyses of the phase 3 VISION trial of [17Lu]Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur. Urol. 85, 382–391 (2024).
Petrylak, D. P. et al. PSMA ADC monotherapy in patients with progressive metastatic castration-resistant prostate cancer following abiraterone and/or enzalutamide: efficacy and safety in open-label single-arm phase 2 study. Prostate 80, 99–108 (2020).
de Bono, J. S. et al. Phase I study of MEDI3726: a prostate-specific membrane antigen-targeted antibody–drug conjugate, in patients with mCRPC after failure of abiraterone or enzalutamide. Clin. Cancer Res. 27, 3602–3609 (2021).
André, F. et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet 401, 1773–1785 (2023).
Sands, J. et al. Analysis of drug-related interstitial lung disease (ILD) inpatients (pts) treated with datopotamab deruxtecan (Dato-DXd) [abstract]. J. Clin. Oncol. 42, 8623 (2024).
Rao, Y. et al. Statins enhance the efficacy of HER2-targeting radioligand therapy in drug-resistant gastric cancers. Proc. Natl Acad. Sci. USA 120, e2220413120 (2023).
Natangelo, S. et al. Radiation therapy, tissue radiosensitization, and potential synergism in the era of novel antibody–drug conjugates. Crit. Rev. Oncol. Hematol. 195, 104270 (2024).
Sridaran, D. et al. Prostate cancer immunotherapy: improving clinical outcomes with a multi-pronged approach. Cell Rep. Med. 4, 101199 (2023).
Abida, W. et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 5, 471–478 (2019).
Antonarakis, E. S. et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 38, 395–405 (2020).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019).
Patel, R. B. et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 13, eabb3631 (2021).
Ertveldt, T. et al. Targeted radionuclide therapy with low and high-dose lutetium-177-labeled single domain antibodies induces distinct immune signatures in a mouse melanoma model. Mol. Cancer Ther. 21, 1136–1148 (2022).
Vardaki, I. et al. Radium-223 treatment increases immune checkpoint expression in extracellular vesicles from the metastatic prostate cancer bone microenvironment. Clin. Cancer Res. 27, 3253–3264 (2021).
Pouget, J. P., Chan, T. A., Galluzzi, L. & Constanzo, J. Radiopharmaceuticals as combinatorial partners for immune checkpoint inhibitors. Trends Cancer 9, 968–981 (2023).
Brudno, J. N. & Kochenderfer, J. N. Current understanding and management of CAR T cell-associated toxicities. Nat. Rev. Clin. Oncol. 21, 501–521 (2024).
Palecki, J. et al. T-cell redirecting bispecific antibodies: a review of a novel class of immuno-oncology for advanced prostate cancer. Cancer Biol. Ther. 25, 2356820 (2024).
Tran, B. et al. Results from a phase I study of AMG 160, a half-life extended (HLE), PSMA-targeted, bispecific T-cell engager (BiTE®) immune therapy for metastatic castration-resistant prostate cancer (mCRPC) [abstract 609O]. Ann. Oncol. 31, S507 (2020).
Kelly, W. K. et al. Interim results from a phase I study of AMG 509 (xaluritamig), a STEAP1 x CD3 XmAb 2+1 immune therapy in patients with metastatic castration-resistant prostate cancer (mCRPC) [abstract 264P]. Ann. Oncol. 34, S1576 (2023).
Carballo, M. & Quiros, R. M. To treat or not to treat: the role of adjuvant radioiodine therapy in thyroid cancer patients. J. Oncol. 2012, 707156 (2012).
Müller, C., van der Meulen, N. P. & Schibli, R. Opportunities and potential challenges of using terbium-161 for targeted radionuclide therapy in clinics. Eur. J. Nucl. Med. Mol. Imaging 50, 3181–3184 (2023).
Wang, X. et al. Druggability of targets for diagnostic radiopharmaceuticals. ACS Pharmacol. Transl. Sci. 6, 1107–1119 (2023).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Cooper, A. J., Sequist, L. V. & Lin, J. J. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 19, 499–514 (2022).
Bejarano, L., Jordāo, M. J. C. & Joyce, J. A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 11, 933–959 (2021).
Rummel, S., Valente, A. L., Kane, J. L., Shriver, C. D. & Ellsworth, R. E. Genomic (in)stability of the breast tumor microenvironment. Mol. Cancer Res. 10, 1526–1531 (2012).
Qiu, W. et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat. Genet. 40, 650–655 (2008).
Hosein, A. N. et al. Breast carcinoma-associated fibroblasts rarely contain p53 mutations or chromosomal aberrations. Cancer Res. 70, 5770–5777 (2010).
Palumbo, A. Jr., Da Costa Nde, O., Bonamino, M. H., Pinto, L. F. & Nasciutti, L. E. Genetic instability in the tumor microenvironment: a new look at an old neighbor. Mol. Cancer 14, 145 (2015).
van der Heide, C. D. & Dalm, S. U. Radionuclide imaging and therapy directed towards the tumor microenvironment: a multi-cancer approach for personalized medicine. Eur. J. Nucl. Med. Mol. Imaging 49, 4616–4641 (2022).
Galogre, M., Rodin, D., Pyatnitskiy, M., Mackelprang, M. & Koman, I. A review of HER2 overexpression and somatic mutations in cancers. Crit. Rev. Oncol. Hematol. 186, 103997 (2023).
Zhang, T. et al. Carrier systems of radiopharmaceuticals and the application in cancer therapy. Cell Death Discov. 10, 16 (2024).
Albert, N. L. et al. Translating the theranostic concept to neuro-oncology: disrupting barriers. Lancet Oncol. 25, e441–e451 (2024).
Jain, R. K. & Baxter, L. T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 48, 7022–7032 (1988).
van Osdol, W., Fujimori, K. & Weinstein, J. N. An analysis of monoclonal antibody distribution in microscopic tumor nodules: consequences of a “binding site barrier”. Cancer Res. 51, 4776–4784 (1991).
Thurber, G. M., Schmidt, M. M. & Wittrup, K. D. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv. Drug. Deliv. Rev. 60, 1421–1434 (2008).
Thurber, G. M., Zajic, S. C. & Wittrup, K. D. Theoretic criteria for antibody penetration into solid tumors and micrometastases. J. Nucl. Med. 48, 995–999 (2007).
Sgouros, G. Plasmapheresis in radioimmunotherapy of micrometastases: a mathematical modeling and dosimetrical analysis. J. Nucl. Med. 33, 2167–2179 (1992).
Le Rhun, E. et al. Targeted radionuclide therapy for patients with central nervous system metastasis: overlooked potential? Neuro-Oncol. 26, S229–S241 (2024).
Zhang, V., Taparra, K., Fisher, G., Aparici, C. & Soltys, S. G. Definitive treatment of brain metastases from a neuroendocrine tumor with peptide receptor radionuclide therapy with 117Lutetium DOTATATE: a case report. Cureus 15, e45327 (2023).
Sathekge, M. M. et al. Treatment of brain metastases of castration-resistant prostate cancer with 225Ac-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 46, 1756–1757 (2019).
Puttemans, J., Lahoutte, T., D’Huyvetter, M. & Devoogdt, N. Beyond the barrier: targeted radionuclide therapy in brain tumors and metastases. Pharmaceutics 11, 376 (2019).
Amerein, A. et al. Intraarterial administration of peptide receptor radionuclide therapy in patients with advanced meningioma: initial safety and efficacy. J. Nucl. Med. 65, 1911–1916 (2024).
Cheal, S. M., Chung, S. K., Vaughn, B. A., Cheung, N.-K. V. & Larson, S. M. Pretargeting: a path forward for radioimmunotherapy. J. Nucl. Med. 63, 1302–1315 (2022).
Paillas, S. et al. Localized irradiation of cell membrane by Auger electrons is cytotoxic through oxidative stress-mediated nontargeted effects. Antioxid. Redox Signal. 25, 467–484 (2016).
Stock, J. K., Jones, N. P., Hammonds, T., Roffey, J. & Dillon, C. Addressing the right targets in oncology: challenges and alternative approaches. SLAS Discov. 20, 305–317 (2015).
Tomuleasa, C. et al. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Signal. Transduct. Target. Ther. 9, 201 (2024).
Akhoundova, D. & Rubin, M. A. Clinical application of advanced multi-omics tumor profiling: shaping precision oncology of the future. Cancer Cell 40, 920–938 (2022).
Chang, K. et al. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 45, 1113–1120 (2013).
Lonsdale, J. et al. The genotype-tissue expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Edwards, N. J. et al. The CPTAC data portal: a resource for cancer proteomics research. J. Proteome Res. 14, 2707–2713 (2015).
Aksoy, B. A. et al. CTD2 dashboard: a searchable web interface to connect validated results from the cancer target discovery and development network. Database 2017, bax054 (2017).
Ghoussaini, M. et al. Open targets genetics: systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucleic Acids Res. 49, D1311–D1320 (2020).
Piñero, J. et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 48, D845–D855 (2019).
Knox, C. et al. DrugBank 6.0: the DrugBank knowledgebase for 2024. Nucleic Acids Res. 52, D1265–D1275 (2024).
Wenteler, A., Cabrera, C. P., Wei, W., Neduva, V. & Barnes, M. R. AI approaches for the discovery and validation of drug targets. Camb. Prisms: Precis. Med. 2, e7 (2024).
You, Y. et al. Artificial intelligence in cancer target identification and drug discovery. Signal. Transduct. Target. Ther. 7, 156 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Krishna, R. et al. Generalized biomolecular modeling and design with RoseTTAFold all-atom. Science 384, eadl2528 (2024).
Wallach, I., Dzamba, M. & Heifets, A. AtomNet: a deep convolutional neural network for bioactivity prediction in structure-based drug discovery. Preprint at ArXiv abs/1510.02855 (2015).
Lee, J. et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics 36, 1234–1240 (2020).
Huang, K. et al. A foundation model for clinician-centered drug repurposing. Nat. Med. 30, 3601–3613 (2024).
Zeng, X. et al. deepDR: a network-based deep learning approach to in silico drug repositioning. Bioinformatics 35, 5191–5198 (2019).
Mullowney, M. W. et al. Artificial intelligence for natural product drug discovery. Nat. Rev. Drug. Discov. 22, 895–916 (2023).
Baratto, L. et al. PSMA- and GRPR-targeted PET: results from 50 patients with biochemically recurrent prostate cancer. J. Nucl. Med. 62, 1545–1549 (2021).
Schollhammer, R. et al. Comparison of 68Ga-PSMA-617 PET/CT and 68Ga-RM2 PET/CT in patients with localized prostate cancer who are candidates for radical prostatectomy: a prospective, single-arm, single-center, phase II study. J. Nucl. Med. 64, 379–385 (2023).
Witzig, T. E. et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 20, 2453–2463 (2002).
Kaminski, M. S. et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J. Clin. Oncol. 19, 3918–3928 (2001).
Ertveldt, T. et al. Targeted α-therapy using 225Ac radiolabeled single-domain antibodies induces antigen-specific immune responses and instills immunomodulation both systemically and at the tumor microenvironment. J. Nucl. Med. 64, 751–758 (2023).
Kang, L. et al. Noninvasive evaluation of CD20 expression using 64Cu-labeled F(ab’)2 fragments of obinutuzumab in lymphoma. J. Nucl. Med. 62, 372–378 (2021).
Longtine, M. et al. Radiolabeling of anti-CD20 ofatumumab with actinium-225 (225Ac) and therapeutic efficacy in a preclinical disseminated lymphoma model. J. Nucl. Med. 63, 4031–4031 (2022).
Pryma, D. A. et al. Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J. Nucl. Med. 60, 623–630 (2019).
de Kraker, J. et al. Iodine-131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. Eur. J. Cancer 44, 551–556 (2008).
Maric, I. et al. Efficacy and safety of 124I-MIBG dosimetry-guided high-activity 11)I-MIBG therapy of advanced pheochromocytoma or neuroblastoma. J. Nucl. Med. 64, 885–891 (2023).
Society of Nuclear Medicine and Molecular Imaging. Lantheus to discontinue production of Azedra. SNMMI https://snmmi.org/Web/News/Articles/Lantheus-to-Discontinue-Production-of-Azedra.aspx (2023).
US Food and Drug Administration. NDA 209607: Azedra. fda.gov https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=209607 (2024).
Strosberg, J. R., Al-Toubah, T., El-Haddad, G., Reidy Lagunes, D. & Bodei, L. Sequencing of somatostatin-receptor-based therapies in neuroendocrine tumor patients. J. Nucl. Med. 65, 340–348 (2024).
Strosberg, J. R. et al. 177Lu-dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 22, 1752–1763 (2021).
Strosberg, J. et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with 177Lu-dotatate: an analysis of the NETTER-1 study. Eur. J. Nucl. Med. Mol. Imaging 47, 2372–2382 (2020).
Singh, S. et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): an open-label, randomised, phase 3 study. Lancet 403, 2807–2817 (2024).
Singh, S. et al. First-line efficacy of [177Lu]Lu-DOTA-TATE in patients with advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors by tumor grade and primary origin: subgroup analysis of the phase III NETTER-2 study [abstract 211MO]. Ann. Oncol. 35, S92–S93 (2024).
Gaze, M. N. et al. Safety and dosimetry of [177Lu]Lu-DOTA-TATE in adolescent patients with somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumours, or pheochromocytomas and paragangliomas: primary analysis of the phase II NETTER-P study. Eur. J. Nucl. Med. Mol. Imaging https://doi.org/10.1007/s00259-025-07246-7 (2025).
Sen, I., Malik, D., Thakral, P. & Schultz, M. 212Pb-VMT-α-NET targeted alpha therapy in metastatic neuroendocrine tumors: first in human study on safety and efficacy [abstract]. J. Nucl. Med. 65, 242556 (2024).
Sgouros, G. et al. 225Ac-DOTATATE dosimetry results from part 1 of the ACTION-1 trial. J. Nucl. Med. 64, P129–P129 (2023).
Reidy-Lagunes, D. et al. Phase I trial of well-differentiated neuroendocrine tumors (NETs) with radiolabeled somatostatin antagonist 177Lu-satoreotide tetraxetan. Clin. Cancer Res. 25, 6939–6947 (2019).
Wild, D. et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J. Nucl. Med. 55, 1248–1252 (2014).
Wild, D. et al. A phase I/II study of the safety and efficacy of [177Lu]Lu-satoreotide tetraxetan in advanced somatostatin receptor-positive neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 51, 183–195 (2023).
Fricke, J. et al. First-in-human administration of terbium-161-labelled somatostatin receptor subtype 2 antagonist ([161Tb]Tb-DOTA-LM3) in a patient with a metastatic neuroendocrine tumour of the ileum. Eur. J. Nucl. Med. Mol. Imaging 51, 2517–2519 (2024).
Nock, B. A., Kanellopoulos, P., Joosten, L., Mansi, R. & Maina, T. Peptide radioligands in cancer theranostics: agonists and antagonists. Pharmaceuticals 16, 674 (2023).
Dalm, S. U. et al. Comparison of the therapeutic response to treatment with a 177Lu-labeled somatostatin receptor agonist and antagonist in preclinical models. J. Nucl. Med. 57, 260–265 (2016).
Krebs, S. et al. Comparison of 68Ga-DOTA-JR11 PET/CT with dosimetric 177Lu-satoreotide tetraxetan (177Lu-DOTA-JR11) SPECT/CT in patients with metastatic neuroendocrine tumors undergoing peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 47, 3047–3057 (2020).
Somvanshi, R. K. & Kumar, U. Pathophysiology of GPCR homo- and heterodimerization: special emphasis on somatostatin receptors. Pharmaceuticals 5, 417–446 (2012).
Cantone, M. C., Dicitore, A. & Vitale, G. Somatostatin-dopamine chimeric molecules in neuroendocrine neoplasms. J. Clin. Med. 10, 501 (2021).
Liu, S. et al. EP14.01-020 trial in progress: [177Lu]Lu-DOTA-TATE combination therapy in treatment-naive extensive stage small cell lung cancer. J. Thorac. Oncol. 17, S534 (2022).
Kurz, S. C. et al. Evaluation of the SSTR2-targeted radiopharmaceutical 177Lu-DOTATATE and SSTR2-specific 68Ga-DOTATATE PET as imaging biomarker in patients with intracranial meningioma. Clin. Cancer Res. 30, 680–686 (2024).
Curium Pharma. Curium announces ECLIPSE trial has met primary endpoint, demonstrating a statistically significant and clinically meaningful benefit for patients with PSMA-positive metastatic castration resistant prostate cancer. Curium https://www.curiumpharma.com/2024/11/13/eclipse-tiral-psma-prostate-cancer/ (2024).
Kim, H. et al. PSMAddition: phase 3 trial of [177Lu]Lu-PSMA-617 plus standard of care (SoC) vs. Soc alone in patients with metastatic hormone-sensitive prostate cancer. Int. J. Radiat. Oncol. Biol., Phys. 120, e546 (2024).
Emmett, L. et al. [(177)Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 25, 563–571 (2024).
Emmett, L. et al. Overall survival and quality of life with [177Lu]Lu-PSMA-617 plus enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer (ENZA-p): secondary outcomes from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 26, 291–299 (2025).
Azad, A. A. et al. Sequential [177Lu]Lu-PSMA-617 and docetaxel versus docetaxel in patients with metastatic hormone-sensitive prostate cancer (UpFrontPSMA): a multicentre, open-label, randomised, phase 2 study. Lancet Oncol. 25, 1267–1276 (2024).
Eapen, R. S. et al. Administering [177Lu]Lu-PSMA-617 prior to radical prostatectomy in men with high-risk localised prostate cancer (LuTectomy): a single-centre, single-arm, phase 1/2 study. Eur. Urol. 85, 217–226 (2024).
Tagawa, S. T. et al. Phase 1/2 study of fractionated dose lutetium-177–labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Cancer 125, 2561–2569 (2019).
Tagawa, S. T. et al. Prostate-specific membrane antigen-targeting alpha emitter via antibody delivery for metastatic castration-resistant prostate cancer: a phase I dose-escalation study of 225Ac-J591. J. Clin. Oncol. 42, 842–851 (2024).
Pandit-Taskar, N. et al. A phase 0 study to assess the biodistribution and pharmacokinetics of a radiolabeled antibody targeting human kallikrein 2 in participants with metastatic castration-resistant prostate cancer. J. Nucl. Med. 65, 1051–1056 (2024).
Vaz, S. et al. Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 47, 9–15 (2020).
Fanti, S. et al. EAU-EANM consensus statements on the role of prostate-specific membrane antigen positron emission tomography/computed tomography in patients with prostate cancer and with respect to [177Lu]Lu-PSMA radioligand therapy. Eur. Urol. Oncol. 5, 530–536 (2022).
Lauri, C., Chiurchioni, L., Russo, V. M., Zannini, L. & Signore, A. PSMA expression in solid tumors beyond the prostate gland: ready for theranostic applications? J. Clin. Med. 11, 6590 (2022).
Baratto, L., Duan, H., Mäcke, H. & Iagaru, A. Imaging the distribution of gastrin-releasing peptide receptors in cancer. J. Nucl. Med. 61, 792–798 (2020).
Minamimoto, R. et al. Pilot comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J. Nucl. Med. 57, 557–562 (2016).
Duan, H. et al. 68Ga-RM2 PET-MRI versus MRI alone for evaluation of patients with biochemical recurrence of prostate cancer: a single-centre, single-arm, phase 2/3 imaging trial. Lancet Oncol. 25, 501–508 (2024).
Gruber, L. et al. MITIGATE-NeoBOMB1, a phase I/IIa study to evaluate safety, pharmacokinetics, and preliminary imaging of 68Ga-NeoBOMB1, a gastrin-releasing peptide receptor antagonist, in GIST patients. J. Nucl. Med. 61, 1749–1755 (2020).
Ruigrok, E. A. M. et al. Safety of [177Lu]Lu-NeoB treatment: a preclinical study characterizing absorbed dose and acute, early, and late organ toxicity. Eur. J. Nucl. Med. Mol. Imaging 49, 4440–4451 (2022).
Damiana, T. S. T. et al. Side-by-side comparison of the two widely studied GRPR radiotracers, radiolabeled NeoB and RM2, in a preclinical setting. Eur. J. Nucl. Med. Mol. Imaging 50, 3851–3861 (2023).
Nordquist, L. et al. COMBAT: a study of 64Cu-SAR-BBN and 67Cu-SAR-BBN for identification and treatment of GRPr-expressing metastatic castrate resistant prostate cancer ineligible for therapy with 177Lu-PSMA-617 [abstract TPS247]. J. Nucl. Med. 65, 241478 (2024).
Kurth, J. et al. GRPr antagonist [177Lu]Lu-AMTG for treatment of castration resistant prostate cancer: first in-human biodistribution and dosimetry [abstract]. J. Nucl. Med. 65, 242300 (2024).
Holzleitner, N. et al. Preclinical evaluation of gastrin-releasing peptide receptor antagonists labeled with 161Tb and 177Lu: a comparative study. J. Nucl. Med. 65, 481–484 (2024).
McDevitt, M. R. et al. Feed-forward alpha particle radiotherapy ablates androgen receptor-addicted prostate cancer. Nat. Commun. 9, 1629 (2018).
Morris, M. J. et al. A phase 1 study of JNJ-69086420 (JNJ-6420), an actinium-225 (225Ac)-labeled antibody targeting human kallikrein 2 (hK2), for metastatic castration-resistant prostate cancer (mCRPC) [abstract]. J. Clin. Oncol. 42, 5010 (2024).
Lezaic, L. et al. [111In]In-CP04 as a novel cholecystokinin-2 receptor ligand with theranostic potential in patients with progressive or metastatic medullary thyroid cancer: final results of a GRAN-T-MTC phase I clinical trial. Eur. J. Nucl. Med. Mol. Imaging 50, 892–907 (2023).
Klingler, M. et al. DOTA-MGS5, a new cholecystokinin-2 receptor-targeting peptide analog with an optimized targeting profile for theranostic use. J. Nucl. Med. 60, 1010–1016 (2019).
Rottenburger, C. et al. Cholecystokinin 2 receptor agonist 177Lu-PP-F11N for radionuclide therapy of medullary thyroid carcinoma: results of the lumed phase 0a study. J. Nucl. Med. 61, 520–526 (2020).
Rottenburger, C. et al. In-vivo inhibition of neutral endopeptidase 1 results in higher absorbed tumor doses of [177Lu]Lu-PP-F11N in humans: the lumed phase 0b study. EJNMMI Res. 14, 37 (2024).
Christou, N. et al. Neurotensin pathway in digestive cancers and clinical applications: an overview. Cell Death Dis. 11, 1027 (2020).
Baum, R. P. et al. (177)Lu-3BP-227 for neurotensin receptor 1-targeted therapy of metastatic pancreatic adenocarcinoma: first clinical results. J. Nucl. Med. 59, 809–814 (2018).
Liu, F. et al. Development of 177Lu-FL-091 for the treatment of NTSR1-positive cancers [abstract]. J. Nucl. Med. 65, 241875 (2024).
Sahlmann, C. O. et al. Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: safety, feasibility, and long-term efficacy results of a prospective phase 2 study. Cancer 123, 638–649 (2017).
Li, L. et al. Immuno-PET of colorectal cancer with a CEA-targeted [68 Ga]Ga-nanobody: from bench to bedside. Eur. J. Nucl. Med. Mol. Imaging 50, 3735–3749 (2023).
Xiao, Y. et al. Identification of a CEACAM5 targeted nanobody for positron emission tomography imaging and near-infrared fluorescence imaging of colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 50, 2305–2318 (2023).
Minnix, M. et al. Improved tumor responses with sequential targeted α-particles followed by interleukin 2 immunocytokine therapies in treatment of CEA-positive breast and colon tumors in CEA transgenic mice. J. Nucl. Med. 63, 1859–1864 (2022).
Akhavan, D. et al. Phase I study of yttrium-90 radiolabeled M5A anti-carcinoembryonic antigen humanized antibody in patients with advanced carcinoembryonic antigen producing malignancies. Cancer Biother Radiopharm. 35, 10–15 (2020).
Buck, A. K. et al. Imaging of C-X-C motif chemokine receptor 4 expression in 690 patients with solid or hematologic neoplasms using 68Ga-pentixafor PET. J. Nucl. Med. 63, 1687–1692 (2022).
Lapa, C. et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed multiple myeloma. Theranostics 7, 1589–1597 (2017).
Werner, R. A. et al. CXCR4-directed imaging in solid tumors. Front. Oncol. 9, 770 (2019).
Rosenblat, T. L. et al. Treatment of patients with acute myeloid leukemia with the targeted alpha-particle nanogenerator actinium-225-lintuzumab. Clin. Cancer Res. 28, 2030–2037 (2022).
Orchard, K. et al. Efficient bone marrow irradiation and low uptake by non-haematological organs with an yttrium-90-anti-CD66 antibody prior to haematopoietic stem cell transplantation. Bone Marrow Transplant. 59, 1247–1257 (2024).
Tuazon, S. A. et al. 90Y-labeled anti-CD45 antibody allogeneic hematopoietic cell transplantation for high-risk multiple myeloma. Bone Marrow Transpl. 56, 202–209 (2021).
Lindén, O. et al. 227Th-labeled anti-CD22 antibody (BAY 1862864) in relapsed/refractory CD22-positive non-Hodgkin lymphoma: a first-in-human, phase I study. Cancer Biother Radiopharm. 36, 672–681 (2021).
Kolstad, A. et al. Phase 1/2a study of 177Lu-lilotomab satetraxetan in relapsed/refractory indolent non-Hodgkin lymphoma. Blood Adv. 4, 4091–4101 (2020).
Rioja-Blanco, E. et al. 161Tb radioimmunotherapy as a treatment for CD30-positive lymphomas. J. Nucl. Med. 66, 909–915 (2025).
Gayton, J. et al. Dose escalation study to evaluate safety, dosimetry, and PD of the HER2-directed radioligand CAM-H2 in patients with advanced HER2+ breast, gastric, GEJ, and other HER2+ solid tumors (trial ID: NCT04467515) [abstract]. J. Nucl. Med. 65, 242035 (2024).
Liu, Y. et al. Evaluation of a novel 177Lu-labelled therapeutic Affibody molecule with a deimmunized ABD domain and improved biodistribution profile. Eur. J. Nucl. Med. Mol. Imaging 51, 4038–4048 (2024).
Tikum, T. et al. Effectiveness of 225Ac-labeled anti-EGFR radioimmunoconjugate in EGFR-positive kirsten rat sarcoma viral oncogene and BRAF mutant colorectal cancer models. J. Nucl. Med. https://doi.org/10.2967/jnumed.123.266204 (2024).
Ulaner, G. et al. A phase 1, first-in-human, multicentre, open-label, dose-escalation study of [225Ac]-FPI-2068 in adult patients with advanced solid tumors[abstract]. J. Nucl. Med. 65, 242181 (2024).
Li, C. et al. 177Lu-labeled bispecific antibodies targeting EGFR and c-MET as radiotheranostics in murine NSCLC tumor model [abstract]. J. Nucl. Med. 65, 241901 (2024).
Pandit-Taskar, N. et al. Dose-escalation study of [225Ac]-FPI-1434 (FPI-1434) in patients (pts) with IGF-1R expressing advanced solid tumors: preliminary pharmacology and dosimetry results [abstract]. J. Nucl. Med. 64, P630 (2023).
Feng, X. et al. Clinical translation of a 68Ga-labeled integrin αvβ6)-targeting cyclic radiotracer for PET imaging of pancreatic cancer. J. Nucl. Med. 61, 1461–1467 (2020).
Quigley, N. G. et al. PET/CT imaging of head-and-neck and pancreatic cancer in humans by targeting the “cancer integrin” αvβ6 with Ga-68-trivehexin. Eur. J. Nucl. Med. Mol. Imaging 49, 1136–1147 (2022).
Davis, R. et al. First-in-human study of the theranostic pair [68Ga]Ga DOTA-5G and [177Lu]Lu DOTA-ABM-5G in pancreatic adenocarcinoma [abstract]. J. Nucl. Med. 64, P1300 (2023).
Koivisto, L., Bi, J., Häkkinen, L. & Larjava, H. Integrin αvβ6: structure, function and role in health and disease. Int. J. Biochem. Cell Biol. 99, 186–196 (2018).
Quigley, N. G. et al. Tracking a TGF-β activator in vivo: sensitive PET imaging of αvβ8-integrin with the Ga-68-labeled cyclic RGD octapeptide trimer Ga-68-triveoctin. EJNMMI Res. 10, 133 (2020).
Kossatz, S., Beer, A. J. & Notni, J. It’s time to shift the paradigm: translation and clinical application of non-αvβ3 integrin targeting radiopharmaceuticals. Cancers 13, 5958 (2021).
Immunomedics. Immunomedics provides update on phase 3 PANCRIT-1 trial of clivatuzumab tetraxetan in patients with advanced pancreatic cancer. MarketScreener https://www.marketscreener.com/quote/stock/IMMUNOMEDICS-INC-9671/news/Immunomedics-Inc-Provides-Update-on-Phase-3-PANCRIT-1-Trial-of-Clivatuzumab-Tetraxetan-in-Patients-38065612/ (2016).
Hull, A. et al. The expression profile and textural characteristics of C595-reactive MUC1 in pancreatic ductal adenocarcinoma for targeted radionuclide therapy. Cancers 13, 61 (2020).
Hull, A. et al. In vitro characterisation of [177Lu]Lu-DOTA-C595 as a novel radioimmunotherapy for MUC1-CE positive pancreatic cancer. EJNMMI Radiopharm. Chem. 8, 18 (2023).
Mack, K. N. et al. Interrogating the theranostic capacity of a MUC16-targeted antibody for ovarian cancer. J. Nucl. Med. 65, 580–585 (2024).
Broer, L. N. et al. 89Zr-3,2-HOPO-mesothelin antibody PET imaging reflects tumor uptake of mesothelin-targeted 227Th-conjugate therapy in mice. J. Nucl. Med. 63, 1715–1721 (2022).
Lu, Z. et al. Preclinical evaluation of 89Zr/177Lu-labeled amatuximab for theranostic application in pancreatic ductal adenocarcinoma. Int. J. Pharm. 667, 124946 (2024).
Wang, X. et al. Construction and preclinical evaluation of 211At labeled anti-mesothelin antibodies as potential targeted alpha therapy drugs. J. Radiat. Res. 61, 684–690 (2020).
Bayer. An open-label, first-in-human, multi-center study to evaluate the safety, tolerability, pharmacokinetics and anti-tumor activity of a thorium-227 labeled antibody-chelator conjugate, BAY2287411 injection, in patients with tumors known to express mesothelin. Bayer https://clinicaltrials.bayer.com/study/18795 (2023).
Guzik, P. et al. Promising potential of [(177)Lu]Lu-DOTA-folate to enhance tumor response to immunotherapy — a preclinical study using a syngeneic breast cancer model. Eur. J. Nucl. Med. Mol. Imaging 48, 984–994 (2021).
Müller, C. et al. Direct in vitro and in vivo comparison of 161Tb and 177Lu using a tumour-targeting folate conjugate. Eur. J. Nucl. Med. Mol. Imaging 41, 476–485 (2014).
Zhang, C. et al. Preclinical melanoma imaging with 68Ga-labeled α-melanocyte-stimulating hormone derivatives using PET. Theranostics 7, 805–813 (2017).
Wharton, L. et al. Preclinical evaluation of MC1R targeting theranostic pair [155Tb]Tb-crown-αMSH and [161Tb]Tb-crown-αMSH. Nucl. Med. Biol. 136-137, 108925 (2024).
Zhang, C. et al. 177Lu melanoma therapy with a novel, metabolically stabilized MC1R-targeting ligand in a human melanoma model [abstract]. J. Nucl. Med. 63, 2872 (2022).
Orcutt, K. D. et al. Dosimetry of [212Pb]VMT01, a MC1R-targeted alpha therapeutic compound, and effect of free 208Tl on tissue absorbed doses. Molecules 27, 5831 (2022).
Morris, Z. S. et al. Interim safety and efficacy data of 212Pb-VMT01 in MC1R expressing melanoma [abstract]. J. Clin. Oncol. 43, 3099 (2025).
Khushalani, N. I. et al. Preliminary results of first-in-human study of 225-actinium MTI-201 (225Ac-MTI-201) in metastatic uveal melanoma [abstract 1128P]. Ann. Oncol. 35, S742 (2024).
Zhang, J. et al. Translational PET imaging of nectin-4 expression in multiple different cancers with 68Ga-N188. J. Nucl. Med. 65, 12S–18S (2024).
Esposito, T. V. et al. Adaptation of the antibody-drug conjugate PADCEV into an ImmunoPET agent against Nectin-4 [abstract]. J. Nucl. Med. 65, 242152 (2024).
Babeker, H. et al. 225Ac/89Zr-labeled N4MU01 radioimmunoconjugates as theranostics against nectin-4–positive triple-negative breast cancer. J. Nucl. Med. 66, 592–598 (2025).
Sathekge, M. et al. 10 Oral: AKY-1189, a novel, first-in-class miniprotein radiopharmaceutical designed to deliver Actinium-225 (225Ac) to nectin-4 expressing tumors with broad therapeutic applications in metastatic urothelial carcinoma (mUC) and other Nectin-4 expressing tumors. Eur. J. Cancer 211, 114539 (2024).
Aktis Oncology. Aktis Oncology initiates phase 1b clinical trial of its nectin-4-targeting radiopharmaceutical product candidate, AKY-1189, across multiple tumor types. Aktis Oncology https://www.aktisoncology.com/news/aktis-initiates-clinical-trial-of-potential-nectin-4-targeting-radiopharmaceutical-product-candidate/ (2025).
Ahn, M.-J. et al. Tarlatamab for patients with previously treated small-cell lung cancer. N. Engl. J. Med. 389, 2063–2075 (2023).
Tendler, S. et al. Imaging with [89Zr]Zr-DFO-SC16.56 anti-DLL3 antibody in patients with high-grade neuroendocrine tumours of the lung and prostate: a phase 1/2, first-in-human trial. Lancet Oncol. 25, 1015–1024 (2024).
Tully, K. M. et al. Radioimmunotherapy targeting delta-like ligand 3 in small cell lung cancer exhibits antitumor efficacy with low toxicity. Clin. Cancer Res. 28, 1391–1401 (2022).
Korsen, J. A. et al. Delta-like ligand 3-targeted radioimmunotherapy for neuroendocrine prostate cancer. Proc. Natl Acad. Sci. USA 119, e2203820119 (2022).
Lizak, C. et al. Lead-212 radio-DARPin therapeutic (RDT) targeting delta-like ligand 3 (DLL3) shows promising preclinical antitumor efficacy and tolerability in small cell lung cancer (SCLC) [abstract]. J. Nucl. Med. 65, 241995 (2024).
Baum, R. P. et al. Systemic endoradiotherapy with carrier-added 4-[131I]iodo-L-phenylalanine: clinical proof-of-principle in refractory glioma. Nucl. Med. Mol. Imaging 45, 299–307 (2011).
Pichler, J. et al. Results from a phase I study of 4-l-[131I]iodo-phenylalanine ([131I]IPA) with external radiation therapy in patients with recurrent glioblastoma (IPAX-1). Neurooncol. Adv. 6, vdae130 (2024).
Telix Pharmaceuticals. FDA grants orphan drug designation for TLX102. Telix https://telixpharma.com/news-views/fda-grants-orphan-drug-designation-for-tlx102/ (2020).
Królicki, L. et al. Dose escalation study of targeted alpha therapy with [225Ac]Ac-DOTA-substance P in recurrence glioblastoma — safety and efficacy. Eur. J. Nucl. Med. Mol. Imaging 48, 3595–3605 (2021).
Królicki, L. et al. Safety and efficacy of targeted alpha therapy with 213Bi-DOTA-substance P in recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 46, 614–622 (2019).
Kramer, K. et al. A phase II study of radioimmunotherapy with intraventricular 131I-3F8 for medulloblastoma. Pediatr. Blood Cancer https://doi.org/10.1002/pbc.26754 (2018).
Liatsou, I. et al. Therapeutic efficacy of an alpha-particle emitter labeled anti-GD2 humanized antibody against osteosarcoma — a proof of concept study. Eur. J. Nucl. Med. Mol. Imaging 51, 1409–1420 (2024).
Santich, B. H. et al. A self-assembling and disassembling (SADA) bispecific antibody (BsAb) platform for curative two-step pretargeted radioimmunotherapy. Clin. Cancer Res. 27, 532–541 (2021).
Radiopharm Theranostics. Radiopharm Theranostics and Cyclotek sign clinical supply agreement for 161Tb-KLK3-mAb phase I clinical trial in Australia. GlobeNewswire https://www.globenewswire.com/news-release/2025/06/24/3104176/0/en/Radiopharm-Theranostics-and-Cyclotek-Sign-Clinical-Supply-Agreement-for-Tb-KLK3-mAb-Phase-I-Clinical-Trial-in-Australia.html (2025).
Striese, F. et al. Preclinical characterization of the 177Lu-labeled prostate stem cell antigen (PSCA)-specific monoclonal antibody 7F5. Int. J. Mol. Sci. 24, 9420 (2023).
Carrasquillo, J. A. et al. Imaging patients with metastatic castration-resistant prostate cancer using 89Zr-DFO-MSTP2109A anti-STEAP1 antibody. J. Nucl. Med. 60, 1517–1523 (2019).
Shitara, K. et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 401, 1655–1668 (2023).
Wang, Y. et al. Preparation of radiolabeled zolbetuximab targeting CLDN18.2 and its preliminary evaluation for potential clinical applications. Mol. Pharm. 21, 3838–3847 (2024).
Qi, C. et al. 68Ga-NC-BCH whole-body PET imaging rapidly targets claudin18.2 in lesions in gastrointestinal cancer patients. J. Nucl. Med. 65, 856–863 (2024).
Zeng, Z. et al. [177Lu]Lu-labeled anti-claudin-18.2 antibody demonstrated radioimmunotherapy potential in gastric cancer mouse xenograft models. Eur. J. Nucl. Med. Mol. Imaging 51, 1221–1232 (2024).
Li, D. et al. Exploration of radionuclide labeling of a novel scFv-Fc fusion protein targeting CLDN18.2 for tumor diagnosis and treatment. Eur. J. Med. Chem. 266, 116134 (2024).
Zeng, Z. et al. On the shoulder of ADC: the development of 124I-IMMU-132, an iodine-124-labelled Trop-2-targeting molecular probe for micro-PET imaging. Biomed. Pharmacother. 178, 117151 (2024).
Huang, W. et al. ImmunoPET imaging of Trop2 in patients with solid tumours. EMBO Mol. Med. 16, 1143–1161 (2024).
Chen, H. et al. 68Ga-MY6349 PET/CT imaging to assess Trop2 expression in multiple types of cancer. J. Clin. Invest. https://doi.org/10.1172/JCI185408 (2024).
Wu, Y. et al. Preclinical evaluation of the theranostic potential of 89Zr/177Lu-labeled anti-TROP-2 antibody in triple-negative breast cancer model. EJNMMI Radiopharm. Chem. 9, 5 (2024).
Yamaguchi, A. et al. 177Lu-labeled antibody–drug conjugate: a dual-mechanistic treatment modality in solid tumors. Mol. Cancer Ther. 24, 907–919 (2025).
Steffin, D. et al. Interleukin-15-armoured GPC3 CAR T cells for patients with solid cancers. Nature 637, 940–946 (2025).
Braat, A. et al. [68Ga]Ga-RYZ-GPC3; a glypican-3 targeted diagnostic radiopharmaceutical for hepatocellular carcinoma molecular imaging. A future game-changer in HCC? [abstract]. J. Nucl. Med. 65, 241739 (2024).
Poot, A. J. et al. [68Ga]Ga-RAYZ-8009: a glypican-3-targeted diagnostic radiopharmaceutical for hepatocellular carcinoma molecular imaging-a first-in-human case series. J. Nucl. Med. 65, 1597–1603 (2024).
Lin, F. et al. Peptide binder to glypican-3 as a theranostic agent for hepatocellular carcinoma. J. Nucl. Med. 65, 586–592 (2024).
Liu, Y. et al. 99mTc-labeled SWL specific peptide for targeting EphA2 receptor. Nucl. Med. Biol. 41, 450–456 (2014).
El Fakiri, M. et al. Development and preclinical characterization of a novel radiotheranostic EphA2-targeting bicyclic peptide. Theranostics 14, 4701–4712 (2024).
Sharma, A. K. et al. EphA2-targeted alpha-particle theranostics for enhancing PDAC treatment. Theranostics 15, 4229–4246 (2025).
Chan, C. Y. et al. [123I]CC1: a PARP-targeting, auger electron-emitting radiopharmaceutical for radionuclide therapy of cancer. J. Nucl. Med. 64, 1965–1971 (2023).
Makvandi, M. et al. Targeting PARP-1 with alpha-particles is potently cytotoxic to human neuroblastoma in preclinical models. Mol. Cancer Ther. 18, 1195–1204 (2019).
Welt, S. et al. Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J. Clin. Oncol. 12, 1193–1203 (1994).
Fischer, E. et al. Radioimmunotherapy of fibroblast activation protein positive tumors by rapidly internalizing antibodies. Clin. Cancer Res. 18, 6208–6218 (2012).
Jansen, K. et al. Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J. Med. Chem. 57, 3053–3074 (2014).
Lindner, T. et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med. 59, 1415–1422 (2018).
Watabe, T. et al. Theranostics targeting fibroblast activation protein in the tumor stroma: 64Cu- and 225Ac-labeled FAPI-04 in pancreatic cancer xenograft mouse models. J. Nucl. Med. 61, 563–569 (2020).
Kratochwil, C. et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 60, 801–805 (2019).
Loktev, A. et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J. Nucl. Med. 60, 1421–1429 (2019).
Liu, Y. et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. Mol. Imaging 49, 871–880 (2022).
Fendler, W. P. et al. Safety and efficacy of 90Y-FAPI-46 radioligand therapy in patients with advanced sarcoma and other cancer entities. Clin. Cancer Res. 28, 4346–4353 (2022).
McConathy, J. et al. 177Lu-FAP-2286 in patients with advanced or metastatic solid tumors: initial data from a phase 1/2 study investigating safety, pharmacokinetics, dosimetry, and preliminary antitumor activity (LuMIERE). J. Nucl. Med. 63, 2271–2271 (2022).
Juneau, D. et al. FRONTIER: FAPi radioligand OpeN-label, phase 1 study to evaluate safety, tolerability and dosImetry of [Lu-177]-PNT6555; a dose escalation study for tReatment of patients with select solid tumors [abstract]. J. Nucl. Med. 65, 241162 (2024).
Martin, M. et al. Novel generation of FAP inhibitor-based homodimers for improved application in radiotheranostics. Cancers 15, 1889 (2023).
Kurth, J. et al. Fibroblast activation protein-targeted radionuclide therapy of solid tumors using [177Lu]Lu-DOTAGA-Glu-(FAPi)2: biodistribution and dosimetry [abstract]. J. Nucl. Med. 65, 242022 (2024).
Galbiati, A. et al. Preclinical evaluation of 177Lu-OncoFAP-23, a multivalent FAP-targeted radiopharmaceutical therapeutic for solid tumors. J. Nucl. Med. 65, 1604–1610 (2024).
Fu, H. et al. 177Lu-LNC1004 radioligand therapy in patients with end-stage metastatic cancers: a single-center, single-arm, phase II study. Clin. Cancer Res. 31, 1415–1426 (2025).
Reschke, M. et al. FXX489, a FAP targeting ligand with best-in-class potential for radioligand therapy [abstract]. Cancer Res. 85, ND07 (2025).
Geva, R. et al. Phase I open-label, multi-center study to evaluate the safety, tolerability, dosimetry, and preliminary activity of FXX489 ([177Lu]Lu-NNS309) in patients with pancreatic, lung, breast and colorectal cancers [abstract]. Cancer Res. 85, CT112 (2025).
Storey, C. M. et al. Development of a LRRC15-targeted radio-immunotheranostic approach to deplete pro-tumorigenic mechanisms and immunotherapy resistance. Preprint at bioRxiv https://doi.org/10.1101/2024.01.30.577289 (2024).
Reardon, D. A. et al. Phase II trial of murine 131I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. 20, 1389–1397 (2002).
Aloj, L. et al. Radioimmunotherapy with tenarad, a 131I-labelled antibody fragment targeting the extra-domain A1 of tenascin-C, in patients with refractory Hodgkin’s lymphoma. Eur. J. Nucl. Med. Mol. Imaging 41, 867–877 (2014).
Virotta, G. et al. Radioimmunotherapy with 131I-L19SIP (radretumab) in metastatic solid tumors: preliminary results. J. Nucl. Med. 53, 498–498 (2012).
Gao, S. et al. Synthesis and assessment of ZD2-(68Ga-NOTA) specific to extradomain B fibronectin in tumor microenvironment for PET imaging of pancreatic cancer. Am. J. Nucl. Med. Mol. Imaging 9, 216–229 (2019).
Jailkhani, N. et al. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl Acad. Sci. USA 116, 14181–14190 (2019).
Carlsen, E. A. et al. Prospective phase II trial of prognostication by 68Ga-NOTA-AE105 uPAR PET in patients with neuroendocrine neoplasms: implications for uPAR-targeted therapy. J. Nucl. Med. 63, 1371–1377 (2022).
Azam, A. et al. Prospective phase II trial of [68Ga]Ga-NOTA-AE105 uPAR-PET/MRI in patients with primary gliomas: prognostic value and implications for uPAR-targeted radionuclide therapy. EJNMMI Res. 14, 100 (2024).
Persson, M. et al. uPAR targeted radionuclide therapy with 177Lu-DOTA-AE105 inhibits dissemination of metastatic prostate cancer. Mol. Pharm. 11, 2796–2806 (2014).
Chen, Z. et al. 177Lu-labeled aflibercept for theranostic application in renal cancer models [abstract]. J. Nucl. Med. 64, P633 (2023).
Steiger, K. et al. There is a world beyond αvβ3-integrin: multimeric ligands for imaging of the integrin subtypes αvβ6, αvβ8, αvβ3, and α5β1 by positron emission tomography. EJNMMI Res. 11, 106 (2021).
Chen, Z., Han, F., Du, Y., Shi, H. & Zhou, W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal. Transduct. Target. Ther. 8, 70 (2023).
Mittal, S. & Mallia, M. B. Molecular imaging of tumor hypoxia: evolution of nitroimidazole radiopharmaceuticals and insights for future development. Bioorg. Chem. 139, 106687 (2023).
Kudo, T. et al. Imaging of HIF-1-active tumor hypoxia using a protein effectively delivered to and specifically stabilized in HIF-1-active tumor cells. J. Nucl. Med. 50, 942–949 (2009).
Ueda, M. et al. Development of an oxygen-sensitive degradable peptide probe for the imaging of hypoxia-inducible factor-1-active regions in tumors. Mol. Imaging Biol. 15, 713–721 (2013).
Shuch, B. et al. [89Zr]Zr-girentuximab for PET-CT imaging of clear-cell renal cell carcinoma: a prospective, open-label, multicentre, phase 3 trial. Lancet Oncol. 25, 1277–1287 (2024).
Muselaers, C. H. et al. Phase 2 study of lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur. Urol. 69, 767–770 (2016).
Chafe, S. C. et al. Targeting hypoxia-induced carbonic anhydrase IX enhances immune-checkpoint blockade locally and systemically. Cancer Immunol. Res. 7, 1064–1078 (2019).
Kleinendorst, S. C. et al. Towards effective CAIX-targeted radionuclide and checkpoint inhibition combination therapy for advanced clear cell renal cell carcinoma. Theranostics 14, 3693–3707 (2024).
Ferldman, D. R. STARLITE 2: phase 2 study of nivolumab plus 177Lutetium-labeled anti-carbonic anhydrase IX (CAIX) monoclonal antibody girentuximab (177Lu-girentuximab) in patients (pts) with advanced clear cell renal cell carcinoma (ccRCC) [abstract]. J. Clin. Oncol. 40, TPS460 (2022).
Hasanov, E. et al. STARLITE 1: phase 1b/2 study of combination 177Lu girentuximab plus cabozantinib and nivolumab in treatment naïve patients with advanced clear cell RCC [abstract]. Oncologist 28, S13 (2023).
Merkx, R. I. J. et al. Carbonic anhydrase IX-targeted α-radionuclide therapy with 225Ac inhibits tumor growth in a renal cell carcinoma model. Pharmaceuticals 15, 570 (2022).
Massière, F. et al. Preclinical characterization of DPI-4452: a 68Ga/177Lu theranostic ligand for carbonic anhydrase IX. J. Nucl. Med. 65, 761–767 (2024).
He, H. et al. Imaging diagnosis and efficacy monitoring by [89Zr]Zr-DFO-KN035 immunoPET in patients with PD-L1-positive solid malignancies. Theranostics 14, 392–405 (2024).
Luna-Gutiérrez, M. et al. Lutetium-177 labeled iPD-L1 as a novel immunomodulator for cancer-targeted radiotherapy. EJNMMI Radiopharm. Chem. 10, 5 (2025).
Kramer, K. et al. Phase 1 study of intraventricular 131I-omburtamab targeting B7H3 (CD276)-expressing CNS malignancies. J. Hematol. Oncol. 15, 165 (2022).
Prasad, K. et al. Feasibility of safe outpatient treatment in pediatric patients following intraventricular radioimmunotherapy with 131I-omburtamab for leptomeningeal disease. EJNMMI Res. 14, 70 (2024).
Khatua, S., Düring, M., Lind, C. L. & Nielsen, J. R. A phase I/II dose-escalation and expansion cohort study of intracerebroventricular radioimmunotherapy using 177Lu-DTPA-omburtamab in pediatric and adolescent patients with recurrent or refractory medulloblastoma [abstract 380TiP]. Ann. Oncol. 32, S528 (2021).
Glazer, S. et al. A novel high affinity anti-B7H3 antibody selective for the 4Ig-B7H3 isoform when labeled with yttrium-90 provides long term survivors for established radioresistant colorectal carcinoma when administered as a single-dose treatment [abstract]. J. Nucl. Med. 64, P1021 (2023).
Jiao, X., Hong, H. & Cai, W. Nanoscale radiotheranostics for cancer treatment: from bench to bedside. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 16, e2006 (2024).
Cui, X.-Y. et al. Covalent targeted radioligands potentiate radionuclide therapy. Nature 630, 206–213 (2024).
Li, Y. et al. Dual targeting PET tracer [68Ga]Ga-PSFA-01 in patients with prostate cancers: a pilot exploratory study. Theranostics 15, 4124–4134 (2025).
Bodei, L. et al. Toward individualized dosimetry for radiopharmaceutical therapy in day-to-day clinical practice of nuclear oncology: overcoming heterogeneity of radiation-absorbed dose to tumor and critical organs. Eur. J. Nucl. Med. Mol. Imaging 51, 325–329 (2024).
Del Prete, M. et al. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur. J. Nucl. Med. Mol. Imaging 46, 728–742 (2019).
Maccauro, M. et al. The LUTADOSE trial: tumour dosimetry after the first administration predicts progression free survival in gastro-entero-pancreatic neuroendocrine tumours (GEP NETs) patients treated with [177Lu]Lu-DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 52, 291–304 (2024).
Bodei, L. et al. Dosimetry of [177Lu]Lu-DOTATATE in patients with advanced midgut neuroendocrine tumors: results from a substudy of the phase III NETTER-1 trial. J. Nucl. Med. 66, 449–456 (2025).
Violet, J. et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J. Nucl. Med. 60, 517–523 (2019).
Herrmann, K. et al. Renal and multiorgan safety of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer in the VISION dosimetry substudy. J. Nucl. Med. 65, 71–78 (2024).
Mauguen, A. et al. The use of single-timepoint images to link administered radioiodine activity (MBq) to a prescribed lesion radiation-absorbed dose (cGy): a regression-based prediction interval tool for the management of well-differentiated thyroid cancer patients. Eur. J. Nucl. Med. Mol. Imaging 50, 2971–2983 (2023).
Jewell, K., Kostos, L., Emmerson, B. & Hofman, M. S. Combination strategies and targeted radionuclide therapies. Semin. Nucl. Med. 54, 612–621 (2024).
Guenter, R. et al. Overexpression of somatostatin receptor type 2 in neuroendocrine tumors for improved Ga68-DOTATATE imaging and treatment. Surgery 167, 189–196 (2020).
Emmett, L. et al. Rapid modulation of PSMA expression by androgen deprivation: serial 68Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. J. Nucl. Med. 60, 950–954 (2019).
Batra, J. S. et al. Phase I trial of docetaxel plus lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Urol. Oncol. 38, 848.e9–848.e16 (2020).
Kostos L. et al. Clinical trial protocol for LuCAB: a phase I-II trial evaluating cabazitaxel in combination with [(177)Lu]Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. J. Nucl. Med. 66, 572–578 (2025).
Sandhu, S. et al. LuPARP: phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC) [abstract]. J. Clin. Oncol. 41, 5005 (2023).
Hallqvist, A. et al. 177Lu-DOTATATE in combination with PARP inhibitor olaparib is feasible in patients with somatostatin-positive tumors: results from the LuPARP phase I trial. J. Nucl. Med. 66, 707–712 (2025).
Kerr, C. P. et al. Developments in combining targeted radionuclide therapies and immunotherapies for cancer treatment. Pharmaceutics 15, 128 (2022).
Aggarwal, R. et al. Single-dose 177Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: an open-label, dose-expansion, phase 1 trial. Lancet Oncol. 24, 1266–1276 (2023).
Sandhu, S. et al. 177Lu-PSMA-617 with ipilimumab (ipi) and nivolumab (nivo) in metastatic castration-resistant prostate cancer (mCRPC): an investigator-initiated phase 2 trial (EVOLUTION; ANZUP2001) [abstract]. J. Clin. Oncol. 43, 5016 (2025).
Kostos, L. et al. AlphaBet: combination of radium-223 and [177Lu]Lu-PSMA-I&T in men with metastatic castration-resistant prostate cancer (clinical trial protocol). Front. Med. 9, 1059122 (2022).
Bushnell, D. L. et al. Addition of 131I-MIBG to PRRT (90Y-DOTATOC) for personalized treatment of selected patients with neuroendocrine tumors. J. Nucl. Med. 62, 1274–1277 (2021).
Fizazi, K. et al. Final overall survival and safety analyses of the phase 3 PSMAfore trial of [177Lu]Lu-PSMA-617 versus change of androgen receptor pathway inhibitor in taxane-naive patients with metastatic castration-resistant prostate cancer. Ann. Oncol. https://doi.org/10.1016/j.annonc.2025.07.003 (2025).
Basu, E. et al. Compartmental radioimmunotherapy (cRIT) 131I-OMBURTAMAB in patients with neuroblastoma (NB) central nervous system (CNS) and/or leptomeningeal (LM) metastases: updated results from pivotal trial 101 [abstract LBA3]. Immunooncol. Technol. 16, 100364 (2022).
Acknowledgements
The authors thank all the peer reviewers for their constructive and valuable feedback. They also sincerely thank C. Deroose and W. Fendler for proofreading the manuscript, and S. Himmen for providing images for Fig. 1. The research of I.P. and K.T. is supported by the Accelerate.EU and Thera4Care consortiums through the Innovative Health Initiative Joint Undertaking, under Grant Agreements no. 101173001 and no. 101172788, respectively. A.T. acknowledges the support of an Emmy Noether Award from the German Research Foundation (DFG, 467788900) and the Ministry of Culture and Science of the State of North Rhine-Westphalia (NRW-Nachwuchsgruppenprogramm). A.T. acknowledges the support of an ERC starting grant (METATARGET, 101078355). A.T. holds the Peter Hans Hofschneider endowed Professorship of Molecular Medicine from the Stiftung Experimentelle Biomedizin. The research of K.H. is supported by the ILLUMINATE and Thera4Care consortiums through the Innovative Health Initiative Joint Undertaking, under Grant Agreements no. 101172722 and no. 101172788, respectively.
Author information
Authors and Affiliations
Contributions
I.P. researched data for the article. All authors contributed substantially to discussion of the content, and wrote, reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.T. has received honoraria as a speaker from Danone and MSD. K.H. has received consultant fees from Actithera, Advanced Accelerator Applications, a Novartis company, Amgen, AstraZeneca, Bain Capital, Bayer, Boston Scientific, Convergent, Curium, Debiopharm, EcoR1, Fusion, GE Healthcare, Immedica, Isotopen Technologien München, Janssen, Merck, Molecular Partners, MSD, NVision, Pentixapharm, Pfizer, POINT Biopharma, Radiopharm Theranostics, Rhine Pharma, Siemens Healthineers, Sofie Biosciences, Telix, Theragnostics and Ymabs; research grants from Advanced Accelerator Applications, a Novartis company, Boston Scientific and Janssen; and has stock or other ownership interests with AdvanCell, Aktis Oncology, Convergent, NVision, Sofie Biosciences and Yellowbird Diagnostics. I.P., K.T. and S.B. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks R. Hicks, M. Sathekge and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Primac, I., Tabury, K., Tasdogan, A. et al. The molecular blueprint of targeted radionuclide therapy. Nat Rev Clin Oncol 22, 869–894 (2025). https://doi.org/10.1038/s41571-025-01069-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01069-z