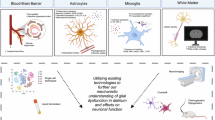
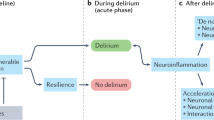
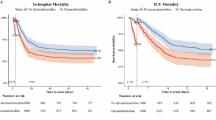
Abstract
Delirium, a syndrome characterized by an acute change in attention, awareness and cognition, is caused by a medical condition that cannot be better explained by a pre-existing neurocognitive disorder. Multiple predisposing factors (for example, pre-existing cognitive impairment) and precipitating factors (for example, urinary tract infection) for delirium have been described, with most patients having both types. Because multiple factors are implicated in the aetiology of delirium, there are likely several neurobiological processes that contribute to delirium pathogenesis, including neuroinflammation, brain vascular dysfunction, altered brain metabolism, neurotransmitter imbalance and impaired neuronal network connectivity. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) is the most commonly used diagnostic system upon which a reference standard diagnosis is made, although many other delirium screening tools have been developed given the impracticality of using the DSM-5 in many settings. Pharmacological treatments for delirium (such as antipsychotic drugs) are not effective, reflecting substantial gaps in our understanding of its pathophysiology. Currently, the best management strategies are multidomain interventions that focus on treating precipitating conditions, medication review, managing distress, mitigating complications and maintaining engagement to environmental issues. The effective implementation of delirium detection, treatment and prevention strategies remains a major challenge for health-care organizations globally.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Change history
01 December 2020
An Erratum to this paper has been published: https://doi.org/10.1038/s41572-020-00236-z.
References
Adamis, D., Treloar, A., Martin, F. C. & Macdonald, A. J. A brief review of the history of delirium as a mental disorder. Hist. Psychiatry 18, 459–469 (2007).
Williams, S. T., Dhesi, J. K. & Partridge, J. S. L. Distress in delirium: causes, assessment and management. Eur. Geriatr. Med. 11, 63–70 (2020).
Pandharipande, P. P. et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 369, 1306–1316 (2013). This prospective longitudinal cohort study demonstrated that critically ill patients are at risk of LTCI after critical illness, that this new LTCI can persist at 3 and 12 months follow-up, and that it is associated with duration of delirium.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edn (DSM-5) (American Psychiatric Association Publishing, 2013).
Cole, M. G. et al. Partial and no recovery from delirium in older hospitalized adults: frequency and baseline risk factors. J. Am. Geriatr. Soc. 63, 2340–2348 (2015).
Ouimet, S. et al. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 33, 1007–1013 (2007).
Cole, M., McCusker, J., Dendukuri, N. & Han, L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J. Am. Geriatr. Soc. 51, 754–760 (2003).
Slooter, A. J. C. et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten societies. Intensive Care Med. 46, 1020–1022 (2020). A joint position paper of ten international societies on the near complete segregation of the literature on delirium from that on encephalopathy, with recommendations to separate the underlying brain pathological state, namely encephalopathy, from the manifest clinical features, namely delirium.
Casey, P. et al. Hospital discharge data under-reports delirium occurrence: results from a point prevalence survey of delirium in a major Australian health service. Intern. Med. J. 49, 338–344 (2019).
Oldham, M. A. & Holloway, R. G. Delirium disorder: Integrating delirium and acute encephalopathy. Neurology 95, 173–178 (2020).
Ely, E. W. et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 27, 1892–1900 (2001).
Teodorczuk, A. & MacLullich, A. New waves of delirium understanding. Int. J. Geriatr. Psychiatry 33, 1417–1419 (2018).
Lipowski, Z. J. Transient cognitive disorders (delirium, acute confusional states) in the elderly. Am. J. Psychiatry 140, 1426–1436 (1983).
Khachaturian, A. S. et al. International drive to illuminate delirium: a developing public health blueprint for action. Alzheimers Dement. 16, 711–725 (2020).
Davis, D. H. et al. The epidemiology of delirium: challenges and opportunities for population studies. Am. J. Geriatr. Psychiatry 21, 1173–1189 (2013).
Gibb, K. et al. The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: a systematic review and meta-analysis study. Age Ageing 49, 352–360 (2020).
Marcantonio, E. R. Delirium in hospitalized older adults. N. Engl. J. Med. 377, 1456–1466 (2017).
Smith, T. O. et al. Factors predicting incidence of post-operative delirium in older people following hip fracture surgery: a systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 32, 386–396 (2017).
Watt, J. et al. Identifying older adults at risk of delirium following elective surgery: a systematic review and meta-analysis. J. Gen. Intern. Med. 33, 500–509 (2018).
Greaves, D. et al. Cognitive outcomes following coronary artery bypass grafting: a systematic review and meta-analysis of 91,829 patients. Int. J. Cardiol. 289, 43–49 (2019).
Abawi, M. et al. Postoperative delirium in individuals undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis. J. Am. Geriatr. Soc. 66, 2417–2424 (2018).
Shaw, R. C., Walker, G., Elliott, E. & Quinn, T. J. Occurrence rate of delirium in acute stroke settings: systematic review and meta-analysis. Stroke 50, 3028–3036 (2019).
Watt, C. L. et al. The incidence and prevalence of delirium across palliative care settings: a systematic review. Palliat. Med. 33, 865–877 (2019).
Hosie, A., Davidson, P. M., Agar, M., Sanderson, C. R. & Phillips, J. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: a systematic review. Palliat. Med. 27, 486–498 (2013).
Morichi, V. et al. A point prevalence study of delirium in Italian nursing homes. Dement. Geriatr. Cogn. Disord. 46, 27–41 (2018).
Andrew, M. K., Freter, S. H. & Rockwood, K. Prevalence and outcomes of delirium in community and non-acute care settings in people without dementia: a report from the Canadian study of health and aging. BMC Med. 4, 15 (2006).
Inouye, S. K., Westendorp, R. G. & Saczynski, J. S. Delirium in elderly people. Lancet 383, 911–922 (2014).
Krewulak, K. D., Stelfox, H. T., Leigh, J. P., Ely, E. W. & Fiest, K. M. Incidence and prevalence of delirium subtypes in an adult ICU: a systematic review and meta-analysis. Crit. Care Med. 46, 2029–2035 (2018).
Almeida, I. C. et al. The impact of acute brain dysfunction in the outcomes of mechanically ventilated cancer patients. PLoS ONE 9, e85332 (2014).
Janssen, N. J. et al. On the utility of diagnostic instruments for pediatric delirium in critical illness: an evaluation of the pediatric anesthesia emergence delirium scale, the delirium rating scale 88, and the delirium rating scale-revised R-98. Intensive Care Med. 37, 1331–1337 (2011).
Larsen, G. Y., Donaldson, A. E., Parker, H. B. & Grant, M. J. Preventable harm occurring to critically ill children. Pediatr. Crit. Care Med. 8, 331–336 (2007).
Schieveld, J. N. et al. Pediatric delirium in critical illness: phenomenology, clinical correlates and treatment response in 40 cases in the pediatric intensive care unit. Intensive Care Med. 33, 1033–1040 (2007).
Silver, G. et al. Detecting pediatric delirium: development of a rapid observational assessment tool. Intensive Care Med. 38, 1025–1031 (2012).
Smith, H. A. et al. Diagnosing delirium in critically ill children: validity and reliability of the pediatric confusion assessment method for the intensive care unit. Crit. Care Med. 39, 150–157 (2011).
Traube, C. et al. Cornell assessment of pediatric delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit. Care Med. 42, 656–663 (2014).
Smith, H. A. et al. The preschool confusion assessment method for the ICU: valid and reliable delirium monitoring for critically Ill infants and children. Crit. Care Med. 44, 592–600 (2016).
Gross, A. L. et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch. Intern. Med. 172, 1324–1331 (2012).
Silver, G. et al. Pediatric delirium and associated risk factors: a single-center prospective observational study. Pediatr. Crit. Care Med. 16, 303–309 (2015).
Smith, P. J., Attix, D. K., Weldon, B. C., Greene, N. H. & Monk, T. G. Executive function and depression as independent risk factors for postoperative delirium. Anesthesiology 110, 781–787 (2009).
Wilson, K., Broadhurst, C., Diver, M., Jackson, M. & Mottram, P. Plasma insulin growth factor-1 and incident delirium in older people. Int. J. Geriatr. Psychiatry 20, 154–159 (2005).
Velayati, A., Vahdat Shariatpanahi, M., Shahbazi, E. & Vahdat Shariatpanahi, Z. Association between preoperative nutritional status and postoperative delirium in individuals with coronary artery bypass graft surgery: a prospective cohort study. Nutrition 66, 227–232 (2019).
Sanford, A. M. & Flaherty, J. H. Do nutrients play a role in delirium? Curr. Opin. Clin. Nutr. Metab. Care 17, 45–50 (2014).
Persico, I. et al. Frailty and delirium in older adults: a systematic review and meta-analysis of the literature. J. Am. Geriatr. Soc. 66, 2022–2030 (2018).
Jung, P. et al. The impact of frailty on postoperative delirium in cardiac surgery patients. J. Thorac. Cardiovasc. Surg. 149, 869–875.e1-2 (2015).
Davis, D. H. et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am. J. Geriatr. Psychiatry 23, 403–415 (2015). This paper represents the clearest demonstration that progressive cognitive decline is a progressively increasing risk factor for delirium and also demonstrates, in mice, that this decline is correlated with increasing synaptic loss and can precede frank neurodegeneration. The study also validates the first animal model of delirium superimposed on dementia.
Nitchingham, A., Kumar, V., Shenkin, S., Ferguson, K. J. & Caplan, G. A. A systematic review of neuroimaging in delirium: predictors, correlates and consequences. Int. J. Geriatr. Psychiatry 33, 1458–1478 (2018).
Ferguson, K. J. & MacLullich, A. M. J. in Brain disorders in Critical Illness (eds Stevens, R. D., Sharshar, T., & Ely, E. W.) (Cambridge University Press, 2013).
McCoy, T. H. Jr. Hart, K., Pellegrini, A. & Perlis, R. H. Genome-wide association identifies a novel locus for delirium risk. Neurobiol. Aging 68, 160.e9–160.e14 (2018).
Adamis, D., Meagher, D., Williams, J., Mulligan, O. & McCarthy, G. A systematic review and meta-analysis of the association between the apolipoprotein E genotype and delirium. Psychiatr. Genet. 26, 53–59 (2016).
Laurila, J. V., Laakkonen, M. L., Laurila, J. V., Timo, S. E. & Reijo, T. S. Predisposing and precipitating factors for delirium in a frail geriatric population. J. Psychosom. Res. 65, 249–254 (2008).
Cirbus, J. et al. Delirium etiology subtypes and their effect on six-month function and cognition in older emergency department patients. Int. Psychogeriatr. 31, 267–276 (2019).
Clegg, A. & Young, J. B. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing 40, 23–29 (2011).
Sampson, E. L., West, E. & Fischer, T. Pain and delirium: mechanisms, assessment, management. Eur. Geriatr. Med. 11, 45–52 (2020).
Van Rompaey, B. et al. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit. Care 13, R77 (2009).
Shehabi, Y. et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit. Care Med. 38, 2311–2318 (2010).
Ely, E. W. et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286, 2703–2710 (2001).
Pandharipande, P. et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J. Trauma 65, 34–41 (2008).
Sharma, A., Malhotra, S., Grover, S. & Jindal, S. K. Incidence, prevalence, risk factor and outcome of delirium in intensive care unit: a study from India. Gen. Hosp. Psychiatry 34, 639–646 (2012).
Tsuruta, R. et al. Prevalence and associated factors for delirium in critically ill patients at a Japanese intensive care unit. Gen. Hosp. Psychiatry 32, 607–611 (2010).
Lindroth, H. et al. Systematic review of prediction models for delirium in the older adult inpatient. BMJ Open 8, e019223 (2018).
Rudolph, J. L. et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation 119, 229 (2009).
O’Keeffe, S. & Lavan, J. Predicting delirium in elderly patients: development and validation of a risk-stratification model. Age Ageing 25, 317–321 (1996).
Fisher, B. W. & Flowerdew, G. A simple model for predicting postoperative delirium in older patients undergoing elective orthopedic surgery. J. Am. Geriatr. Soc. 43, 175–178 (1995).
Kalisvaart, K. J. et al. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: Implementation and validation of a medical risk factor model. J. Am. Geriatr. Soc. 54, 817–822 (2006).
Böhner, H. et al. Predicting delirium after vascular surgery: a model based on pre-and intraoperative data. Ann. Surg. 238, 149 (2003).
Kennedy, M. et al. Delirium risk prediction, healthcare use and mortality of elderly adults in the emergency department. J. Am. Geriatr. Soc. 62, 462–469 (2014).
Wassenaar, A. et al. Multinational development and validation of an early prediction model for delirium in ICU patients. Intensive Care Med. 41, 1048–1056 (2015).
Van den Boogaard, M. et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 344, e420 (2012).
Linkaite, G., Riauka, M., Buneviciute, I. & Vosylius, S. Evaluation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for the patients in the intensive care unit. Acta Med. Litu. 25, 14–22 (2018).
Paton, L., Elliott, S. & Chohan, S. Utility of the PRE-DELIRIC delirium prediction model in a Scottish ICU cohort. J. Intensive Care Soc. 17, 202–206 (2016).
Sosa, F. A. et al. Assessment of delirium using the PRE-DELIRIC model in an intensive care unit in Argentina. Rev. Bras. Ter. Intensiva 30, 50–56 (2018).
Rudberg, M. A., Pompei, P., Foreman, M. D., Ross, R. E. & Cassel, C. K. The natural history of delirium in older hospitalized patients: a syndrome of heterogeneity. Age Ageing 26, 169–174 (1997).
Cole, M. G., Ciampi, A., Belzile, E. & Zhong, L. Persistent delirium in older hospital patients: a systematic review of frequency and prognosis. Age Ageing 38, 19–26 (2009).
Meagher, D., Adamis, D., Trzepacz, P. & Leonard, M. Features of subsyndromal and persistent delirium. Br. J. Psychiatry 200, 37–44 (2012).
Witlox, J. et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 304, 443–451 (2010). This meta-analysis provides evidence that, in elderly patients, delirium is associated with poor outcomes (mortality, institutionalization and dementia), independent of important confounders.
Jackson, T. A., Wilson, D., Richardson, S. & Lord, J. M. Predicting outcome in older hospital patients with delirium: a systematic literature review. Int. J. Geriatr. Psychiatry 31, 392–399 (2016).
Davis, D. H. et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain 135, 2809–2816 (2012).
Goldberg, T. E. et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.2273 (2020).
Engel, G. L. & Romano, J. Delirium II Reversibility of the electroencephalogram with experimental procedures. Arch. Neuro Psychiatr. 51, 378–392 (1944).
Itil, T. & Fink, M. Anticholinergic drug-induced delirium: experimental modification, quantitative EEG and behavioral correlations. J. Nerv. Ment. Dis. 143, 492–507 (1966).
Girard, T. D. et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir. Med. 6, 213–222 (2018).
Inouye, S. K. & Charpentier, P. A. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 275, 852–857 (1996).
Tijms, B. M. et al. Alzheimer’s disease: connecting findings from graph theoretical studies of brain networks. Neurobiol. Aging 34, 2023–2036 (2013).
Cunningham, C., Wilcockson, D. C., Campion, S., Lunnon, K. & Perry, V. H. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 25, 9275–9284 (2005).
Hennessy, E., Griffin, E. W. & Cunningham, C. Astrocytes are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1beta and TNF-alpha. J. Neurosci. 35, 8411–8422 (2015).
Hasel, P. et al. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat. Commun. 8, 15132 (2017).
Sweeney, M. D., Kisler, K., Montagne, A., Toga, A. W. & Zlokovic, B. V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 21, 1318–1331 (2018).
Yang, A. C. et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430 (2020).
Alagiakrishnan, K. & Wiens, C. A. An approach to drug induced delirium in the elderly. Postgrad. Med. J. 80, 388–393 (2004).
Engel, G. L. & Romano, J. Delirium, a syndrome of cerebral insufficiency. J. Chronic Dis. 9, 260–277 (1959). This perspective piece gathers many key observations on clinical and volunteer neurophysiology studies detailing the relationship between EEG and delirium symptoms as well as making a compelling case for the role of disturbed brain energy metabolism as a driver of delirium.
Taccone, F. S. et al. Cerebral microcirculation is impaired during sepsis: an experimental study. Crit. Care 14, R140 (2010).
Yokota, H., Ogawa, S., Kurokawa, A. & Yamamoto, Y. Regional cerebral blood flow in delirium patients. Psychiatry Clin. Neurosci. 57, 337–339 (2003).
Pfister, D. et al. Cerebral perfusion in sepsis-associated delirium. Crit. Care 12, R63 (2008).
Caplan, G. A. et al. Cerebrospinal fluid in long-lasting delirium compared with Alzheimer’s dementia. J. Gerontol. A Biol. Sci. Med. Sci. 65, 1130–1136 (2010).
Kealy, J. et al. Acute inflammation alters brain energy metabolism in mice and humans: role in suppressed spontaneous activity, impaired cognition, and delirium. J. Neurosci. 40, 5681–5696 (2020).
Bendahan, N., Neal, O., Ross-White, A., Muscedere, J. & Boyd, J. G. Relationship between near-infrared spectroscopy-derived cerebral oxygenation and delirium in critically ill patients: a systematic review. J. Intensive Care Med. 34, 514–520 (2019).
Rosengarten, B. et al. Microcirculatory dysfunction in the brain precedes changes in evoked potentials in endotoxin-induced sepsis syndrome in rats. Cerebrovasc. Dis. 23, 140–147 (2007).
Polito, A. et al. Pattern of brain injury in the acute setting of human septic shock. Crit. Care 17, R204 (2013).
Reddy, P. H. & Beal, M. F. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 14, 45–53 (2008).
Shehab, N. et al. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA 316, 2115–2125 (2016).
Sejling, A. S. et al. Hypoglycemia-associated changes in the electroencephalogram in patients with type 1 diabetes and normal hypoglycemia awareness or unawareness. Diabetes 64, 1760–1769 (2015).
Gugger, J. J., Geocadin, R. G. & Kaplan, P. W. A multimodal approach using somatosensory evoked potentials for prognostication in hypoglycemic encephalopathy. Clin. Neurophysiol. Pract. 4, 194–197 (2019).
Sonneville, R. et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 43, 1075–1084 (2017).
Thorell, A., Efendic, S., Gutniak, M., Haggmark, T. & Ljungqvist, O. Insulin resistance after abdominal surgery. Br. J. Surg. 81, 59–63 (1994).
Virkamaki, A., Puhakainen, I., Koivisto, V. A., Vuorinen-Markkola, H. & Yki-Jarvinen, H. Mechanisms of hepatic and peripheral insulin resistance during acute infections in humans. J. Clin. Endocrinol. Metab. 74, 673–679 (1992).
Meltzer, C. C. et al. Regional hypometabolism in Alzheimer’s disease as measured by positron emission tomography after correction for effects of partial volume averaging. Neurology 47, 454–461 (1996).
Holscher, C. Insulin signalling impairment in the brain as a risk factor in Alzheimer’s disease. Front. Aging Neurosci. 11, 88 (2019).
Semmler, A. et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J. Neuroinflammation 5, 38 (2008).
Haggstrom, L. R., Nelson, J. A., Wegner, E. A. & Caplan, G. A. 2-(18)F-fluoro-2-deoxyglucose positron emission tomography in delirium. J. Cereb. Blood Flow Metab. 37, 3556–3567 (2017).
Zimmer, E. R. et al. [18F]FDG PET signal is driven by astroglial glutamate transport. Nat. Neurosci. 20, 393–395 (2017).
Maclullich, A. M., Ferguson, K. J., Miller, T., de Rooij, S. E. & Cunningham, C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J. Psychosom. Res. 65, 229–238 (2008).
Yu, M. et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26, 174–179 (2006).
Deiner, S. et al. Human plasma biomarker responses to inhalational general anaesthesia without surgery. Br. J. Anaesth. https://doi.org/10.1016/j.bja.2020.04.085 (2020).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 (2008).
Cunningham, C. Microglia and neurodegeneration: the role of systemic inflammation. Glia 61, 71–90 (2013).
Subramaniyan, S. & Terrando, N. Neuroinflammation and perioperative neurocognitive disorders. Anesth. Analg. 128, 781–788 (2019).
Banks, W. A., Farr, S. A. & Morley, J. E. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation 10, 319–327 (2002).
Cerejeira, J., Firmino, H., Vaz-Serra, A. & Mukaetova-Ladinska, E. B. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 119, 737–754 (2010).
van Gool, W. A., van de Beek, D. & Eikelenboom, P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375, 773–775 (2010).
van den Boogaard, M. et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit. Care 15, R297 (2011).
Vasunilashorn, S. M. et al. High C-reactive protein predicts delirium incidence, duration, and feature severity after major noncardiac surgery. J. Am. Geriatr. Soc. 65, e109–e116 (2017).
Vasunilashorn, S. M. et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1289–1295 (2015).
Henjum, K. et al. CSF sTREM2 in delirium-relation to Alzheimer’s disease CSF biomarkers Aβ42, t-tau and p-tau. J. Neuroinflammation 15, 304 (2018).
van Munster, B. C. et al. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. 14, 615–622 (2011).
Feng, X. et al. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight 2, e91229 (2017).
Cibelli, M. et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann. Neurol. 68, 360–368 (2010).
Frank, M. G. et al. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav. Immun. 24, 254–262 (2010).
Pugh, C. R. et al. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav. Brain Res. 106, 109–118 (1999).
Skelly, D. T. et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol. Psychiatry 24, 1533–1548 (2019). This paper provides a partial mechanistic basis for acute lipopolysaccharide-induced delirium-like deficits selectively in mice with prior neurodegeneration. The data suggest that acute cognitive deficits and acute brain injury may be dissociable, driven by systemic and centrally produced IL-1β, respectively.
Cape, E. et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1beta in delirium after hip fracture. J. Psychosom. Res. 77, 219–225 (2014).
Serantes, R. et al. Interleukin-1beta enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J. Biol. Chem. 281, 14632–14643 (2006).
Liu, X. et al. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity 50, 764–766 (2019).
Lopez-Rodriguez, A. B. et al. Microglial and astrocyte priming in the APP/PS1 model of Alzheimer’s disease: increased vulnerability to acute inflammation and cognitive deficits. Preprint at bioRxiv https://doi.org/10.1101/344218 (2018).
Andonegui, G. et al. Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight https://doi.org/10.1172/jci.insight.99364 (2018).
Vacas, S., Degos, V., Tracey, K. J. & Maze, M. High-mobility group Box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology 120, 1160–1167 (2014).
Waltl, I. et al. Macrophage depletion by liposome-encapsulated clodronate suppresses seizures but not hippocampal damage after acute viral encephalitis. Neurobiol. Dis. 110, 192–205 (2018).
MacLullich, A. M. et al. Cerebrospinal fluid interleukin-8 levels are higher in people with hip fracture with perioperative delirium than in controls. J. Am. Geriatr. Soc. 59, 1151–1153 (2011).
van Munster, B. C. et al. Time-course of cytokines during delirium in elderly patients with hip fractures. J. Am. Geriatr. Soc. 56, 1704–1709 (2008).
Hall, R. J. et al. CSF biomarkers in delirium: a systematic review. Int. J. Geriatr. Psychiatry 33, 1479–1500 (2018).
Skrede, K., Wyller, T. B., Watne, L. O., Seljeflot, I. & Juliebo, V. Is there a role for monocyte chemoattractant protein-1 in delirium? Novel observations in elderly hip fracture patients. BMC Res. Notes 8, 186 (2015).
Campbell, S. J. et al. Sickness behaviour is induced by a peripheral CXC-chemokine also expressed in multiple sclerosis and EAE. Brain Behav. Immun. 24, 738–746 (2010).
Le Thuc, O. et al. Central CCL2 signaling onto MCH neurons mediates metabolic and behavioral adaptation to inflammation. EMBO Rep. 17, 1738–1752 (2016).
Marciniak, E. et al. The chemokine MIP-1alpha/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci. Rep. 5, 15862 (2015).
Varatharaj, A. & Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 60, 1–12 (2017).
Hov, K. R. et al. Blood-cerebrospinal fluid barrier integrity in delirium determined by Q-Albumin. Dement. Geriatr. Cognit. Disord. 41, 192–198 (2016).
The Lancet Haematology. COVID-19 coagulopathy: an evolving story. Lancet Haematol. 7, e425 (2020).
Forsberg, A. et al. The immune response of the human brain to abdominal surgery. Ann. Neurol. 81, 572–582 (2017).
Ebert, U. & Kirch, W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur. J. Clin. Invest. 28, 944–949 (1998).
Trzepacz, P. T. Anticholinergic model for delirium. Semin. Clin. Neuropsychiatry 1, 294–303 (1996).
Tune, L. E. Serum anticholinergic activity levels and delirium in the elderly. Semin. Clin. Neuropsychiatry 5, 149–153 (2000).
Carnahan, R. M., Lund, B. C., Perry, P. J., Pollock, B. G. & Culp, K. R. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J. Clin. Pharmacol. 46, 1481–1486 (2006).
Liptzin, B., Laki, A., Garb, J. L., Fingeroth, R. & Krushell, R. Donepezil in the prevention and treatment of post-surgical delirium. Am. J. Geriatr. Psychiatry 13, 1100–1106 (2005).
Marcantonio, E. R., Palihnich, K., Appleton, P. & Davis, R. B. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J. Am. Geriatr. Soc. 59 (Suppl. 2), S282–S288 (2011).
van Eijk, M. M. et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet 376, 1829–1837 (2010).
Field, R. H., Gossen, A. & Cunningham, C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J. Neurosci. 32, 6288–6294 (2012).
McKeith, I. et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 356, 2031–2036 (2000).
Roy, R., Niccolini, F., Pagano, G. & Politis, M. Cholinergic imaging in dementia spectrum disorders. Eur. J. Nucl. Med. Mol. Imaging 43, 1376–1386 (2016).
Agar, M. R. et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern. Med. 177, 34–42 (2017).
Girard, T. D. et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N. Engl. J. Med. 379, 2506–2516 (2018). This randomized, double-blind, placebo-controlled trial (MIND-USA) evaluated haloperidol, ziprasidone or placebo for treating delirium in critically ill patients with respiratory failure or shock, finding no effect of these antipsychotic drugs on days alive without delirium or coma, or duration of delirium or coma.
Page, V. J. et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 1, 515–523 (2013).
van den Boogaard, M. et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 319, 680–690 (2018). In the multisite, randomized, double-blinded, placebo-controlled REDUCE trial comparing prophylactic haloperidol with placebo for delirium prevention in critically ill adults, haloperidol did not improve survival at 28 days; thus, prophylactic haloperidol is not recommended for reducing mortality in critically ill adults.
Gainetdinov, R. R., Jones, S. R. & Caron, M. G. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol. Psychiatry 46, 303–311 (1999).
Scammell, T. E., Jackson, A. C., Franks, N. P., Wisden, W. & Dauvilliers, Y. Histamine: neural circuits and new medications. Sleep https://doi.org/10.1093/sleep/zsy183 (2019).
Chazot, P. L., Johnston, L., McAuley, E. & Bonner, S. Histamine and delirium: current opinion. Front. Pharmacol. 10, 299 (2019).
Aston-Jones, G. & Cohen, J. D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
Arnsten, A. F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422 (2009).
Matthews, K. L. et al. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol. Psychiatry 51, 407–416 (2002).
Hahn, P. Y. et al. Sustained elevation in circulating catecholamine levels during polymicrobial sepsis. Shock 4, 269–273 (1995).
Buhler, H. U., da Prada, M., Haefely, W. & Picotti, G. B. Plasma adrenaline, noradrenaline and dopamine in man and different animal species. J. Physiol. 276, 311–320 (1978).
Deiner, S., Lin, H. M., Bodansky, D., Silverstein, J. & Sano, M. Do stress markers and anesthetic technique predict delirium in the elderly? Dement. Geriatr. Cogn. Disord. 38, 366–374 (2014).
Cursano, S. et al. A CRHR1 antagonist prevents synaptic loss and memory deficits in a trauma-induced delirium-like syndrome. Mol. Psychiatry https://doi.org/10.1038/s41380-020-0659-y (2020).
Hawley, R. J. et al. Neurochemical correlates of sympathetic activation during severe alcohol withdrawal. Alcohol. Clin. Exp. Res. 18, 1312–1316 (1994).
Smith, A. J., Brent, P. J., Henry, D. A. & Foy, A. Plasma noradrenaline, platelet alpha 2-adrenoceptors, and functional scores during ethanol withdrawal. Alcohol. Clin. Exp. Res. 14, 497–502 (1990).
Muzyk, A. J., Fowler, J. A., Norwood, D. K. & Chilipko, A. Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Ann. Pharmacother. 45, 649–657 (2011).
Maldonado, J. R. Novel algorithms for the prophylaxis and management of alcohol withdrawal syndromes-beyond benzodiazepines. Crit. Care Clin. 33, 559–599 (2017).
Skrobik, Y., Duprey, M. S., Hill, N. S. & Devlin, J. W. Low-dose nocturnal dexmedetomidine prevents ICU delirium. A randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 197, 1147–1156 (2018).
Su, X. et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 388, 1893–1902 (2016).
Liu, X. et al. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: a meta-analysis with trial sequential analysis of randomized controlled trials. J. Crit. Care 38, 190–196 (2017).
Ventura, R., Alcaro, A. & Puglisi-Allegra, S. Prefrontal cortical norepinephrine release is critical for morphine-induced reward, reinstatement and dopamine release in the nucleus accumbens. Cereb. Cortex 15, 1877–1886 (2005).
Sanders, R. D. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med. Hypotheses 77, 140–143 (2011).
Pandharipande, P. et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 104, 21–26 (2006).
Zaal, I. J. et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 41, 2130–2137 (2015).
Vlisides, P. & Avidan, M. Recent Advances in Preventing and Managing Postoperative Delirium. F1000Res https://doi.org/10.12688/f1000research.16780.1 (2019).
Yoshitaka, S., Egi, M., Kanazawa, T., Toda, Y. & Morita, K. The association of plasma gamma-aminobutyric acid concentration with postoperative delirium in critically ill patients. Crit. Care Resusc. 16, 269–273 (2014).
Morandi, A. et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study. Crit. Care Med. 40, 2182–2189 (2012).
Cavallari, M. et al. Neural substrates of vulnerability to postsurgical delirium as revealed by presurgical diffusion MRI. Brain 139, 1282–1294 (2016).
Murray, C. et al. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol. Aging 33, 603–616.e3 (2012).
Peters van Ton, A. M., Verbeek, M. M., Alkema, W., Pickkers, P. & Abdo, W. F. Downregulation of synapse-associated protein expression and loss of homeostatic microglial control in cerebrospinal fluid of infectious patients with delirium and patients with Alzheimer’s disease. Brain Behav. Immun. https://doi.org/10.1016/j.bbi.2020.06.027 (2020).
van Montfort, S. J. T. et al. Brain network disintegration as a final common pathway for delirium: a systematic review and qualitative meta-analysis. Neuroimage Clin. 23, 101809 (2019).
Stam, C. J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 15, 683–695 (2014).
Choi, S. H. et al. Neural network functional connectivity during and after an episode of delirium. Am. J. Psychiatry 169, 498–507 (2012).
Raichle, M. E. The brain’s default mode network. Annu. Rev. Neurosci. 38, 433–447 (2015).
Fleischmann, R. et al. Delirium is associated with frequency band specific dysconnectivity in intrinsic connectivity networks: preliminary evidence from a large retrospective pilot case-control study. Pilot. Feasibility Stud. 5, 2 (2019).
Numan, T. et al. Functional connectivity and network analysis during hypoactive delirium and recovery from anesthesia. Clin. Neurophysiol. 128, 914–924 (2017).
van Montfort, S. J. T. et al. Resting-state fMRI reveals network disintegration during delirium. Neuroimage Clin. 20, 35–41 (2018).
Zhang, L. J., Wu, S., Ren, J. & Lu, G. M. Resting-state functional magnetic resonance imaging in hepatic encephalopathy: current status and perspectives. Metab. Brain Dis. 29, 569–582 (2014).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th edn (American Psychiatric Association, 1994).
European Delirium Association & American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 12, 141 (2014).
Cole, M. G., Dendukuri, N., McCusker, J. & Han, L. An empirical study of different diagnostic criteria for delirium among elderly medical inpatients. J. Neuropsychiatry Clin. Neurosci. 15, 200–207 (2003).
Laurila, J. V., Pitkala, K. H., Strandberg, T. E. & Tilvis, R. S. The impact of different diagnostic criteria on prevalence rates for delirium. Dement. Geriatr. Cogn. Disord. 16, 156–162 (2003).
Trzepacz, P. T. A review of delirium assessment instruments. Gen. Hosp. Psychiatry 16, 397–405 (1994).
Tieges, Z., Evans, J. J., Neufeld, K. J. & MacLullich, A. M. J. The neuropsychology of delirium: advancing the science of delirium assessment. Int. J. Geriatr. Psychiatry 33, 1501–1511 (2018).
Network for Investigation of Delirium: Unifying Scientists (NIDUS). Delirium Measurement Info Cards https://deliriumnetwork.org/measurement/delirium-info-cards/ (2020).
van Velthuijsen, E. L. et al. Psychometric properties and feasibility of instruments for the detection of delirium in older hospitalized patients: a systematic review. Int. J. Geriatr. Psychiatry 31, 974–989 (2016).
De, J. & Wand, A. P. Delirium screening: a systematic review of delirium screening tools in hospitalized patients. Gerontologist 55, 1079–1099 (2015).
Neufeld, K. J. et al. Delirium diagnosis methodology used in research: a survey-based study. Am. J. Geriatr. Psychiatry 22, 1513–1521 (2014).
Inouye, S. K. et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 113, 941–948 (1990). This study reports the creation and validation of the CAM; a multitude of CAM-based assessment tools for use in specific patient populations have since been validated and used worldwide.
Shenkin, S. D. et al. Delirium detection in older acute medical inpatients: a multicentre prospective comparative diagnostic test accuracy study of the 4AT and the confusion assessment method. BMC Med. 17, 138 (2019). This STARD-compliant randomized controlled diagnostic trial compared the performance of two of the most widely-used delirium detection tools employed in general settings, the 4AT and the CAM.
Heinrich, T. W., Kato, H., Emanuel, C. & Denson, S. Improving the validity of nurse-based delirium screening: a head-to-head comparison of nursing delirium-screening scale and short confusion assessment method. Psychosomatics 60, 172–178 (2019).
Hshieh, T. T., Inouye, S. K. & Oh, E. S. Delirium in the elderly. Clin. Geriatr. Med. 36, 183–199 (2020).
Inouye, S. K., Foreman, M. D., Mion, L. C., Katz, K. H. & Cooney, L. M. Jr. Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch. Intern. Med. 161, 2467–2473 (2001).
Rohatgi, N. et al. Initiative for prevention and early identification of delirium in medical-surgical units: lessons learned in the past five years. Am. J. Med. 132, 1421–1430.e8 (2019).
Reynish, E. L. et al. Epidemiology and outcomes of people with dementia, delirium, and unspecified cognitive impairment in the general hospital: prospective cohort study of 10,014 admissions. BMC Med. 15, 140 (2017).
Marcantonio, E. R. et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann. Intern. Med. 161, 554–561 (2014).
Han, J. H. et al. A quick and easy delirium assessment for nonphysician research personnel. Am. J. Emerg. Med. 34, 1031–1036 (2016).
Bellelli, G. et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 43, 496–502 (2014).
Tieges, Z. M., et al. Diagnostic accuracy of the 4AT for delirium detection: systematic review and meta-analysis. Preprint at medRxiv https://doi.org/10.1101/2020.06.11.20128280 (2020).
Royal College of Physicians. National hip fracture database annual report September 2018. NHFD https://www.nhfd.co.uk/files/2018ReportFiles/NHFD-2018-Annual-Report-v101.pdf (2018).
Maldonado, J. R. et al. A study of the psychometric properties of the “Stanford Proxy Test for Delirium” (S-PTD): a new screening tool for the detection of delirium. Psychosomatics 61, 116–126 (2020).
Ely, E. W. et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond agitation-sedation scale (RASS). JAMA 289, 2983–2991 (2003).
Chester, J. G., Beth Harrington, M., Rudolph, J. L. & VA Delirium Working Group. Serial administration of a modified Richmond agitation and sedation scale for delirium screening. J. Hosp. Med. 7, 450–453 (2012).
Royal College of Physicians. National Early Warning Score (NEWS) 2. Standardising the assessment of acute-illness severity in the NHS: additional implementation guidance. RCP https://www.rcplondon.ac.uk/file/20716/download (2020).
Voyer, P. et al. Recognizing acute delirium as part of your routine [RADAR]: a validation study. BMC Nurs. 14, 19 (2015).
Detroyer, E. et al. Detection of delirium in palliative care unit patients: a prospective descriptive study of the delirium observation screening scale administered by bedside nurses. Palliat. Med. 28, 79–86 (2014).
Gaudreau, J. D., Gagnon, P., Harel, F., Tremblay, A. & Roy, M.-A. Fast, systematic,and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J. Pain Symptom Manage. 29, 368–375 (2005).
Gélinas, C. et al. Delirium assessment tools for use in critically ill adults: a psychometric analysis and systematic review. Crit. Care Nurse 38, 38–49 (2018).
Ely, E. W. et al. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit. Care Med. 29, 1370–1379 (2001).
Gusmao-Flores, D., Salluh, J. I., Chalhub, R. A. & Quarantini, L. C. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit. Care 16, R115 (2012).
Bergeron, N., Dubois, M.-J., Dumont, M., Dial, S. & Skrobik, Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 27, 859–864 (2001).
Fick, D. M. & Marcantonio, E. R. In response to “Preliminary development of an ultrabrief two-item bedside test for delirium”. J. Hosp. Med. 11, 155 (2016).
Lin, H. S. et al. Screening in delirium: a pilot study of two screening tools, the simple query for easy evaluation of consciousness and simple question in delirium. Australas. J. Ageing 34, 259–264 (2015).
Trzepacz, P. T. et al. Validation of the delirium rating scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J. Neuropsychiatry Clin. Neurosci. 13, 229–242 (2001).
Adamis, D. et al. Reliability of delirium rating scale (DRS) and delirium rating scale-revised-98 (DRS-R98) using variance-based multivariate modelling. J. Psychiatr. Res. 47, 966–971 (2013).
Hart, R. P. et al. Validation of a cognitive test for delirium in medical ICU patients. Psychosomatics 37, 533–546 (1996).
Tieges, Z. et al. A smartphone-based test for the assessment of attention deficits in delirium: A case-control diagnostic test accuracy study in older hospitalised patients. PLoS ONE 15, e0227471 (2020).
Tang, E. et al. Development and feasibility of a smartphone-based test for the objective detection and monitoring of attention impairments in delirium in the ICU. J. Crit. Care 48, 104–111 (2018).
Jones, R. N. et al. Assessment of instruments for measurement of delirium severity: a systematic review. JAMA Intern. Med. 179, 231–239 (2019).
Breitbart, W. et al. The memorial delirium assessment scale. J. Pain Symptom Manage. 13, 128–137 (1997).
Inouye, S. K. et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann. Intern. Med. 160, 526–533 (2014).
Ely, E. W. et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291, 1753–1762 (2004).
Scottish Intercollegiate Guidelines Network (SIGN). SIGN 157. Risk reduction and management of delirium: a national clinical guideline. SIGN https://www.sign.ac.uk/media/1423/sign157.pdf (2019).
Hijazi, Z., Lange, P., Watson, R. & Maier, A. B. The use of cerebral imaging for investigating delirium aetiology. Eur. J. Intern. Med. 52, 35–39 (2018).
Haggstrom, L., Welschinger, R. & Caplan, G. A. Functional neuroimaging offers insights into delirium pathophysiology: a systematic review. Australas. J. Ageing 36, 186–192 (2017).
Caplan, G. A., Haggstrom, L. R., Nelson, J. A. & Wegner, E. A. Novel unique pattern of cerebral glucose hypometabolism seen on 2-18F-Fluoro-2-deoxyglucose positron emission tomography in delirium. Alzheimers Dement. 13, P1533 (2017).
Thomas, C. et al. Serum anticholinergic activity and cerebral cholinergic dysfunction: an EEG study in frail elderly with and without delirium. BMC Neurosci. 9, 86 (2008).
Van Der Kooi, A. W. et al. Delirium detection using EEG. Chest 147, 94–101 (2015).
Fleischmann, R. et al. Diagnostic performance and utility of quantitative EEG analyses in delirium: confirmatory results from a large retrospective case-control study. Clin. EEG Neurosci. 50, 111–120 (2019).
Geriatric Medicine Research Collaborative. Delirium is prevalent in older hospital inpatients and associated with adverse outcomes: results of a prospective multi-centre study on World Delirium Awareness Day. BMC Med. 17, 229 (2019).
Copeland, C. & Barron, D. T. “Delirium: an essential component in undergraduate training?”. Nurse Educ. Today 85, 104211 (2020).
Teodorczuk, A., Mukaetova-Ladinska, E., Corbett, S. & Welfare, M. Reconceptualizing models of delirium education: findings of a grounded theory study. Int. Psychogeriatr. 25, 645–655 (2013). Delirium detection, treatment and preventive strategies remain poorly implemented at scale in clinical practice. This study provides novel and crucial insights into the status of delirium in health-care settings, including the necessity for attitudinal change and ownership of responsibility for delirium care in addition to standard educational strategies.
Richardson, S. J., Fisher, J. M. & Teodorczuk, A. The future hospital: a blueprint for effective delirium care. Future Hosp. J. 3, 178–181 (2016).
Vardy, E. et al. Use of a digital delirium pathway and quality improvement to improve delirium detection in the emergency department and outcomes in an acute hospital. Age Ageing 49, 672–678 (2020).
Dormandy, L., Mufti, S., Higgins, E., Bailey, C. & Dixon, M. Shifting the focus: a QI project to improve the management of delirium in patients with hip fracture. Future Healthc. J. 6, 215–219 (2019).
Inouye, S. K. et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N. Engl. J. Med. 340, 669–676 (1999).
Hshieh, T. T., Yang, T., Gartaganis, S. L., Yue, J. & Inouye, S. K. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am. J. Geriatr. Psychiatry 26, 1015–1033 (2018).
National Institute for Health and Care Excellence (NICE). Delirium: prevention, diagnosis and management. NICE https://www.nice.org.uk/guidance/cg103 (2019).
Marcantonio, E. R., Flacker, J. M., Wright, R. J. & Resnick, N. M. Reducing delirium after hip fracture: a randomized trial. J. Am. Geriatr. Soc. 49, 516–522 (2001).
Ludolph, P. et al. Non-pharmacologic multicomponent interventions preventing delirium in hospitalized people. J. Am. Geriatr. Soc. https://doi.org/10.1111/jgs.16565 (2020).
Smith, J. et al. Investigation of ward fidelity to a multicomponent delirium prevention intervention during a multicentre, pragmatic, cluster randomised, controlled feasibility trial. Age Ageing 49, 648–655 (2020).
Woodhouse, R. et al. Interventions for preventing delirium in older people in institutional long-term care. Cochrane Database Syst. Rev. 4, CD009537 (2019).
Ely, E. W., Siegel, M. D. & Inouye, S. K. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin. Respir. Crit. Care Med. 22, 115–126 (2001).
Devlin, J. et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 46, e825–e873 (2018).
Schweickert, W. D. et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 373, 1874–1882 (2009).
Schaller, S. J. et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet 388, 1377–1388 (2016).
Marra, A., Ely, E. W., Pandharipande, P. P. & Patel, M. B. The ABCDEF bundle in critical care. Crit. Care Clin. 33, 225–243 (2017).
Pun, B. T. et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit. Care Med. 47, 3–14 (2019).
Trogrlic´, Z. et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit. Care 19, 157 (2015).
Oh, E. S. et al. Antipsychotics for preventing delirium in hospitalized adults: a systematic review. Ann. Intern. Med. 171, 474–484 (2019).
Asleson, D. R. & Chiu, A. W. Melatonin for delirium prevention in acute medically ill, and perioperative geriatric patients. Aging Med. 3, 132–137 (2020).
Siddiqi, N. et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst. Rev. 3, CD005563 (2016). This Cochrane review examined the evidence base on delirium prevention and concluded that moderate-to-strong evidence exists that such interventions are effective and should be implemented.
Pitkala, K. H., Laurila, J. V., Strandberg, T. E. & Tilvis, R. S. Multicomponent geriatric intervention for elderly inpatients with delirium: a randomized, controlled trial. J. Gerontol. A Biol. Sci. Med. Sci 61, 176–181 (2006).
Bauernfreund, Y., Butler, M., Ragavan, S. & Sampson, E. L. TIME to think about delirium: improving detection and management on the acute medical unit. BMJ Open Qual. 7, e000200 (2018).
Lee, S. Y. et al. Developing delirium best practice: a systematic review of education interventions for healthcare professionals working in inpatient settings. Eur. Geriatr. Med. 11, 1–32 (2020).
Burry, L. et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst. Rev. 6, CD005594 (2018).
Neufeld, K. J., Yue, J., Robinson, T. N., Inouye, S. K. & Needham, D. M. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J. Am. Geriatrics Soc. 64, 705–714 (2016).
Finucane, A. M. et al. Drug therapy for delirium in terminally ill adults. Cochrane Database Syst. Rev. 1, CD004770 (2020).
Tampi, R. R., Tampi, D. J. & Ghori, A. K. Acetylcholinesterase inhibitors for delirium in older adults. Am. J. Alzheimers Dis. Other Demen. 31, 305–310 (2016).
Yu, A. et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst. Rev. 6, CD012494 (2018).
Hov, K. R. et al. The Oslo study of clonidine in elderly patients with delirium; LUCID: a randomised placebo-controlled trial. Int. J. Geriatr. Psychiatry 34, 974–981 (2019).
Carrasco, G. et al. Dexmedetomidine for the treatment of hyperactive delirium refractory to haloperidol in nonintubated ICU patients: a nonrandomized controlled trial. Crit. Care Med. 44, 1295–1306 (2016).
Patel, R. P. et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit. Care Med. 37, 825–832 (2009).
Girard, T. D. et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit. Care Med. 38, 428–437 (2010).
Nikooie, R. et al. Antipsychotics for treating delirium in hospitalized adults: a systematic review. Ann. Intern. Med. 171, 485–495 (2019). This systematic review concluded that there is insufficient evidence supporting the routine use of antipsychotic agents for the treatment of delirium.
Devlin, J. W. et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit. Care Med. 38, 419–427 (2010).
Pandharipande, P. P. et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients. JAMA 298, 2644–2653 (2007).
Riker, R. R. et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 301, 489–499 (2009).
Shehabi, Y. et al. Early sedation with dexmedetomidine in critically ill ventilated patients. N. Engl. J. Med. 380, 2506–2517 (2019). The SPICE-III trial comparing dexmedetomidine sedation with usual-care regimens (propofol, midazolam or other sedatives) in 4,000 critically ill patients ventilated >24 hours; death rate at 90 days was similar for both treatments with more days alive, free of delirium and coma, and ventilator free, with more adverse events reported in dexmedetomidine-treated patients.
Reade, M. C. et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA 315, 1460–1468 (2016).
Balas, M. C. et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit. Care Med. 42, 1024–1036 (2014).
Barnes-Daly, M. A., Phillips, G. & Ely, E. W. Improving hospital survival and reducing brain dysfunction at seven california community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit. Care Med. 45, 171–178 (2017).
O’Neal, H. R. Jr. Lin, J. C., Devlin, J. W. & Ely, E. W. Coronavirus disease 2019: harnessing healthy fear via knowledge, attitudes, and behavior. Crit. Care Explor. 2, e0149 (2020).
Devlin, J. W. et al. Strategies to optimize ICU liberation (A to F) bundle performance in critically ill adults with coronavirus disease 2019. Crit. Care Explor. 2, e0139 (2020).
Helms, J. et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 382, 2268–2270 (2020).
Kotfis, K. et al. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care 24, 176 (2020).
O’Hanlon, S. & Inouye, S. K. Delirium: a missing piece in the COVID-19 pandemic puzzle. Age Ageing 49, 497–498 (2020).
LaHue, S. C. et al. Collaborative delirium prevention in the age of COVID-19. J. Am. Geriatr. Soc. 68, 947–949 (2020).
Sillner, A. Y., Holle, C. L. & Rudolph, J. L. The overlap between falls and delirium in hospitalized older adults: a systematic review. Clin. Geriatr. Med. 35, 221–236 (2019).
Dharmarajan, K., Swami, S., Gou, R. Y., Jones, R. N. & Inouye, S. K. Pathway from delirium to death: potential in-hospital mediators of excess mortality. J. Am. Geriatr. Soc. 65, 1026–1033 (2017).
Israni, J., Lesser, A., Kent, T. & Ko, K. Delirium as a predictor of mortality in US Medicare beneficiaries discharged from the emergency department: a national claims-level analysis up to 12 months. BMJ Open 8, e021258 (2018).
Pisani, M. A. et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am. J. Respir. Crit. Care Med. 180, 1092–1097 (2009).
Altman, M. T. et al. Association of intensive care unit delirium with sleep disturbance and functional disability after critical illness: an observational cohort study. Ann. Intensive Care 8, 63 (2018).
Todd, A. et al. Reduced level of arousal and increased mortality in adult acute medical admissions: a systematic review and meta-analysis. BMC Geriatr. 17, 283 (2017).
Hayhurst, C. J. et al. Association of hypoactive and hyperactive delirium with cognitive function after critical illness. Crit. Care Med. 48, e480–e488 (2020).
Herridge, M. S. et al. Functional disability 5 years after acute respiratory distress syndrome. N. Engl. J. Med. 364, 1293–1304 (2011).
Pitkala, K. H. et al. Multicomponent geriatric intervention for elderly inpatients with delirium: effects on costs and health-related quality of life. J. Gerontol. A Biol. Sci. Med. Sci. 63, 56–61 (2008).
Hshieh, T. T. et al. Trajectory of functional recovery after postoperative delirium in elective surgery. Ann. Surg. 265, 647–653 (2017).
Van Rompaey, B. et al. Long term outcome after delirium in the intensive care unit. J. Clin. Nurs. 18, 3349–3357 (2009).
Abelha, F. J. et al. Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit. Care 17, R257 (2013).
Fann, J. R., Alfano, C. M., Roth-Roemer, S., Katon, W. J. & Syrjala, K. L. Impact of delirium on cognition, distress, and health-related quality of life after hematopoietic stem-cell transplantation. J. Clin. Oncol. 25, 1223–1231 (2007).
Naidech, A. M. et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am. J. Respir. Crit. Care Med. 188, 1331–1337 (2013).
van den Boogaard, M. et al. Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit. Care Med. 40, 112–118 (2012).
Wolters, A. E. et al. Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Crit. Care 18, R125 (2014).
Gill, T. M. & Feinstein, A. R. A critical appraisal of the quality of quality-of-life measurements. JAMA 272, 619–626 (1994).
Bergner, M. Quality of life, health status, and clinical research. Med. Care 27, S148–S156 (1989).
Cella, D. F. Quality of life: concepts and definition. J. Pain Symptom Manage. 9, 186–192 (1994).
Ware, J. E. Jr. & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30, 473–483 (1992).
Ware, J. E. SF-36 Physical and Mental Health Summary Scales: A User’s Manual Vol. 5 (Health Assessment Lab, New England Medical Center, 1994).
Ware, J. Jr. Kosinski, M. & Keller, S. D. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med. Care 34, 220–233 (1996).
Ware, J. E., Kosinski, M. & Keller, S. D. SF-12: how to Score the SF-12 Physical and Mental Health Summary Scales 3rd edn Vol. 3 (Qualitymetric, 1998).
Yang, F., Dawes, P., Leroi, I. & Gannon, B. Measurement tools of resource use and quality of life in clinical trials for dementia or cognitive impairment interventions: a systematically conducted narrative review. Int. J. Geriatr. Psychiatry 33, e166–e176 (2018).
Jackson, J. C., Mitchell, N. & Hopkins, R. O. Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Psychiatr. Clin. North. Am. 38, 91–104 (2015).
Bickel, H., Gradinger, R., Kochs, E. & Forstl, H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement. Geriatr. Cogn. Disord. 26, 26–31 (2008).
Fong, T. G. et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 72, 1570–1575 (2009).
Fong, T. G. et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann. Intern. Med. 156, 848–856, W296 (2012).
Saczynski, J. S. et al. Cognitive trajectories after postoperative delirium. N. Engl. J. Med. 367, 30–39 (2012).
Hopkins, R. O. et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 160, 50–56 (1999).
Girard, T. D. et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit. Care Med. 38, 1513–1520 (2010).
Rothenhausler, H. B., Ehrentraut, S., Stoll, C., Schelling, G. & Kapfhammer, H. P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen. Hosp. Psychiatry 23, 90–96 (2001).
Mikkelsen, M. E. et al. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology 14, 76–82 (2009).
Hopkins, R. O. et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 171, 340–347 (2005).
Phillips-Bute, B. et al. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom. Med. 68, 369–375 (2006).
Lewis, M. B. & Howdle, P. D. Cognitive dysfunction and health-related quality of life in long-term liver transplant survivors. Liver Transplant. 9, 1145–1148 (2003).
Norman, B. C. et al. Employment outcomes after critical illness: an analysis of the bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors cohort. Crit. Care Med. 44, 2003–2009 (2016).
Myhren, H., Ekeberg, O. & Stokland, O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit. Care Med. 38, 1554–1561 (2010).
Girard, T. D. et al. Risk factors for posttraumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit. Care 11, R28 (2007).
Jackson, J. C. et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir. Med. 2, 369–379 (2014).
Hopkins, R. O., Weaver, L. K., Chan, K. J. & Orme, J. F. Jr. Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J. Int. Neuropsychol. Soc. 10, 1005–1017 (2004).
Weinert, C. R., Gross, C. R., Kangas, J. R., Bury, C. L. & Marinelli, W. A. Health-related quality of life after acute lung injury. Am. J. Respir. Crit. Care Med. 156, 1120–1128 (1997).
Kapfhammer, H. P., Rothenhausler, H. B., Krauseneck, T., Stoll, C. & Schelling, G. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am. J. Psychiatry 161, 45–52 (2004).
Duggan, M. C. et al. The relationship between executive dysfunction, depression, and mental health-related quality of life in survivors of critical illness: results from the BRAIN-ICU investigation. J. Crit. Care 37, 72–79 (2017).
Wilson, I. B. & Cleary, P. D. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 273, 59–65 (1995).
Dinglas, V. D. et al. Perspectives of survivors, families and researchers on key outcomes for research in acute respiratory failure. Thorax 73, 7–12 (2018).
O’Keeffe, S. & Lavan, J. The prognostic significance of delirium in older hospital patients. J. Am. Geriatr. Soc. 45, 174–178 (1997).
Brummel, N. E. et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit. Care Med. 42, 369–377 (2014).
Oldham, M. A., Flaherty, J. H. & Maldonado, J. R. Refining delirium: a transtheoretical model of delirium disorder with preliminary neurophysiologic subtypes. Am. J. Geriatr. Psychiatry 26, 913–924 (2018).
Meagher, D. J. et al. A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, etiology, medication exposure and prognosis. J. Psychosom. Res. 71, 395–403 (2011).
Gual, N. et al. Delirium subtypes and associated characteristics in older patients with exacerbation of chronic conditions. Am. J. Geriatr. Psychiatry 26, 1204–1212 (2018).
Kiely, D. K., Jones, R. N., Bergmann, M. A. & Marcantonio, E. R. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J. Gerontol. A Biol. Sci. Med. Sci. 62, 174–179 (2007).
Robinson, T. N., Raeburn, C. D., Tran, Z. V., Brenner, L. A. & Moss, M. Motor subtypes of postoperative delirium in older adults. Arch. Surg. 146, 295–300 (2011).
Bellelli, G., Speciale, S., Barisione, E. & Trabucchi, M. Delirium subtypes and 1-year mortality among elderly patients discharged from a post-acute rehabilitation facility. J. Gerontol. A Biol. Sci. Med. Sci. 62, 1182–1183 (2007).
Peritogiannis, V., Bolosi, M., Lixouriotis, C. & Rizos, D. V. Recent insights on prevalence and corelations of hypoactive delirium. Behav. Neurol. 2015, 416792 (2015).
Yang, F. M. et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics 50, 248–254 (2009).
Mudge, A. M. et al. CHERISH (collaboration for hospitalised elders reducing the impact of stays in hospital): protocol for a multi-site improvement program to reduce geriatric syndromes in older inpatients. BMC Geriatr. 17, 11 (2017).
Saper, C. B., Scammell, T. E. & Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263 (2005).
Halaas, N. B. et al. Neurofilament light in serum and cerebrospinal fluid of hip fracture patients with delirium. Dement. Geriatr. Cogn. Disord. 46, 346–357 (2018).
Idland, A. V. et al. CSF neurofilament light levels predict hippocampal atrophy in cognitively healthy older adults. Neurobiol. Aging 49, 138–144 (2017).
Casey, C. P. et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain 143, 47–54 (2019).
Semmler, A. et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J. Neurol. Neurosurg. Psychiatry 84, 62–69 (2013).
Torvell, M. et al. A single systemic inflammatory insult causes acute motor deficits and accelerates disease progression in a mouse model of human tauopathy. Alzheimers Dement. 5, 579–591 (2019).
Acknowledgements
J.E.W. acknowledges salary support from the Vanderbilt Clinical and Translational Research Scholars program (1KL2TR002245) and from NIH grants (GM120484 and HL111111). M.F.M. acknowledges training and salary support from the Vanderbilt Clinical and Translational Training Program in Pulmonary Medicine (NIH 5T32HL087738) and research funding from the Vanderbilt University Medical Center Arthur and Lisa Wheeler Critical Care Research Fund. C.C. acknowledges research grants from the NIH (AG050626), WT SRF090907 and ARUK. Y.S. acknowledges research grants from the National Health and Medical Research Council of Australia. T.D.G. acknowledges support from NIH grants (HL135144 and HL143507). A.M.J.M. acknowledges funding from the Medical Research Council and the National Institute for Health Research. E.W.E. discloses additional funding for his time from NIH grant AG027472 and salary support from the Tennessee Valley Healthcare System Geriatric Research Education and Clinical Center (GRECC).
Author information
Authors and Affiliations
Contributions
Introduction (J.E.W., A.J.C.S., A.M.J.M. and E.W.E.); Epidemiology (J.E.W., A.M.J.M. and E.W.E.); Mechanisms/pathophysiology (C.C. and A.J.C.S.); Diagnosis, screening and prevention (J.E.W., Y.S., A.M.J.M. and E.W.E.); Management (J.E.W., T.D.G., A.M.J.M. and E.W.E.); Quality of life (J.E.W., M.F.M. and E.W.E.); Outlook (J.E.W., A.M.J.M. and E.W.E.); Overview of Primer (J.E.W.). All authors listed above have contributed substantially to the conception or design of the work or to the acquisition, analysis, or interpretation of data for the work and have participated in drafting the work or revising it critically for important intellectual content. Additionally, each author has given their approval to the final version of the manuscript and has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
C.C. acknowledges a current research collaboration with IONIS pharmaceuticals. Y.S. acknowledges unrestricted research grants from Pfizer, Orion Pharma, and Brainstem Biometrics and travel reimbursements and speaker honorarium for educational non-promotional symposia from Pfizer and Orion Pharma. A.J.C.S. is an adviser for Prolira, a start-up company that develops an EEG-based delirium monitor; any (future) profits from EEG-based delirium monitoring will be used for future scientific research only. A.M.J.M. is the main author of the 4AT delirium assessment tool (this tool is free to use and there is no financial interest) and holds patents for computerized delirium assessment tools (any future profits will be used for future scientific research only). E.W.E. received honoraria from Orion and Hospira for continuing medical education activity but does not hold stock or consultant relationships with these companies. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks K. Neufeld, Y. Skrobik, M. Agar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Network for Investigation of Delirium: Unifying Scientists (NIDUS): https://deliriumnetwork.org/
Preserving your brain health during illness or surgery: GCBH recommendations to prevent and treat delirium: https://www.aarp.org/health/brain-health/global-council-on-brain-health/delirium/
The Critical Illness, Brain Dysfunction and Survivorship (CIBS) Center: https://www.icudelirium.org/cibs-center/overview
The Hospital Elder Life Program (HELP): https://www.hospitalelderlifeprogram.org
Rights and permissions
About this article
Cite this article
Wilson, J.E., Mart, M.F., Cunningham, C. et al. Delirium. Nat Rev Dis Primers 6, 90 (2020). https://doi.org/10.1038/s41572-020-00223-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-020-00223-4
This article is cited by
-
The role of diabetes mellitus on delirium onset: a systematic review and meta-analysis
Cardiovascular Diabetology (2025)
-
Effect of preoperative oral carbohydrate on postoperative delirium in elderly patients undergoing lower extremity orthopedic surgery: a prospective randomized trial
Journal of Orthopaedic Surgery and Research (2025)
-
Serum biomarkers of delirium in critical illness: a systematic review of mechanistic and diagnostic evidence
Intensive Care Medicine Experimental (2025)
-
Effectiveness of perioperative remimazolam in preventing postoperative delirium: a systematic review and meta-analysis
European Journal of Medical Research (2025)
-
Risk factors and outcomes of hyperactive delirium in older medical inpatients admitted to non-intensive care unit: a prospective cohort study
BMC Psychiatry (2025)