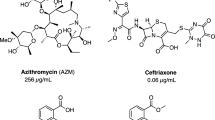
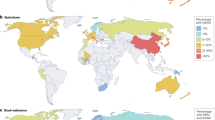
Abstract
The bacterium Neisseria gonorrhoeae causes the sexually transmitted infection (STI) gonorrhoea, which has an estimated global annual incidence of 86.9 million adults. Gonorrhoea can present as urethritis in men, cervicitis or urethritis in women, and in extragenital sites (pharynx, rectum, conjunctiva and, rarely, systemically) in both sexes. Confirmation of diagnosis requires microscopy of Gram-stained samples, bacterial culture or nucleic acid amplification tests. As no gonococcal vaccine is available, prevention relies on promoting safe sexual behaviours and reducing STI-associated stigma, which hinders timely diagnosis and treatment thereby increasing transmission. Single-dose systemic therapy (usually injectable ceftriaxone plus oral azithromycin) is the recommended first-line treatment. However, a major public health concern globally is that N. gonorrhoeae is evolving high levels of antimicrobial resistance (AMR), which threatens the effectiveness of the available gonorrhoea treatments. Improved global surveillance of the emergence, evolution, fitness, and geographical and temporal spread of AMR in N. gonorrhoeae, and improved understanding of the pharmacokinetics and pharmacodynamics for current and future antimicrobials in the treatment of urogenital and extragenital gonorrhoea, are essential to inform treatment guidelines. Key priorities for gonorrhoea control include strengthening prevention, early diagnosis, and treatment of patients and their partners; decreasing stigma; expanding surveillance of AMR and treatment failures; and promoting responsible antimicrobial use and stewardship. To achieve these goals, the development of rapid and affordable point-of-care diagnostic tests that can simultaneously detect AMR, novel therapeutic antimicrobials and gonococcal vaccine(s) in particular is crucial.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Edwards, J. L., Shao, J. Q., Ault, K. A. & Apicella, M. A. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect. Immun. 68, 5354–5363 (2000).
Evans, B. A. Ultrastructural study of cervical gonorrhea. J. Infect. Dis. 136, 248–255 (1977).
Barlow, D. & Phillips, I. Gonorrhoea in women. Diagnostic, clinical, and laboratory aspects. Lancet 1, 761–764 (1978).
Schmale, J. D., Martin, J. E. Jr & Domescik, G. Observations on the culture diagnosis of gonorrhea in women. JAMA 210, 312–314 (1969).
Quillin, S. J. & Seifert, H. S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 16, 226–240 (2018). This review discusses sex-related symptomatic gonorrhoea and provides a detailed overview of the bacterial factors, on molecular levels, that are important for the different stages of pathogenesis, including transmission, colonization and immune evasion.
Elias, J. F. & Vogel, U. in Manual of Clinical Microbiology 12th edn Vol. 1(eds Carroll, C. C. et al.) 640–655 (American Society for Microbiology, 2019).
Adeolu, M. & Gupta, R. S. Phylogenomics and molecular signatures for the order Neisseriales: proposal for division of the order Neisseriales into the emended family Neisseriaceae and Chromobacteriaceae fam. nov. Antonie van Leeuwenhoek 104, 1–24 (2013).
Tønjum, T. & van Putten, J. in Infectious Diseases 4th edn (eds Cohen, J., Powderly, W. G. & Steven M. Opal) 1553-1564 (Elsevier, 2016).
Liu, G., Tang, C. M. & Exley, R. M. Non-pathogenic Neisseria: members of an abundant, multi-habitat, diverse genus. Microbiology 161, 1297–1312 (2015).
Johnson, A. P. The pathogenic potential of commensal species of Neisseria. J. Clin. Pathol. 36, 213–223 (1983).
Seifert, H. S. Location, location, location — commensalism, damage and evolution of the pathogenic Neisseria. J. Mol. Biol 431, 3010–3014 (2019).
Hook, E. W. 3rd & Handsfield, H. H. in Sexually Transmitted Diseases (eds Holmes, K. K. et al.) 4th edn, 627–645 (McGraw-Hill Education, 2008). This comprehensive chapter describes different clinical manifestations of gonorrhoea.
Public Health Agency of Canada. Canadian Guidelines on Sexually Transmitted Infections — Management and treatment of specific infections — Gonococcal Infections (Government of Canada, Ottawa, 2013) (modified Sept 2017).
World Health Organization. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria gonorrhoeae (World Health Organization, 2012).
Wi, T. et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med. 14, e1002344 (2017).
Unemo, M. & Shafer, W. M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin. Microbiol. Rev. 27, 587–613 (2014). This review provides an extensive overview regarding gonorrhoea treatment regimens and emerging antimicrobial resistance, including genetic and phenotypic AMR determinants.
Wadsworth, C. B., Arnold, B. J., Sater, M. R. A. & Grad, Y. H. Azithromycin resistance through interspecific acquisition of an epistasis-dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae. mBio 9, e01419–18 (2018).
Rouquette-Loughlin, C. E. et al. Mechanistic basis for decreased antimicrobial susceptibility in a clinical isolate of Neisseria gonorrhoeae possessing a mosaic-like mtr efflux pump locus. mBio 9, e02281–18 (2018).
Kunz, A. N. et al. Impact of fluoroquinolone resistance mutations on gonococcal fitness and in vivo selection for compensatory mutations. J. Infect. Dis. 205, 1821–1829 (2012).
Warner, D. M., Folster, J. P., Shafer, W. M. & Jerse, A. E. Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J. Infect. Dis. 196, 1804–1812 (2007).
Warner, D. M., Shafer, W. M. & Jerse, A. E. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol. Microbiol. 70, 462–478 (2008).
Rowley, J. et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull. World Health Organ. 97, 548–562P (2019).
Adler, M., Foster, S., Richens, J. & Slavin, H. Sexual Health and Care. Sexually Transmitted Infections, Guidelines for Prevention and Treatment. Health and Population Division Occasional Paper 136 (Overseas Development Administration, London, 1996).
Aral, S. O. et al. in Sexually Transmitted Diseases, 4th edn (eds Holmes, K. K. et al.) 54–92 (McGraw-Hill, 2008).
Dallabetta, G. A., Laga, M. & Lamptey, P. R. Control of Sexually Transmitted Diseases: A Handbook for the Design and Management of Programs (AIDSCAP/Family Health International, 1996).
Aral, S. O., Fenton, K. A. & Holmes, K. K. Sexually transmitted diseases in the USA: temporal trends. Sex. Transm. Infect. 83, 257–266 (2007).
Fenton, K. A. & Lowndes, C. M. Recent trends in the epidemiology of sexually transmitted infections in the European union. Sex. Transm. Infect. 80, 255–263 (2004).
Mohammed, H. et al. 100 years of STIs in the UK: a review of national surveillance data. Sex. Transm. Infect. 94, 553–558 (2018).
Centers for Disease Control and Prevention. Tracking the hidden epidemics, trends in STDs in the United States 2000. CDC www.cdc.gov/std/trends2000/trends2000.pdf (2000).
Centers for Disease Control and Prevention. STDs in men who have sex with men. CDC https://www.cdc.gov/std/stats17/msm.htm. (2017).
Centers for Disease Control and Prevention. New CDC analysis shows steep and sustained increases in STDs in recent years. CDC https://www.cdc.gov/media/releases/2018/p0828-increases-in-stds.html (2018).
Centers for Disease Control and Prevention. Gonorrhea. CDC https://www.cdc.gov/std/stats17/gonorrhea.htm (2017).
European Centre for Disease Prevention and Control Surveillance Atlas of Infectious Diseases. Surveillance atlas of infectious diseases. ECDC https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases (2017).
Public Health England. Health Protection Report volume 12 issue 20: news (8 June). PHE https://www.gov.uk/government/publications/health-protection-report-volume-12-2018/hpr-volume-12-issue-20-news-8-june (2018).
Torrone, E. A. et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med. 15, e1002511 (2018).
Dehne, K. L. et al. A survey of STI policies and programmes in Europe: preliminary results. Sex. Transm. Infect. 78, 380–384 (2002).
Kojima, N., Davey, D. J. & Klausner, J. D. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. AIDS 30, 2251–2252 (2016).
Traeger, M. W. et al. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA 321, 1380–1390 (2019).
World Health Organization. Prevention and control of seually transmittted infections (STIs) in the era of oral pre-exposure prophylaxis (PrEP) for HIV. Technical Brief. WHO https://apps.who.int/iris/bitstream/handle/10665/325908/WHO-CDS-HIV-19.9-eng.pdf?ua=1 (2019).
Celum C. Oral pre-exposure prophylaxis (PrEP) for prevention [MOSA3401]. 22nd International AIDS Conference (AIDS 2018) http://programme.aids2018.org/People/PeopleDetailStandalone/7599 (2018).
McCormack, S. et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 387, 53–60 (2016).
Morse, S. A. Neisseria gonorrhoeae: physiology and metabolism. Sex. Transm. Dis. 6, 28–37 (1979).
Rohde, K. H. & Dyer, D. W. Mechanisms of iron acquisition by the human pathogens Neisseria meningitidis and Neisseria gonorrhoeae. Front. Biosci. 8, d1186–d1218 (2003).
Cole, J. A. Legless pathogens: how bacterial physiology provides the key to understanding pathogenicity. Microbiology 158, 1402–1413 (2012).
Sanchez-Buso, L. et al. The impact of antimicrobials on gonococcal evolution. Nat. Microbiol. 4, 1941–1950 (2019). This genomics paper provides evidence that the modern gonococcal population is not as old as previously anticipated and has been formed by antimicrobial treatment, leading to the emergence of one multidrug-resistant lineage and one multisusceptible lineage with different evolutionary strategies.
Tobiason, D. M. & Seifert, H. S. The obligate human pathogen, Neisseria gonorrhoeae, is polyploid. PLoS Biol. 4, 1069–1078 (2006).
Unemo, M. et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J. Antimicrob. Chemother. 71, 3096–3108 (2016).
Goodman, S. D. & Scocca, J. J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc. Natl Acad. Sci. USA 85, 6982–6986 (1988).
Berry, J. L., Cehovin, A., McDowell, M. A., Lea, S. M. & Pelicic, V. Functional analysis of the interdependence between DNA uptake sequence and its cognate ComP receptor during natural transformation in Neisseria species. PLoS Genet. 9, e1004014 (2013).
Bennett, J. S. et al. Species status of Neisseria gonorrhoeae: evolutionary and epidemiological inferences from multilocus sequence typing. BMC Biol. 5, 35 (2007).
Maiden, M. C. et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl Acad. Sci. USA 95, 3140–3145 (1998).
Goire, N. et al. Mixed gonococcal infections in a high-risk population, Sydney, Australia 2015: implications for antimicrobial resistance surveillance? J. Antimicrob. Chemother. 72, 407–409 (2017).
Martin, I. M. & Ison, C. A. Detection of mixed infection of Neisseria gonorrhoeae. Sex. Transm. Infect. 79, 56–58 (2003).
Unemo, M. & Shafer, W. M. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann. NY Acad. Sci. 1230, E19–E28 (2011).
Piekarowicz, A. et al. Characterization of the dsDNA prophage sequences in the genome of Neisseria gonorrhoeae and visualization of productive bacteriophage. BMC Microbiol. 7, 66 (2007).
Stohl, E. A., Dale, E. M., Criss, A. K. & Seifert, H. S. Neisseria gonorrhoeae metalloprotease NGO1686 is required for full piliation, and piliation is required for resistance to H2O2- and neutrophil-mediated killing. mBio 4, e00399–13 (2013).
Biswas, G. D., Sox, T., Blackman, E. & Sparling, P. F. Factors affecting genetic transformation of Neisseria gonorrhoeae. J. Bacteriol. 129, 983–992 (1977).
Dehio, C., Gray-Owen, S. D. & Meyer, T. F. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 6, 489–495 (1998).
Sadarangani, M., Pollard, A. J. & Gray-Owen, S. D. Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria. FEMS Microbiol. Rev. 35, 498–514 (2011).
Deo, P. et al. Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLoS Pathog. 14, e1006945 (2018).
Massari, P., Ram, S., Macleod, H. & Wetzler, L. M. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 11, 87–93 (2003).
Madico, G. et al. Factor H binding and function in sialylated pathogenic Neisseriae is influenced by gonococcal, but not meningococcal, porin. J. Immunol. 178, 4489–4497 (2007).
Olesky, M., Zhao, S., Rosenberg, R. L. & Nicholas, R. A. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J. Bacteriol. 188, 2300–2308 (2006).
Shafer, W. M. et al. in Efflux-Mediated Antimicrobial Resistance in Bacteria (eds Li, X. Z., Elkins,C. & Zgurskaya, H.) 439-469 (Adis, Cham, 2016).
Hagman, K. E. et al. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141, 611–622 (1995).
Lee, E. H. & Shafer, W. M. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 33, 839–845 (1999).
Hooper, R. R. et al. Cohort study of venereal disease. I: the risk of gonorrhea transmission from infected women to men. Am. J. Epidemiol. 108, 136–144 (1978).
Cohen, M. S. et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 349, 1868–1873 (1997).
Price, M. A. et al. Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: a randomized clinical trial. Sex. Transm. Dis. 30, 516–522 (2003).
Melly, M. A., Gregg, C. R. & McGee, Z. A. Studies of toxicity of Neisseria gonorrhoeae for human fallopian tube mucosa. J. Infect. Dis. 143, 423–431 (1981).
Melly, M. A., McGee, Z. A. & Rosenthal, R. S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 149, 378–386 (1984).
Escobar, A., Rodas, P. I. & Acuña-Castillo, C. Macrophage–Neisseria gonorrhoeae interactions: a better understanding of pathogen mechanisms of immunomodulation. Front. Immunol. 9, 3044 (2018).
Criss, A. K. & Seifert, H. S. A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nat. Rev. Microbiol. 10, 178–190 (2012).
Massari, P., Ho, Y. & Wetzler, L. M. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl Acad. Sci. USA 97, 9070–9075 (2000).
Muller, A. et al. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 19, 5332–5343 (2000).
Shaughnessy, J., Ram, S. & Rice, P. A. Biology of the gonococcus: disease and pathogenesis. Methods Mol. Biol. 1997, 1–27 (2019). This review describes gonorrhoea, its epidemiology, the structure and function of major surface components involved in pathogenesis, and mechanisms that gonococci use to evade immune responses.
Densen, P. Interaction of complement with Neisseria meningitidis and Neisseria gonorrhoeae. Clin. Microbiol. Rev. 2, S11–S17 (1989).
Crew, P. E. et al. Unusual Neisseria species as a cause of infection in patients taking eculizumab. J. Infect. 78, 113–118 (2019).
Liu, Y., Feinen, B. & Russell, M. W. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front. Microbiol. 2, 52 (2011).
Boslego, J. W. et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 9, 154–162 (1991).
Rotman, E. & Seifert, H. S. The genetics of Neisseria species. Annu. Rev. Genet. 48, 405–431 (2014).
Bignell, C. & Unemo, M., European STI Guidelines Editorial Board. European guideline on the diagnosis and treatment of gonorrhoea in adults. Int. J. STD & AIDS 24, 85–92 (2012).
Ghanem, K. G. Clinical manifestations and diagnosis of Neisseria gonorrhoeae infection in adults and adolescents UpToDate.com https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-neisseria-gonorrhoeae-infection-in-adults-and-adolescents/print (2019).
Ison, C. A. Laboratory methods in genitourinary medicine. Methods of diagnosing gonorrhoea. Genitourin. Med. 66, 453–459 (1990).
Unemo, M. & Ison, C. in Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus (eds Unemo, M. et al.) 21–54 (World Health Organization, 2013). This comprehensive chapter describes biological sampling, different methods for laboratory detection and antimicrobial susceptibility testing of N. gonorrhoeae.
Taylor, S. N., DiCarlo, R. P. & Martin, D. H. Comparison of methylene blue/gentian violet stain to Gram's stain for the rapid diagnosis of gonococcal urethritis in men. Sex. Transm. Dis. 38, 995–996 (2011).
Papp, J. R. S. J., Gaydos, C. A. & Van Der Pol, B. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae — 2014. MMWR Recomm. Rep. 63, 1–19 (2014).
Dillon, J. R. Sustainable antimicrobial surveillance programs essential for controlling Neisseria gonorrhoeae superbug. Sex. Transm. Dis. 38, 899–901 (2011).
Starnino, S. D., J. R. Laboratory manual: identification and antimicrobial susceptibility testing of Neisseria gonorrhoeae 2nd edn Co-ordinating Centre for the Gonococcal Antimicrobial Susceptibility Surveillance Program in Latin America and the Caribbean (2002).
Starnino, S. & Dillon, J. R. in Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus (eds Unemo, M. et al.)199–218 (World Health Organization, 2013).
Dillon, J. R., Carballo, M. & Pauze, M. Evaluation of eight methods for identification of pathogenic Neisseria species: Neisseria-Kwik, RIM-N, Gonobio-Test, Minitek, Gonochek II, GonoGen, Phadebact Monoclonal GC OMNI Test, and Syva MicroTrak Test. J. Clin. Microbiol. 26, 493–497 (1988).
Kellogg, J. A. & Orwig, L. K. Comparison of GonoGen, GonoGen II, and MicroTrak direct fluorescent-antibody test with carbohydrate fermentation for confirmation of culture isolates of Neisseria gonorrhoeae. J. Clin. Microbiol. 33, 474–476 (1995).
Kulkarni, S., Bala, M. & Risbud, A. Performance of tests for identification of Neisseria gonorrhoeae. Indian J. Med. Res. 141, 833–835 (2015).
Centers for Disease Control and Prevention. Acid Detection Test http://www.cdc.gov/std/gonorrhea/lab/tests/acid.htm (CDC, 2013).
Buchanan, R., Ball, D., Dolphin, H. & Dave, J. Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry for the identification of Neisseria gonorrhoeae. Clin. Microbiol. Infect. 22, 815.e815–815.e817 (2016).
Ilina, E. N. et al. Direct bacterial profiling by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry for identification of pathogenic Neisseria. J. Mol. Diagn. 11, 75–86 (2009).
Morel, F. et al. Use of Andromas and Bruker MALDI-TOF MS in the identification of Neisseria. Eur. J. Clin. Microbiol. Infect. Dis 37, 2273–2277 (2018).
Schmidt, K. et al. Identification of bacterial pathogens and antimicrobial resistance directly from clinical urines by nanopore-based metagenomic sequencing. J. Antimicrob. Chemother. 72, 104–114 (2017).
Hughes, G. I. et al. Guidance for the detection of gonorrhoea in England. (Public Health England, London, 2014).
Tabrizi, S. N. et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J. Clin. Microbiol. 49, 3610–3615 (2011).
Murtagh, M. M. The point-of-care diagnostic landscape for sexually transmitted infections (STIs). WHO https://www.who.int/reproductivehealth/topics/rtis/Diagnostic_Landscape_2018.pdf (2018). This extensive report details point-of-care diagnostic tests for STIs, with special focus on tests in the development pipeline.
Whiley, D. M., Tapsall, J. W. & Sloots, T. P. Nucleic acid amplification testing for Neisseria gonorrhoeae: an ongoing challenge. J. Mol. Diagn. 8, 3–15 (2006).
Alexander, S., da Silva, Coelho, Manuel, F., Varma, R. & Ison, R. C. Evaluation of strategies for confirming Neisseria gonorrhoeae nucleic acid amplification tests. J. Med. Microbiol. 60, 909–912 (2011).
Venter, J. M. E. et al. Comparison of an in-house real-time duplex PCR assay with commercial HOLOGIC(R) APTIMA assays for the detection of Neisseria gonorrhoeae and Chlamydia trachomatis in urine and extra-genital specimens. BMC Infect. Dis. 19, 6 (2019).
United States Food and Drug Administration. Nucleic Acid Based Tests. FDA https://www.fda.gov/medical-devices/vitro-diagnostics/nucleic-acid-based-tests (2019).
Schachter, J., Moncada, J., Liska, S., Shayevich, C. & Klausner, J. D. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex. Transm. Dis. 35, 637–642 (2008).
Chernesky, M. et al. Head-to-head comparison of second-generation nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae on urine samples from female subjects and self-collected vaginal swabs. J. Clin. Microbiol. 52, 2305–2310 (2014).
Jang, D. et al. Comparison of workflow, maintenance, and consumables in the genexpert infinity 80 and panther instruments while testing for chlamydia trachomatis and Neisseria gonorrhoeae. Sex. Transm. Dis. 43, 377–381 (2016).
World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae (WHO, 2016).
Public Health Agency Canada. National Surveillance of Antimicrobial Susceptibilities of Neisseria gonorrhoeae - 2016. (Government of Canada 2018).
Thakur, S. D. & Dillon, J. R. High levels of susceptibility to new and older antibiotics in Neisseria gonorrhoeae isolates from Saskatchewan (2003–15): time to consider point-of-care or molecular testing for precision treatment? Authors' response. J. Antimicrob. Chemother. 73, 829–830 (2018).
Allan-Blitz, L. T. et al. Implementation of a rapid genotypic assay to promote targeted ciprofloxacin therapy of Neisseria gonorrhoeae in a large health system. Clin. Infect. Dis. 64, 1268–1270 (2017).
Ellis, O. et al. A multisite implementation of a real-time polymerase chain reaction assay to predict ciprofloxacin susceptibility in Neisseria gonorrhoeae. Diagn. Microbiol. Infect. Dis. 94, 213–217 (2019).
Fifer, H., Saunders, J., Soni, S., Sadiq, S. T. & FitzGerald, M. British Association for Sexual Health and HIV national guideline for the management of infection with Neisseria gonorrhoeae (BASHH, 2019).
Badman, S. G. et al. A diagnostic evaluation of a molecular assay used for testing and treating anorectal chlamydia and gonorrhoea infections at the point-of-care in Papua New Guinea. Clin. Microbiol. Infect. 25, 623–627 (2018).
Wi, T. E. et al. Diagnosing sexually transmitted infections in resource-constrained settings: challenges and ways forward. J. Int. AIDS Soc. 22 (Suppl. 6), e25343 (2019).
Pai, M., Ghiasi., M. & Pai, N. P. Point-of-care diagnostic testing in global health: what is the point? Microbe 10, 103–107 (2015).
Pai, N. P., Vadnais, C., Denkinger, C., Engel, N. & Pai, M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 9, e1001306 (2012).
Peeling, R. W., Holmes, K. K., Mabey, D. & Ronald, A. Rapid tests for sexually transmitted infections (STIs): the way forward. Sex. Transm. Infect. 82 (Suppl. 5), v1–v6 (2006).
Watchirs Smith, L. A. et al. Point-of-care tests for the diagnosis of Neisseria gonorrhoeae infection: a systematic review of operational and performance characteristics. Sex. Transm. Infect. 89, 320–326 (2013).
Cristillo, A. D. et al. Point-of-care sexually transmitted infection diagnostics: proceedings of the STAR Sexually Transmitted Infection–Clinical Trial Group Programmatic Meeting. Sex. Transm. Dis. 44, 211–218 (2017).
Herbst de Cortina, S., Bristow, C. C., Joseph Davey, D. & Klausner, J. D. A systematic review of point of care testing for chlamydia trachomatis, Neisseria gonorrhoeae, and trichomonas vaginalis. Infect. Dis. Obstet. Gynecol. 2016, 4386127 (2016).
Guy, R. J. et al. Performance and operational characteristics of point-of-care tests for the diagnosis of urogenital gonococcal infections. Sex. Transm. Infect. 93, S16–S21 (2017).
Vickerman, P., Watts, C., Alary, M., Mabey, D. & Peeling, R. W. Sensitivity requirements for the point of care diagnosis of chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex. Transm. Infect. 79, 363–367 (2003).
Causer, L. M. et al. A field evaluation of a new molecular-based point-of-care test for chlamydia and gonorrhoea in remote aboriginal health services in Australia. Sex. Health 12, 27–33 (2015).
Garrett, N. et al. Diagnostic accuracy of the Xpert CT/NG and OSOM trichomonas rapid assays for point-of-care STI testing among young women in South Africa: a cross-sectional study. BMJ Open 9, e026888 (2019).
LeFevre, M. L. Screening for chlamydia and gonorrhea: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 161, 902–910 (2014).
Workowski, K. A. & Bolan, G. A., Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. Recomm. Rep. 64, 1–137 (2015).
Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2017 update. (US Public Health Service, 2017).
World Health Organization. Global strategy for the prevention and control of sexually transmitted infections: 2006 – 2015 Breaking the chain of transmission. WHO https://www.who.int/hiv/pub/toolkits/stis_strategy%5B1%5Den.pdf (2007).
Gottlieb, S. L. & Johnston, C. Future prospects for new vaccines against sexually transmitted infections. Curr. Opin. Infect. Dis. 30, 77–86 (2017).
Jerse, A. E. & Deal, C. D. Vaccine research for gonococcal infections: where are we? Sex. Transm. Infect. 89, iv63–iv68 (2013).
Zhu, W. et al. Vaccines for gonorrhea: can we rise to the challenge? Front. Microbiol. 2, 124 (2011).
Edwards, J. L., Jennings, M. P., Apicella, M. A. & Seib, K. L. Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development. Crit. Rev. Microbiol. 42, 928–941 (2016). This review describes the status of gonococcal vaccine development and, in particular, focuses on the model systems available to evaluate drug and vaccine candidates.
Tramont, E. C. Gonococcal vaccines. Clin. Microbiol. Rev. 2, S74–S77 (1989).
Paynter, J. et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine in preventing hospitalization from gonorrhea in New Zealand: a retrospective cohort study. Vaccines 7, E5 (2019).
Petousis-Harris, H. Impact of meningococcal group B OMV vaccines, beyond their brief. Hum. Vaccin. Immunother. 14, 1058–1063 (2018).
Petousis-Harris, H. et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 390, 1603–1610 (2017). This study provides a first proof-of-principle for vaccine protection against gonorrhoea, owing to cross-protection by the outer membrane vesicle Neisseria meningitidis serogroup B vaccine (MeNZB).
Hadad, R. et al. Novel meningococcal 4CMenB vaccine antigens — prevalence and polymorphisms of the encoding genes in Neisseria gonorrhoeae. APMIS 120, 750–760 (2012).
Beernink, P. T. et al. A meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and overexpressed factor h binding protein elicits gonococcal bactericidal antibodies. J. Infect. Dis. 219, 1130–1137 (2019).
Centers for Disease Control and Prevention. Expedited partner therapy in the management of sexually transmitted diseases. (US Department of Health and Human Services, 2006).
Parran, T. Shadow on the Land: Syphilis. (Reynal & Hitchcock, 1937).
Golden, M. R. et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N. Engl. J. Med. 352, 676–685 (2005).
Romanowski, B., Robinson, J. & Wong, T. Canadian Guidelines on Sexually Transmitted Infections - Gonococcal Infections Chapter. Phac-aspc.gc.ca http://www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/assets/pdf/section-5-6-eng.pdf (2013).
Australasian Sexual Health Alliance (ASHA). Gonorrhoea. ASHA http://www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea#management (2016).
Japanese Society for Sexually Transmitted Infections. Gonococcal infection. Sexually transmitted infections, diagnosis and treatment guidelines 2011. Jpn J. Sex. Transm. Dis. 22 (Suppl. 1), 52–59 (2011). In Japanese.
Bignell, C. & Fitzgerald, M., Guideline Development Group, British Association for Sexual Health and HIV UK. UK national guideline for the management of gonorrhoea in adults, 2011. Int. J. STD & AIDS 22, 541–547 (2011).
Boiko, I. et al. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates and treatment of gonorrhoea patients in ternopil and dnipropetrovsk regions of Ukraine, 2013–2018. APMIS 127, 503–509 (2019).
Unemo, M., Shipitsyna, E. & Domeika, M. Eastern European Sexual and Reproductive Health (EE SRH) Network Antimicrobial Resistance Group. Recommended antimicrobial treatment of uncomplicated gonorrhoea in 2009 in 11 East European countries: implementation of a Neisseria gonorrhoeae antimicrobial susceptibility programme in this region is crucial. Sex. Transm. Infect. 86, 442–444 (2010).
Leonard, C. A., Schoborg, R. V., Low, N., Unemo, M. & Borel, N. Pathogenic interplay between chlamydia trachomatis and Neisseria gonorrhoeae that influences management and control efforts — more questions than answers? Curr. Clin. Microbiol. Rep. 6, 182–191 (2019).
Handsfield, H. H., McCutchan, J. A., Corey, L. & Ronald, A. R. Evaluation of new anti-infective drugs for the treatment of uncomplicated gonorrhea in adults and adolescents. infectious diseases society of america and the food and drug administration. Clin. Infect. Dis. 15 (Suppl. 1), S123–S130 (1992).
Hook, E. W. 3rd & Kirkcaldy, R. D. A brief history of evolving diagnostics and therapy for gonorrhea: lessons learned. Clin. Infect. Dis. 67, 1294–1299 (2018).
Unemo, M. et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex. Health 16, 412–425 (2019). This paper reports the WHO GASP data from 2015 to 2016, confirmed gonorrhoea treatment failures with recommended therapy and international collaborative actions and research efforts essential for the effective management and control of gonorrhoea.
Cole, M. J. et al. Is the tide turning again for cephalosporin resistance in Neisseria gonorrhoeae in Europe? Results from the 2013 European surveillance. BMC Infect. Dis. 15, 321 (2015).
Day, M. J. et al. Stably high azithromycin resistance and decreasing ceftriaxone susceptibility in Neisseria gonorrhoeae in 25 European countries, 2016. BMC Infect. Dis. 18, 609 (2018).
Harris, S. R. et al. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey. Lancet Infect. Dis. 18, 758–768 (2018). This genomics paper provides the first use of joint analysis of WGS and epidemiological data in an international surveillance programme for STIs and a framework for genomic surveillance of gonococci through standardized sampling, use of WGS, and a shared information architecture for interpretation and dissemination by use of open-access software.
Kirkcaldy, R. D. et al. Neisseria gonorrhoeae antimicrobial susceptibility surveillance — the gonococcal isolate surveillance project, 27 sites, United States, 2014. MMWR 65, 1–19 (2016).
Kirkcaldy, R. D., Kidd, S., Weinstock, H. S., Papp, J. R. & Bolan, G. A. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: the Gonococcal Isolate Surveillance Project (GISP), January 2006–June 2012. Sex. Transm. Infect. 89, iv5–iv10 (2013).
Ford, J. V. et al. The need to promote sexual health in America: a new vision for public health action. Sex. Transm. Dis. 44, 579–585 (2017).
Reed, J. L. et al. Adolescent patient preferences surrounding partner notification and treatment for sexually transmitted infections. Acad. Emerg. Med. 22, 61–66 (2015).
Goffman, E. Stigma: notes on the management of spoiled identity (Aronson, J., 1974).
Fortenberry, J. D. et al. Relationships of stigma and shame to gonorrhea and HIV screening. Am. J. Public Health 92, 378–381 (2002).
Lichtenstein, B. Stigma as a barrier to treatment of sexually transmitted infection in the American deep south: issues of race, gender and poverty. Soc. Sci. Med. 57, 2435–2445 (2003).
Tsadik, M., Berhane, Y., Worku, A. & Terefe, W. The magnitude of, and factors associated with, loss to follow-up among patients treated for sexually transmitted infections: a multilevel analysis. BMJ Open 7, e016864 (2017).
Tshokey, T. et al. Antibiotic resistance in Neisseria gonorrhoea and treatment outcomes of gonococcal urethritis suspected patients in two large hospitals in Bhutan, 2015. PLoS One 13, e0201721 (2018).
Schwartz, R. M. et al. Coping with a diagnosis of C. trachomatis or N. gonorrhoeae: psychosocial and behavioral correlates. J. Health Psychol. 13, 921–929 (2008).
Wong, J. P. H., Chan, K. B. K., Bio-Doku, R. & Mcwatt, S. Risk discourse and sexual stigma: barriers to STI testing, treatment and care among young heterosexual women in disadvantaged neighbourhoods in Toronto. Can. J. Hum. Sex. 21, 74–89 (2012).
Morris, J. L. et al. Sexually transmitted infection related stigma and shame among African American male youth: implications for testing practices, partner notification, and treatment. AIDS Patient Care STDS 28, 499–506 (2014).
Crenshaw, K. Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanf. Law Rev. 43, 1241–1299 (1991).
Unemo, M. et al. Sexually transmitted infections: challenges ahead. Lancet Infect. Dis. 17, e235–e279 (2017). This very extensive Commission discusses the current key challenges facing the field of STIs and outlines new approaches to improve the clinical management of STIs and public health.
Carlton, T. O. & Mayes, S. M. Gonorrhea: not a ‘second-class’ disease. Health Soc. Work. 7, 301–313 (1982).
Wu, D., Hawkes, S. & Buse, K. Prevention of mother-to-child transmission of syphilis and HIV in China: what drives political prioritization and what can this tell us about promoting dual elimination? Int. J. Gynaecol. Obstet. 130, S32–S36 (2015).
Cook, J. E., Purdie-Vaughns, V., Meyer, I. H. & Busch, J. T. A. Intervening within and across levels: a multilevel approach to stigma and public health. Soc. Sci. Med. 103, 101–109 (2014).
Demczuk, W. et al. Whole-genome phylogenomic heterogeneity of Neisseria gonorrhoeae isolates with decreased cephalosporin susceptibility collected in Canada between 1989 and 2013. J. Clin. Microbiol. 53, 191–200 (2015).
Demczuk, W. et al. Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J. Clin. Microbiol. 54, 1304–1313 (2016).
Grad, Y. H. et al. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect. Dis. 14, 220–226 (2014).
Grad, Y. H. et al. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J. Infect. Dis. 214, 1579–1587 (2016).
Jacobsson, S. et al. WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014. J. Antimicrob. Chemother. 71, 3109–3116 (2016).
De Silva, D. et al. Whole-genome sequencing to determine transmission of Neisseria gonorrhoeae: an observational study. Lancet Infect. Dis. 16, 1295–1303 (2016).
Ezewudo, M. N. et al. Population structure of Neisseria gonorrhoeae based on whole genome data and its relationship with antibiotic resistance. PeerJ. 3, e806 (2015).
Ryan, L. et al. Antimicrobial resistance and molecular epidemiology using whole-genome sequencing of Neisseria gonorrhoeae in Ireland, 2014–2016: focus on extended-spectrum cephalosporins and azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 37, 1661–1672 (2018).
Eyre, D. W. et al. WGS to predict antibiotic MICs for Neisseria gonorrhoeae. J. Antimicrob. Chemother. 72, 1937–1947 (2017). This genomics paper provides strong evidence that WGS can relatively successfully predict MICs of antimicrobials and AMR in N. gonorrhoeae.
Golparian, D. et al. Antimicrobial resistance prediction and phylogenetic analysis of Neisseria gonorrhoeae isolates using the Oxford Nanopore MinION sequencer. Sci. Rep. 8, 17596 (2018).
Eyre, D. W., Golparian, D. & Unemo, M. Prediction of minimum inhibitory concentrations of antimicrobials for Neisseria gonorrhoeae using whole-genome sequencing. Methods Mol. Biol. 1997, 59–76 (2019).
Unemo, M. & Althaus, C. L. Fitness cost and benefit of antimicrobial resistance in Neisseria gonorrhoeae: multidisciplinary approaches are needed. PLoS Med. 14, e1002423 (2017).
Fingerhuth, S. M., Low, N., Bonhoeffer, S. & Althaus, C. L. Detection of antibiotic resistance is essential for gonorrhoea point-of-care testing: a mathematical modelling study. BMC Med. 15, 142 (2017).
Jacobsson, S. et al. WHO laboratory validation of Xpert((R)) CT/NG and Xpert((R)) TV on the GeneXpert system verifies high performances. APMIS. 126, 907–912 (2018).
Nudel, K. et al. Transcriptome analysis of Neisseria gonorrhoeae during natural infection reveals differential expression of antibiotic resistance determinants between men and women. mSphere 3, e00312–e00318 (2018).
Zielke, R. A. et al. Proteomics-driven antigen discovery for development of vaccines against gonorrhea. Mol. Cell Proteomics 15, 2338–2355 (2016).
El-Rami, F. E., Zielke, R. A., Wi, T., Sikora, A. E. & Unemo, M. Quantitative proteomics of the 2016 WHO Neisseria gonorrhoeae reference strains surveys vaccine candidates and antimicrobial resistance determinants. Mol. Cell Proteomics 18, 127–150 (2019).
Unemo, M. & Sikora, A. E. Infection: proof of principle for effectiveness of a gonorrhoea vaccine. Nat. Rev. Urol. 14, 643–644 (2017).
Moreau, M. R., Massari, P. & Genco, C. A. The ironclad truth: how in vivo transcriptomics and in vitro mechanistic studies shape our understanding of Neisseria gonorrhoeae gene regulation during mucosal infection. Pathog. Dis. 75, https://doi.org/10.1093/femspd/ftx057 (2017).
Jerse, A. E. et al. Estradiol-treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Front. Microbiol. 2, 107 (2011).
Sintsova, A. et al. Selection for CEACAM receptor-specific binding phenotype during Neisseria gonorrhoeae infection of the human genital tract. Infect. Immun. 83, 1372–1383 (2015).
Lujan, E., Pajon, R. & Granoff, D. M. Impaired immunogenicity of meningococcal neisserial surface protein A in human complement factor H transgenic mice. Infect. Immun. 84, 452–458 (2016).
Low, N. & Unemo, M. Molecular tests for the detection of antimicrobial resistant Neisseria gonorrhoeae: when, where, and how to use? Curr. Opin. Infect. Dis. 29, 45–51 (2016).
Dona, V., Low, N., Golparian, D. & Unemo, M. Recent advances in the development and use of molecular tests to predict antimicrobial resistance in Neisseria gonorrhoeae. Expert Rev. Mol. Diagn. 17, 845–859 (2017).
Sadiq, S. T., Mazzaferri, F. & Unemo, M. Rapid accurate point-of-care tests combining diagnostics and antimicrobial resistance prediction for Neisseria gonorrhoeae and mycoplasma genitalium. Sex. Transm. Infect. 93, S65–S68 (2017).
Goire, N. et al. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat. Rev. Microbiol. 12, 223–229 (2014).
Basarab, G. S. et al. Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial type II topoisomerases. Sci. Rep. 5, 11827 (2015).
Foerster, S. et al. Genetic resistance determinants, in vitro time-kill curve analysis and pharmacodynamic functions for the novel topoisomerase II inhibitor ETX0914 (AZD0914) in Neisseria gonorrhoeae. Front. Microbiol. 6, 1377 (2015).
Jacobsson, S. et al. High in vitro activity of the novel spiropyrimidinetrione AZD0914, a DNA gyrase inhibitor, against multidrug-resistant Neisseria gonorrhoeae isolates suggests a new effective option for oral treatment of gonorrhea. Antimicrob. Agents Chemother. 58, 5585–5588 (2014).
Taylor, S. N. et al. Single-dose zoliflodacin (ETX0914) for treatment of urogenital gonorrhea. N. Engl. J. Med. 379, 1835–1845 (2018).
Foerster, S. et al. In vitro antimicrobial combination testing and evolution of resistance to the first-in-class spiropyrimidinetrione zoliflodacin combined with six therapeutically relevant antimicrobials for Neisseria gonorrhoeae. J. Antimicrob. Chemother. https://doi.org/10.1093/jac/dkz376 (2019)
Jacobsson, S., Golparian, D., Scangarella-Oman, N. & Unemo, M. In vitro activity of the novel triazaacenaphthylene gepotidacin (GSK2140944) against MDR Neisseria gonorrhoeae. J. Antimicrob. Chemother. 73, 2072–2077 (2018).
Scangarella-Oman, N. E. et al. Microbiological analysis from a phase 2 randomized study in adults evaluating single oral doses of gepotidacin in the treatment of uncomplicated urogenital gonorrhea caused by Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 62, e01221–18 (2018).
Taylor, S. N. et al. Gepotidacin for the treatment of uncomplicated urogenital gonorrhea: a phase 2, randomized, dose-ranging, single-oral dose evaluation. Clin. Infect. Dis. 67, 504–512 (2018).
Jacobsson, S., Paukner, S., Golparian, D., Jensen, J. S. & Unemo, M. In vitro activity of the novel pleuromutilin lefamulin (bc-3781) and effect of efflux pump inactivation on multidrug-resistant and extensively drug-resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 61, e01497–17 (2017).
Paukner, S., Gruss, A. & Jensen, J. S. In vitro activity of lefamulin against sexually transmitted bacterial pathogens. Antimicrob. Agents Chemother. 62, e02380–17 (2018).
Jacobsson, S., Mason, C., Khan, N., Meo, P. & Unemo, M. In vitro activity of the novel oral antimicrobial SMT-571, with a new mechanism of action, against MDR and XDR Neisseria gonorrhoeae: future treatment option for gonorrhoea? J. Antimicrob. Chemother. 74, 1591–1594 (2019).
Kong, F. Y. S., Horner, P., Unemo, M. & Hocking, J. S. Pharmacokinetic considerations regarding the treatment of bacterial sexually transmitted infections with azithromycin: a review. J. Antimicrob. Chemother. 74, 1157–1166 (2019). This paper provides a detailed overview of the pharmacokinetics of antimicrobials used to treat STIs and how factors related to the drug, human and organism can affect treatment outcomes.
Lenz, J. D. & Dillard, J. P. Pathogenesis of Neisseria gonorrhoeae and the host defense in ascending infections of human fallopian tube. Front. Immunol. 9, 2710 (2018).
Lucas, C. T., Chandler, F. Jr., Martin, J. E. Jr & Schmale, J. D. Transfer of gonococcal urethritis from man to chimpanzee. An animal model for gonorrhea. JAMA 216, 1612–1614 (1971).
Cohen, M. S. & Cannon, J. G. Human experimentation with Neisseria gonorrhoeae: progress and goals. J. Infect. Dis. 179 (Suppl. 2), S375–S379 (1999).
Chow, E. P. et al. Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study. Sex. Transm. Infect. 93, 88–93 (2017).
Liu, Y. et al. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. 10, 1594–1608 (2017).
Kenyon, C., Buyze, J., Spiteri, G., Cole, M. J. & Unemo, M. Population-level antimicrobial consumption is associated with decreased antimicrobial susceptibility in Neisseria gonorrhoeae in 24 European countries: an ecological analysis. J. Infect. Dis. https://doi.org/10.1093/infdis/jiz153 (2019).
Tomberg, J. et al. Alanine 501 mutations in penicillin-binding protein 2 from Neisseria gonorrhoeae: structure, mechanism, and effects on cephalosporin resistance and biological fitness. Biochemistry 56, 1140–1150 (2017).
Tomberg, J., Unemo, M., Davies, C. & Nicholas, R. A. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 49, 8062–8070 (2010).
Tomberg, J., Unemo, M., Ohnishi, M., Davies, C. & Nicholas, R. A. Identification of amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from Neisseria gonorrhoeae strain H041. Antimicrob. Agents Chemother. 57, 3029–3036 (2013).
Lee, H. et al. Emergence of decreased susceptibility and resistance to extended-spectrum cephalosporins in Neisseria gonorrhoeae in Korea. J. Antimicrob. Chemother. 70, 2536–2542 (2015).
Olsen, B. et al. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect. Dis. 13, 40 (2013).
Whiley, D. M. et al. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal penicillin-binding protein 2. J. Antimicrob. Chemother. 65, 1615–1618 (2010).
Ohnishi, M. et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55, 3538–3545 (2011). This paper describes the identification and verification of the first global extensively drug-resistant and high-level ceftriaxone-resistant gonococcal strain that caused a ceftriaxone treatment failure in Japan.
Camara, J. et al. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J. Antimicrob. Chemother. 67, 1858–1860 (2012).
Unemo, M. et al. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 56, 1273–1280 (2012).
Gianecini, R., Oviedo, C., Stafforini, G. & Galarza, P. Neisseria gonorrhoeae resistant to ceftriaxone and cefixime, Argentina. Emerg. Infect. Dis. 22, 1139–1141 (2016).
Deguchi, T. et al. New clinical strain of Neisseria gonorrhoeae with decreased susceptibility to ceftriaxone, Japan. Emerg. Infect. Dis. 22, 142–144 (2016).
Nakayama, S. et al. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic pena gene isolated in Japan. Antimicrob. Agents Chemother. 60, 4339–4341 (2016).
Lahra, M. M. et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg. Infect. Dis. 24, https://doi.org/10.3201/eid2404.171873 (2018).
Lefebvre, B. et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017. Emerg. Infect. Dis. 24, https://doi.org/10.3201/eid2402.171756 (2018).
Terkelsen, D. et al. Multidrug-resistant Neisseria gonorrhoeae infection with ceftriaxone resistance and intermediate resistance to azithromycin, Denmark, 2017. Euro Surveill 22, https://doi.org/10.2807/1560-7917.ES.2017.22.42.17-00659 (2017).
Poncin, T. et al. Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017. Euro Surveill 23, https://doi.org/10.2807/1560-7917.ES.2018.23.21.1800264 (2018).
Golparian, D. et al. Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, Ireland, August 2018. Euro Surveill 23, https://doi.org/10.2807/1560-7917.ES.2018.23.47.1800617 (2018).
Eyre, D. W. et al. Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Euro Surveill 24, https://doi.org/10.2807/1560-7917.ES.2019.24.10.1900147 (2019).
Eyre, D. W. et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 23, https://doi.org/10.2807/1560-7917.ES.2018.23.27.1800323 (2018). This paper describes the identification of the first global gonococcal strain with combined ceftriaxone and high-level azithromycin resistance that caused a ceftriaxone treatment failure in the UK.
Whiley, D. M., Jennison, A., Pearson, J. & Lahra, M. M. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect. Dis. 18, 717–718 (2018).
Jennison, A. V. et al. Genetic relatedness of ceftriaxone-resistant and high-level azithromycin-resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Euro Surveill 24, https://doi.org/10.2807/1560-7917.ES.2019.24.8.1900118 (2019).
Ko, K. K. K. et al. First case of ceftriaxone-resistant multidrug-resistant Neisseria gonorrhoeae in Singapore. Antimicrob. Agents Chemother 63, e06224-18 (2019).
Lee, K. et al. Clonal expansion and spread of the ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, identified in Japan in 2015, and closely related isolates. J. Antimicrob. Chemother 74, 1812–1819 (2019).
Fifer, H. et al. Failure of dual antimicrobial therapy in treatment of gonorrhea. N. Engl. J. Med. 374, 2504–2506 (2016). This study reports on the first global failure of dual antimicrobial therapy (ceftriaxone plus azithromycin) in the treatment of gonorrhoea.
Chen, S. C., Han, Y., Yuan, L. F., Zhu, X. Y. & Yin, Y. P. Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg. Infect. Dis. 25, 1427–1429 (2019).
Poncin, T. et al. Two cases of multidrug-resistant Neisseria gonorrhoeae related to travel in south-eastern Asia, France, June 2019. Euro Surveill 24, https://doi.org/10.2807/1560-7917.ES.2019.24.36.1900528 (2019).
Morse, S. A. The biology of the gonococcus. CRC Crit. Rev. Microbiol. 7, 93–189 (1978).
Tonjum, T. & Koomey, M. The pilus colonization factor of pathogenic neisserial species: organelle biogenesis and structure/function relationships — a review. Gene 192, 155–163 (1997).
Maier, B., Potter, L., So, M., Seifert, H. S. & Sheetz, M. P. Single pilus motor forces exceed 100 pN. Proc. Natl Acad. Sci. USA 99, 16012–16017 (2002).
Stern, A., Brown, M., Nickel, P. & Meyer, T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47, 61–71 (1986).
James, J. F. & Swanson, J. Studies on gonococcus infection. XIII. Occurrence color/opacity colonial variants in clinical cultures. Infect. Immun. 19, 332–340 (1978).
Jerse, A. E. et al. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 179, 911–920 (1994).
Rice, P. A., Vayo, H. E., Tam, M. R. & Blake, M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J. Exp. Med. 164, 1735–1748 (1986).
Mandrell, R. E. et al. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J. Exp. Med. 171, 1649–1664 (1990).
Gaydos, C. A. et al. Performance of the Abbott RealTime CT/NG for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol. 48, 3236–3243 (2010).
Levett, P. N. et al. Evaluation of three automated nucleic acid amplification systems for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in first-void urine specimens. J. Clin. Microbiol. 46, 2109–2111 (2008).
Gaydos, C. A. et al. Performance of the cepheid CT/NG xpert rapid PCR test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol. 51, 1666–1672 (2013).
Tabrizi, S. N. et al. Analytical evaluation of GeneXpert CT/NG, the first genetic point-of-care assay for simultaneous detection of Neisseria gonorrhoeae and Chlamydia trachomatis. J. Clin. Microbiol. 51, 1945–1947 (2013).
Bromhead, C., Miller, A., Jones, M. & Whiley, D. Comparison of the cobas 4800 CT/NG test with culture for detecting Neisseria gonorrhoeae in genital and nongenital specimens in a low-prevalence population in New Zealand. J. Clin. Microbiol. 51, 1505–1509 (2013).
Rockett, R. et al. Evaluation of the cobas 4800 CT/NG test for detecting Chlamydia trachomatis and Neisseria gonorrhoeae. Sex. Transm. Infect. 86, 470–473 (2010).
Van Der Pol, B., Williams, J. A., Fuller, D., Taylor, S. N. & Hook, E. W. 3rd Combined testing for chlamydia, gonorrhea, and trichomonas by use of the BD Max CT/GC/TV assay with genitourinary specimen types. J. Clin. Microbiol. 55, 155–164 (2017).
Masek, B. J. et al. Performance of three nucleic acid amplification tests for detection of chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J. Clin. Microbiol. 47, 1663–1667 (2009).
Moncada, J., Schachter, J., Liska, S., Shayevich, C. & Klausner, J. D. Evaluation of self-collected glans and rectal swabs from men who have sex with men for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of nucleic acid amplification tests. J. Clin. Microbiol. 47, 1657–1662 (2009).
Golparian, D., Tabrizi, S. N. & Unemo, M. Analytical specificity and sensitivity of the APTIMA Combo 2 and APTIMA GC assays for detection of commensal Neisseria species and Neisseria gonorrhoeae on the gen-probe panther instrument. Sex. Transm. Dis. 40, 175–178 (2013).
Acknowledgements
The authors are grateful to S. Jacobsson (Örebro University Hospital and Örebro University) and S. Perera and N. Parmar (University of Saskatchewan) for technical assistance with preparing this manuscript.
Reviewer information
Nature Reviews Disease Primers thanks G. Hughes, S. Sood and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (M.U.); Epidemiology (F.N.); Mechanisms/pathophysiology (H S.S.); Diagnosis, screening and prevention (J.-A.R.D.); Management (E.W.H.III); Quality of life (S.H.); Outlook (M.U.); Overview of Primer (M.U.).
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
World Bank Income Classification: https://databank.worldbank.org/reports.aspx?source=2&series=NY.GNP.PCAP.CD&country=
Rights and permissions
About this article
Cite this article
Unemo, M., Seifert, H.S., Hook, E.W. et al. Gonorrhoea. Nat Rev Dis Primers 5, 79 (2019). https://doi.org/10.1038/s41572-019-0128-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-019-0128-6
This article is cited by
-
Investigating the influence of partner attitudes, norms and risk behavior on condom use decision-making during penile-vaginal sex with casual partners: a vignette study among dutch young people
BMC Public Health (2025)
-
Novobiocin primarily targets ParE in Neisseria gonorrhoeae
The Journal of Antibiotics (2025)
-
Modeling gonorrhea and HIV coinfection with predictive analytics for disability and mortality risks
Scientific Reports (2025)
-
Management and prevention of Neisseria meningitidis and Neisseria gonorrhoeae infections in the context of evolving antimicrobial resistance trends
European Journal of Clinical Microbiology & Infectious Diseases (2025)
-
Recurrence of sexually transmitted infections is commonly found in a subpopulation of Austrian users of HIV pre-exposure prophylaxis
Wiener klinische Wochenschrift (2025)