Abstract
Duchenne muscular dystrophy is a severe, progressive, muscle-wasting disease that leads to difficulties with movement and, eventually, to the need for assisted ventilation and premature death. The disease is caused by mutations in DMD (encoding dystrophin) that abolish the production of dystrophin in muscle. Muscles without dystrophin are more sensitive to damage, resulting in progressive loss of muscle tissue and function, in addition to cardiomyopathy. Recent studies have greatly deepened our understanding of the primary and secondary pathogenetic mechanisms. Guidelines for the multidisciplinary care for Duchenne muscular dystrophy that address obtaining a genetic diagnosis and managing the various aspects of the disease have been established. In addition, a number of therapies that aim to restore the missing dystrophin protein or address secondary pathology have received regulatory approval and many others are in clinical development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mercuri, E., Bonnemann, C. G. & Muntoni, F. Muscular dystrophies. Lancet 394, 2025–2038 (2019). Comprehensive overview of the clinical and genetic aspects of muscular dystrophies.
Aartsma-Rus, A., Van Deutekom, J. C. T., Fokkema, I. F., Van Ommen, G. J. B. & Den Dunnen, J. T. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 34, 135–144 (2006).
Monaco, A. P., Bertelson, C. J., Liechti-Gallati, S., Moser, H. & Kunkel, L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 2, 90–95 (1988).
García-Rodríguez, R. et al. Premature termination codons in the DMD gene cause reduced local mRNA synthesis. Proc. Natl Acad. Sci. USA 117, 16456–16464 (2020).
Mendell, J. R. et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann. Neurol. 71, 304–313 (2012).
Ryder, S. et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J. Rare Dis. 12, 79 (2017).
Mah, J. K. et al. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul. Disord. 24, 482–491 (2014). Systematic study into the global epidemiology of DMD and BMD.
Bladen, C. L. et al. The TREAT-NMD Duchenne muscular dystrophy registries: conception, design, and utilization by industry and academia. Hum. Mutat. 34, 1449–1457 (2013).
Kieny, P. et al. Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann. Phys. Rehabil. Med. 56, 443–454 (2013).
Ferrier, P., Bamatter, F. & Klein, D. Muscular dystrophy (Duchenne) in a girl with Turner’s syndrome. J. Med. Genet. 2, 38–46 (1965).
Chelly, J. et al. De novo DNA microdeletion in a girl with Turner syndrome and Duchenne muscular dystrophy. Hum. Genet. 74, 193–196 (1986).
Satre, V., Monnier, N., Devillard, F., Amblard, F. & Lunardi, J. Prenatal diagnosis of DMD in a female foetus affected by Turner syndrome. Prenat. Diagn. 24, 913–917 (2004).
Takeshita, E. et al. Duchenne muscular dystrophy in a female with compound heterozygous contiguous exon deletions. Neuromuscul. Disord. 27, 569–573 (2017).
Ishizaki, M., Kobayashi, M., Adachi, K., Matsumura, T. & Kimura, E. Female dystrophinopathy: review of current literature. Neuromuscul. Disord. 28, 572–581 (2018).
Holloway, S. M. et al. Life expectancy and death from cardiomyopathy amongst carriers of Duchenne and Becker muscular dystrophy in Scotland. Heart 94, 633–636 (2008).
Bladen, C. L. et al. The TREAT-NMD DMD global database: analysis of more than 7000 Duchenne muscular dystrophy mutations. Hum. Mutat. 36, 395–402 (2015). Systematic analysis of different mutation types and locations of mutations in patients around the world.
Magri, F. et al. Genotype and phenotype characterization in a large dystrophinopathic cohort with extended follow-up. J. Neurol. 258, 1610–1623 (2011).
Garcia, S. et al. Identification of de novo mutations of Duchenne/Becker muscular dystrophies in southern Spain. Int. J. Med. Sci. 11, 988–993 (2014).
Kesari, A. et al. Integrated DNA, cDNA, and protein studies in Becker muscular dystrophy show high exception to the reading frame rule. Hum. Mutat. 29, 728–737 (2008).
Nakamura, A. et al. Comparison of the phenotypes of patients harboring in-frame deletions starting at exon 45 in the Duchenne muscular dystrophy gene indicates potential for the development of exon skipping therapy. J. Hum. Genet. 62, 459–463 (2017).
Nakamura, A. et al. Deletion of exons 3-9 encompassing a mutational hot spot in the DMD gene presents an asymptomatic phenotype, indicating a target region for multiexon skipping therapy. J. Hum. Genet. 61, 663–667 (2016).
Nevin, N. C., Hughes, A. E., Calwell, M. & Lim, J. H. Duchenne muscular dystrophy in a female with a translocation involving Xp21. J. Med. Genet. 23, 171–173 (1986).
Chen, W. J. et al. Molecular analysis of the dystrophin gene in 407 Chinese patients with Duchenne/Becker muscular dystrophy by the combination of multiplex ligation-dependent probe amplification and Sanger sequencing. Clin. Chim. Acta 423, 35–38 (2013).
Yu, H., Chen, Y. C., Liu, G. L. & Wu, Z. Y. A de novo mutation in dystrophin causing muscular dystrophy in a female patient. Chin. Med. J. 130, 2273–2278 (2017).
Caskey, C. T., Nussbaum, R. L., Cohan, L. C. & Pollack, L. Sporadic occurrence of Duchenne muscular dystrophy: evidence for new mutation. Clin. Genet. 18, 329–341 (1980).
Haldane, J. B. The rate of spontaneous mutation of a human gene. 1935. J. Genet. 83, 235–244 (2004).
Helderman-van den Enden, A. T. et al. Recurrence risk due to germ line mosaicism: Duchenne and Becker muscular dystrophy. Clin. Genet. 75, 465–472 (2009).
Dreyfus, J. C., Schapira, G. & Schapira, F. Serum enzymes in the physiopathology of muscle. Ann. N. Y. Acad. Sci. 75, 235–249 (1958).
Moser, H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum. Genet. 66, 17–40 (1984).
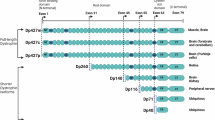
Koenig, M. et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50, 509–517 (1987).
Hoffman, E. P., Brown, R. H. Jr. & Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 (1987). This paper reports the discovery of the dystrophin protein and its absence in DMD.
Chelly, J. et al. Dystrophin gene transcribed from different promoters in neuronal and glial cells. Nature 344, 64–65 (1990).
Górecki, D. C. et al. Expression of four alternative dystrophin transcripts in brain regions regulated by different promoters. Hum. Mol. Genet. 1, 505–510 (1992).
Doorenweerd, N. et al. Timing and localization of human dystrophin isoform expression provide insights into the cognitive phenotype of Duchenne muscular dystrophy. Sci. Rep. 7, 12575 (2017).
D’Souza, V. N. et al. A novel dystrophin isoform is required for normal retinal electrophysiology. Hum. Mol. Genet. 4, 837–842 (1995).
Lidov, H. G., Selig, S. & Kunkel, L. M. Dp140: a novel 140 kDa CNS transcript from the dystrophin locus. Hum. Mol. Genet. 4, 329–335 (1995).
Byers, T. J., Lidov, H. G. & Kunkel, L. M. An alternative dystrophin transcript specific to peripheral nerve. Nat. Genet. 4, 77–81 (1993).
Hugnot, J. P. et al. Distal transcript of the dystrophin gene initiated from an alternative first exon and encoding a 75-kDa protein widely distributed in nonmuscle tissues. Proc. Natl Acad. Sci. USA 89, 7506–7510 (1992).
Tinsley, J. M., Blake, D. J. & Davies, K. E. Apo-dystrophin-3: a 2.2kb transcript from the DMD locus encoding the dystrophin glycoprotein binding site. Hum. Mol. Genet. 2, 521–524 (1993).
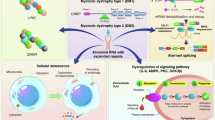
Gao, Q. Q. & McNally, E. M. The dystrophin complex: structure, function, and implications for therapy. Compr. Physiol. 5, 1223–1239 (2015).
Ervasti, J. M. & Sonnemann, K. J. Biology of the striated muscle dystrophin-glycoprotein complex. Int. Rev. Cytol. 265, 191–225 (2008).
Zhao, J. et al. Dystrophin contains multiple independent membrane-binding domains. Hum. Mol. Genet. 25, 3647–3653 (2016).
Amann, K. J., Renley, B. A. & Ervasti, J. M. A cluster of basic repeats in the dystrophin rod domain binds F-actin through an electrostatic interaction. J. Biol. Chem. 273, 28419–28423 (1998).
Prins, K. W. et al. Dystrophin is a microtubule-associated protein. J. Cell Biol. 186, 363–369 (2009).
Nelson, D. M. et al. Rapid, redox-mediated mechanical susceptibility of the cortical microtubule lattice in skeletal muscle. Redox Biol. 37, 101730 (2020).
Stone, M. R., O’Neill, A., Catino, D. & Bloch, R. J. Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol. Biol. Cell 16, 4280–4293 (2005).
Bhosle, R. C., Michele, D. E., Campbell, K. P., Li, Z. & Robson, R. M. Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochem. Biophys. Res. Commun. 346, 768–777 (2006).
Huang, X. et al. Structure of a WW domain containing fragment of dystrophin in complex with beta-dystroglycan. Nat. Struct. Biol. 7, 634–638 (2000).
Ayalon, G., Davis, J. Q., Scotland, P. B. & Bennett, V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135, 1189–1200 (2008).
Rezniczek, G. A. et al. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J. Cell Biol. 176, 965–977 (2007).
Yamashita, K. et al. The 8th and 9th tandem spectrin-like repeats of utrophin cooperatively form a functional unit to interact with polarity-regulating kinase PAR-1b. Biochem. Biophys. Res. Commun. 391, 812–817 (2010).
Lai, Y. et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest. 119, 624–635 (2009). This paper showed, for the first time, the direct binding of dystrophin to nNOS.
Anderson, J. T., Rogers, R. P. & Jarrett, H. W. Ca2+-calmodulin binds to the carboxyl-terminal domain of dystrophin. J. Biol. Chem. 271, 6605–6610 (1996).
Reynolds, J. G., McCalmon, S. A., Donaghey, J. A. & Naya, F. J. Deregulated protein kinase A signaling and myospryn expression in muscular dystrophy. J. Biol. Chem. 283, 8070–8074 (2008).
Constantin, B. Dystrophin complex functions as a scaffold for signalling proteins. Biochim. Biophys. Acta 1838, 635–642 (2014).
Allen, D. G., Whitehead, N. P. & Froehner, S. C. Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol. Rev. 96, 253–305 (2016).
Lai, Y., Zhao, J., Yue, Y. & Duan, D. α2 and α3 helices of dystrophin R16 and R17 frame a microdomain in the alpha1 helix of dystrophin R17 for neuronal NOS binding. Proc. Natl Acad. Sci. USA 110, 525–530 (2013).
Chang, N. C. et al. The dystrophin glycoprotein complex regulates the epigenetic activation of muscle stem cell commitment. Cell Stem Cell 22, 755–768.e6 (2018).
Lumeng, C. et al. Interactions between β2-syntrophin and a family of microtubule- associated serine/threonine kinases. Nat. Neurosci. 2, 611–617 (1999).
Sarparanta, J. Biology of myospryn: what’s known? J. Muscle Res. Cell Motil. 29, 177–180 (2008).
Sugita, S. et al. A stoichiometric complex of neurexins and dystroglycan in brain. J. Cell Biol. 154, 435–445 (2001).
Mokri, B. & Engel, A. G. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology 25, 1111–1120 (1975).
Aartsma-Rus, A. & van Putten, M. Assessing functional performance in the mdx mouse model. J. Vis. Exp. 85, 51303 (2014).
Stedman, H. H. et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352, 536–539 (1991).
Duan, D. Systemic AAV micro-dystrophin gene therapy for Duchenne muscular dystrophy. Mol. Ther. 26, 2337–2356 (2018). Comprehensive overview of microdystrophin gene therapy development, its opportunities and challenges.
Bradley, W. G., Hudgson, P., Larson, P. F., Papapetropoulos, T. A. & Jenkison, M. Structural changes in the early stages of Duchenne muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 35, 451–455 (1972).
Pearson, C. M. Histopathological features of muscle in the preclinical stages of muscular dystrophy. Brain 85, 109–120 (1962).
Brenman, J. E., Chao, D. S., Xia, H., Aldape, K. & Bredt, D. S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82, 743–752 (1995).
Sander, M. et al. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc. Natl Acad. Sci. USA 97, 13818–13823 (2000).
Patel, A. et al. Dystrophin R16/17-syntrophin PDZ fusion protein restores sarcolemmal nNOSmu. Skelet. Muscle 8, 36 (2018).
Kodippili, K. et al. Dual AAV gene therapy for Duchenne muscular dystrophy with a 7-kb mini-dystrophin gene in the canine model. Hum. Gene Ther. 29, 299–311 (2018).
Prosser, B. L., Ward, C. W. & Lederer, W. J. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333, 1440–1445 (2011).
Khairallah, R. J. et al. Microtubules underlie dysfunction in Duchenne muscular dystrophy. Sci. Signal. 5, ra56 (2012).
Li, D., Yue, Y., Lai, Y., Hakim, C. H. & Duan, D. Nitrosative stress elicited by nNOSmu delocalization inhibits muscle force in dystrophin-null mice. J. Pathol. 223, 88–98 (2011).
Kim, J. H., Kwak, H. B., Thompson, L. V. & Lawler, J. M. Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy. J. Muscle Res. Cell Motil. 34, 1–13 (2013).
Grounds, M. D. et al. Biomarkers for Duchenne muscular dystrophy: myonecrosis, inflammation and oxidative stress. Dis. Models Mech. 13, dmm.043638 (2020).
Rando, T. A. Role of nitric oxide in the pathogenesis of muscular dystrophies: a “two hit” hypothesis of the cause of muscle necrosis. Microsc. Res. Tech. 55, 223–235 (2001).
Dudley, R. W. et al. Dynamic responses of the glutathione system to acute oxidative stress in dystrophic mouse (mdx) muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R704–710 (2006).
Petrillo, S. et al. Oxidative stress in Duchenne muscular dystrophy: focus on the NRF2 redox pathway. Hum. Mol. Genet. 26, 2781–2790 (2017).
Turner, P. R., Westwood, T., Regen, C. M. & Steinhardt, R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335, 735–738 (1988).
Millay, D. P. et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med. 14, 442–447 (2008).
Phillips, M. F. & Quinlivan, R. Calcium antagonists for Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 4, CD004571 (2008).
Kyrychenko, S. et al. Hierarchical accumulation of RyR post-translational modifications drives disease progression in dystrophic cardiomyopathy. Cardiovasc. Res. 97, 666–675 (2013).
Bellinger, A. M. et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 15, 325–330 (2009).
Kushnir, A., Wajsberg, B. & Marks, A. R. Ryanodine receptor dysfunction in human disorders. Biochim. Biophys. Acta Mol. Cell Res. 1865, 1687–1697 (2018).
Capogrosso, R. F. et al. Ryanodine channel complex stabilizer compound S48168/ARM210 as a disease modifier in dystrophin-deficient mdx mice: proof-of-concept study and independent validation of efficacy. FASEB J. 32, 1025–1043 (2018).
Voit, A. et al. Reducing sarcolipin expression mitigates Duchenne muscular dystrophy and associated cardiomyopathy in mice. Nat. Commun. 8, 1068 (2017).
Wasala, N. B. et al. Single SERCA2a therapy ameliorated dilated cardiomyopathy for 18 months in a mouse model of Duchenne muscular dystrophy. Mol. Ther. 28, 845–854 (2020).
Dumont, N. A. et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 21, 1455–1463 (2015).
Bello, L. & Pegoraro, E. The “usual suspects”: genes for inflammation, fibrosis, regeneration, and muscle strength modify Duchenne muscular dystrophy. J. Clin. Med. 8, 649 (2019).
Cappellari, O., Mantuano, P. & De Luca, A. “The Social Network” and muscular dystrophies: the lesson learnt about the niche environment as a target for therapeutic strategies. Cells 9, 1659 (2020).
Grounds, M. D. Two-tiered hypotheses for Duchenne muscular dystrophy. Cell. Mol. Life Sci. 65, 1621–1625 (2008).
Sandri, M., Coletto, L., Grumati, P. & Bonaldo, P. Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J. Cell Sci. 126, 5325–5333 (2013).
De Palma, C. et al. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis. 3, e418 (2012).
Pal, R. et al. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat. Commun. 5, 4425 (2014).
Xiong, Y., Zhou, Y. & Jarrett, H. W. Dystrophin glycoprotein complex-associated Gbetagamma subunits activate phosphatidylinositol-3-kinase/Akt signaling in skeletal muscle in a laminin-dependent manner. J. Cell. Physiol. 219, 402–414 (2009).
De Palma, C., Perrotta, C., Pellegrino, P., Clementi, E. & Cervia, D. Skeletal muscle homeostasis in Duchenne muscular dystrophy: modulating autophagy as a promising therapeutic strategy. Front. Aging Neurosci. 6, 188 (2014).
Evans, N. P., Misyak, S. A., Robertson, J. L., Bassaganya-Riera, J. & Grange, R. W. Immune-mediated mechanisms potentially regulate the disease time-course of Duchenne muscular dystrophy and provide targets for therapeutic intervention. PM R 1, 755–768 (2009).
Tidball, J. G., Welc, S. S. & Wehling-Henricks, M. Immunobiology of inherited muscular dystrophies. Compr. Physiol. 8, 1313–1356 (2018).
Rosenberg, A. S. et al. Immune-mediated pathology in Duchenne muscular dystrophy. Sci. Transl. Med. 7, 299rv294 (2015).
Bello, L. et al. Functional changes in Becker muscular dystrophy: implications for clinical trials in dystrophinopathies. Sci. Rep. 6, 32439 (2016).
Wasala, L. et al. Cardiac specific expression of ∆H2-R15 mini-dystrophin normalized all ECG abnormalities and the end-diastolic volume in a 23-m-old mouse model of Duchenne dilated cardiomyopathy. Hum. Gene Ther. 29, 737–748 (2018).
Vo, A. H. & McNally, E. M. Modifier genes and their effect on Duchenne muscular dystrophy. Curr. Opin. Neurol. 28, 528–534 (2015).
Ricotti, V. et al. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev. Med. Child Neurol. 58, 77–84 (2016).
Bello, L. et al. Association study of exon variants in the NF-κB and TGFβ pathways identifies CD40 as a modifier of Duchenne muscular dystrophy. Am. J. Hum. Genet. 99, 1163–1171 (2016).
Spitali, P. et al. TCTEX1D1 is a genetic modifier of disease progression in Duchenne muscular dystrophy. Eur. J. Hum. Genet. 28, 815–825 (2020).
Klietsch, R., Ervasti, J. M., Arnold, W., Campbell, K. P. & Jorgensen, A. O. Dystrophin-glycoprotein complex and laminin colocalize to the sarcolemma and transverse tubules of cardiac muscle. Circ. Res. 72, 349–360 (1993).
Meng, H., Leddy, J. J., Frank, J., Holland, P. & Tuana, B. S. The association of cardiac dystrophin with myofibrils/Z-disc regions in cardiac muscle suggests a novel role in the contractile apparatus. J. Biol. Chem. 271, 12364–12371 (1996).
Johnson, E. K. et al. Proteomic analysis reveals new cardiac-specific dystrophin-associated proteins. PLoS ONE 7, e43515 (2012).
Thangarajh, M. et al. Relationships between DMD mutations and neurodevelopment in dystrophinopathy. Neurology 93, e1597–e1604 (2019).
Doorenweerd, N. et al. Reduced cerebral gray matter and altered white matter in boys with Duchenne muscular dystrophy. Ann. Neurol. 76, 403–411 (2014).
Pilgram, G. S., Potikanond, S., Baines, R. A., Fradkin, L. G. & Noordermeer, J. N. The roles of the dystrophin-associated glycoprotein complex at the synapse. Mol. Neurobiol. 41, 1–21 (2010).
Huard, J., Côté, P. Y., Parent, A., Bouchard, J. P. & Tremblay, J. P. Dystrophin-like immunoreactivity in monkey and human brain areas involved in learning and motor functions. Neurosci. Lett. 141, 181–186 (1992).
Chieffo, D. et al. Early neurodevelopmental findings predict school age cognitive abilities in Duchenne muscular dystrophy: a longitudinal study. PLoS ONE 10, e0133214 (2015).
Cohen, E. J., Quarta, E., Fulgenzi, G. & Minciacchi, D. Acetylcholine, GABA and neuronal networks: a working hypothesis for compensations in the dystrophic brain. Brain Res. Bull. 110, 1–13 (2015).
Doorenweerd, N. Combining genetics, neuropsychology and neuroimaging to improve understanding of brain involvement in Duchenne muscular dystrophy — a narrative review. Neuromuscul. Disord. 30, 437–442 (2020). Comprehensive and clear review on brain involvement of dystrophin.
Birnkrant, D. J. et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 17, 251–267 (2018). Part 1 of a three-part standard-of-care document for DMD.
Aartsma-Rus, A. et al. Evidence-based consensus and systematic review on reducing the time to diagnosis of Duchenne muscular dystrophy. J. Pediatr. 204, 305–313.e314 (2019).
Aartsma-Rus, A., Ginjaar, I. B. & Bushby, K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J. Med. Genet. 53, 145–151 (2016). Educational paper on how different mutations cause DMD and how they can be detected with diagnostic techniques.
Verhaart, I. E. C. & Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 15, 373–386 (2019).
Janssen, B., Hartmann, C., Scholz, V., Jauch, A. & Zschocke, J. MLPA analysis for the detection of deletions, duplications and complex rearrangements in the dystrophin gene: potential and pitfalls. Neurogenetics 6, 29–35 (2005).
Chamberlain, J. S. et al. Diagnosis of Duchenne and Becker muscular dystrophies by polymerase chain reaction. A multicenter study. JAMA 267, 2609–2615 (1992).
Aartsma-Rus, A. et al. Report of a TREAT-NMD/World Duchenne organisation meeting on dystrophin quantification methodology. J. Neuromuscul. Dis. 6, 147–159 (2019).
Scheuerbrandt, G. Screening for Duchenne muscular dystrophy in Germany, 1977–2011: a personal story. Muscle Nerve 57, 185–188 (2018).
Moat, S. J. et al. Characterization of a blood spot creatine kinase skeletal muscle isoform immunoassay for high-throughput newborn screening of Duchenne muscular dystrophy. Clin. Chem. 63, 908–914 (2017).
Gatheridge, M. A. et al. Identifying non-Duchenne muscular dystrophy-positive and false negative results in prior Duchenne muscular dystrophy newborn screening programs: a review. JAMA Neurol. 73, 111–116 (2016).
Wood, M. F. et al. Parental attitudes toward newborn screening for Duchenne/Becker muscular dystrophy and spinal muscular atrophy. Muscle Nerve 49, 822–828 (2014).
Bianchi, D. W. & Chiu, R. W. K. Sequencing of circulating cell-free DNA during pregnancy. N. Engl. J. Med. 379, 464–473 (2018).
Brison, N. et al. Maternal copy-number variations in the DMD gene as secondary findings in noninvasive prenatal screening. Genet. Med. 21, 2774–2780 (2019).
Tuffery-Giraud, S. et al. Genotype-phenotype analysis in 2,405 patients with a dystrophinopathy using the UMD-DMD database: a model of nationwide knowledgebase. Hum. Mutat. 30, 934–945 (2009).
Eagle, M. et al. Managing Duchenne muscular dystrophy–the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul. Disord. 17, 470–475 (2007).
Moxley, R. T. 3rd, Pandya, S., Ciafaloni, E., Fox, D. J. & Campbell, K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J. Child Neurol. 25, 1116–1129 (2010).
Saito, T. et al. Study of Duchenne muscular dystrophy long-term survivors aged 40 years and older living in specialized institutions in Japan. Neuromuscul. Disord. 27, 107–114 (2017).
Birnkrant, D. J. et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 17, 347–361 (2018). Part 2 of a three-part standard-of-care document for DMD.
Birnkrant, D. J. et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 17, 445–455 (2018). Part 3 of a three-part standard-of-care document for DMD.
Passamano, L. et al. Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myol. 31, 121–125 (2012).
Finder, J. D. et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am. J. Respir. Crit. Care Med. 170, 456–465 (2004).
Buddhe, S. et al. Cardiac management of the patient with Duchenne muscular dystrophy. Pediatrics 142 (Suppl. 2), S72–S81 (2018).
McNally, E. M. et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation 131, 1590–1598 (2015).
Duboc, D. et al. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J. Am. Coll. Cardiol. 45, 855–857 (2005).
Duboc, D. et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow-up. Am. Heart J. 154, 596–602 (2007).
Yilmaz, O., Karaduman, A. & Topaloğlu, H. Prednisolone therapy in Duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. Eur. J. Neurol. 11, 541–544 (2004).
Bertrand, L. A., Askeland, E. J., Mathews, K. D., Erickson, B. A. & Cooper, C. S. Prevalence and bother of patient-reported lower urinary tract symptoms in the muscular dystrophies. J. Pediatr. Urol. 12, 398.e391–398.e4 (2016).
Haenggi, T., Schaub, M. C. & Fritschy, J. M. Molecular heterogeneity of the dystrophin-associated protein complex in the mouse kidney nephron: differential alterations in the absence of utrophin and dystrophin. Cell Tissue Res. 319, 299–313 (2005).
Matsumura, T., Saito, T., Fujimura, H. & Sakoda, S. Renal dysfunction is a frequent complication in patients with advanced stage of Duchenne muscular dystrophy [Japanese]. Rinsho Shinkeigaku 52, 211–217 (2012).
Banihani, R. et al. Cognitive and neurobehavioral profile in boys with Duchenne muscular dystrophy. J. Child Neurol. 30, 1472–1482 (2015).
Biggar, W. D. et al. Deflazacort in Duchenne muscular dystrophy: a comparison of two different protocols. Neuromuscul. Disord. 14, 476–482 (2004).
McDonald, C. M. et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet 391, 451–461 (2018).
McDonald, C. M. et al. Deflazacort vs prednisone treatment for Duchenne muscular dystrophy: a meta-analysis of disease progression rates in recent multicenter clinical trials. Muscle Nerve 61, 26–35 (2020).
Bylo, M., Farewell, R., Coppenrath, V. A. & Yogaratnam, D. A review of deflazacort for patients with Duchenne muscular dystrophy. Ann. Pharmacother. 54, 788–794 (2020).
Ricotti, V. et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 84, 698–705 (2013).
Matthews, E., Brassington, R., Kuntzer, T., Jichi, F. & Manzur, A. Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 5, CD003725 (2016).
Connolly, A. M. et al. Twice-weekly glucocorticosteroids in infants and young boys with Duchenne muscular dystrophy. Muscle Nerve 59, 650–657 (2019).
Angelini, C. & Peterle, E. Old and new therapeutic developments in steroid treatment in Duchenne muscular dystrophy. Acta Myol. 31, 9–15 (2012).
Jaisser, F. & Farman, N. Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol. Rev. 68, 49–75 (2016).
Bushby, K. et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve 50, 477–487 (2014).
McDonald, C. M. et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 1489–1498 (2017).
Haas, M. et al. European medicines agency review of ataluren for the treatment of ambulant patients aged 5 years and older with Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene. Neuromuscul. Disord. 25, 5–13 (2015).
Mercuri, E. et al. Safety and effectiveness of ataluren: comparison of results from the STRIDE registry and CINRG DMD Natural History Study. J. Comp. Eff. Res. 9, 341–360 (2020).
Aartsma-Rus, A. & Goemans, N. A sequel to the eteplirsen saga: eteplirsen is approved in the United States but was not approved in Europe. Nucleic Acid Ther. 29, 13–15 (2019).
Niks, E. H. & Aartsma-Rus, A. Exon skipping: a first in class strategy for Duchenne muscular dystrophy. Expert. Opin. Biol. Ther. 17, 225–236 (2017).
Alfano, L. N. et al. Long-term treatment with eteplirsen in nonambulatory patients with Duchenne muscular dystrophy. Medicine 98, e15858 (2019).
Mendell, J. R. et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 79, 257–271 (2016).
Frank, D. E. et al. Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy. Neurology 94, e2270–e2282 (2020).
Roshmi, R. R. & Yokota, T. Viltolarsen for the treatment of Duchenne muscular dystrophy. Drugs Today 55, 627–639 (2019).
Uttley, L., Carlton, J., Woods, H. B. & Brazier, J. A review of quality of life themes in Duchenne muscular dystrophy for patients and carers. Health Qual. Life Outcomes 16, 237 (2018).
Pangalila, R. F. et al. Prevalence of fatigue, pain, and affective disorders in adults with duchenne muscular dystrophy and their associations with quality of life. Arch. Phys. Med. Rehabil. 96, 1242–1247 (2015).
Schara, U., Geers, B., Schmid, J. & Elsenbruch, S. Health-related quality of life in patients with Duchenne muscular dystrophy. Neuromuscul. Disord. 21, 652 (2011).
Elsenbruch, S., Schmid, J., Lutz, S., Geers, B. & Schara, U. Self-reported quality of life and depressive symptoms in children, adolescents, and adults with Duchenne muscular dystrophy: a cross-sectional survey study. Neuropediatrics 44, 257–264 (2013).
Abresch, R. T., Seyden, N. K. & Wineinger, M. A. Quality of life. Issues for persons with neuromuscular diseases. Phys. Med. Rehabil. Clin. N. Am. 9, 233–248 (1998).
Bach, J. R., Campagnolo, D. I. & Hoeman, S. Life satisfaction of individuals with Duchenne muscular dystrophy using long-term mechanical ventilatory support. Am. J. Phys. Med. Rehabil. 70, 129–135 (1991).
Crescimanno, G., Greco, F., D’Alia, R., Messina, L. & Marrone, O. Quality of life in long term ventilated adult patients with Duchenne muscular dystrophy. Neuromuscul. Disord. 29, 569–575 (2019).
Pangalila, R. Quality of life in Duchenne muscular dystrophy: the disability paradox. Dev. Med. Child Neurol. 58, 435–436 (2016).
Albrecht, G. L. & Devlieger, P. J. The disability paradox: high quality of life against all odds. Soc. Sci. Med. 48, 977–988 (1999).
Rose, M. R. et al. Role of disease severity, illness perceptions, and mood on quality of life in muscle disease. Muscle Nerve 46, 351–359 (2012).
Zamani, G. et al. The quality of life in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 26, 423–427 (2016).
Lim, Y., Velozo, C. & Bendixen, R. M. The level of agreement between child self-reports and parent proxy-reports of health-related quality of life in boys with Duchenne muscular dystrophy. Qual. Life Res. 23, 1945–1952 (2014).
Leclerc, T. et al. Prioritisation of ICU treatments for critically ill patients in a COVID-19 pandemic with scarce resources. Anaesth. Crit. Care Pain Med. 39, 333–339 (2020).
de Moura, M. C. et al. Is functional dependence of Duchenne muscular dystrophy patients determinant of the quality of life and burden of their caregivers? Arq. Neuropsiquiatr. 73, 52–57 (2015).
Yilmaz, O., Yildirim, S. A., Oksuz, C., Atay, S. & Turan, E. Mothers’ depression and health-related quality of life in neuromuscular diseases: role of functional independence level of the children. Pediatr. Int. 52, 648–652 (2010).
Barlow, J. H. & Ellard, D. R. The psychosocial well-being of children with chronic disease, their parents and siblings: an overview of the research evidence base. Child Care Health Dev. 32, 19–31 (2006).
Kay, E. & Kingston, H. Feelings associated with being a carrier and characteristics of reproductive decision making in women known to be carriers of X-linked conditions. J. Health Psychol. 7, 169–181 (2002).
Skuk, D. et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J. Neuropathol. Exp. Neurol. 65, 371–386 (2006).
Skuk, D. et al. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul. Disord. 17, 38–46 (2007).
Taylor, M. et al. Cardiac and skeletal muscle effects in the randomized HOPE-Duchenne trial. Neurology 92, e866–e878 (2019).
Duan, D. Micro-dystrophin gene therapy goes systemic in Duchenne muscular dystrophy patients. Hum. Gene Ther. 29, 733–736 (2018).
Mendell, J. R. et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with duchenne muscular dystrophy: a nonrandomized controlled trial. JAMA Neurol. 77, 1122–1131 (2020).
Wang, D., Zhang, F. & Gao, G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell 181, 136–150 (2020).
Chemello, F., Bassel-Duby, R. & Olson, E. N. Correction of muscular dystrophies by CRISPR gene editing. J. Clin. Invest. 130, 2766–2776 (2020).
Nelson, C. E., Robinson-Hamm, J. N. & Gersbach, C. A. Genome engineering: a new approach to gene therapy for neuromuscular disorders. Nat. Rev. Neurol. 13, 647–661 (2017).
Nance, M. E. et al. AAV9 edits muscle stem cells in normal and dystrophic adult mice. Mol. Ther. 27, 1568–1585 (2019).
Kwon, J. B. et al. In vivo gene editing of muscle stem cells with adeno-associated viral vectors in a mouse model of Duchenne muscular dystrophy. Mol. Ther. Methods Clin. Dev. 19, 320–329 (2020).
Ousterout, D. G. et al. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat. Commun. 6, 6244 (2015).
Duchêne, B. L. et al. CRISPR-induced deletion with SaCas9 restores dystrophin expression in dystrophic models in vitro and in vivo. Mol. Ther. 26, 2604–2616 (2018).
Wasala, N. B., Hakim, C. H., Yang, N. N. & Duan, D. Questions answered and unanswered by the first CRISPR editing study in the canine model of Duchenne muscular dystrophy. Hum. Gene Ther. 30, 535–543 (2019).
Mendell, J. R. et al. A phase I/IIa follistatin gene therapy trial for Becker muscular dystrophy. Mol. Ther. 23, 192–201 (2015).
Goemans, N. et al. A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy. Neuromuscul. Disord. 28, 4–15 (2018).
Iyombe-Engembe, J. P. et al. Efficient restoration of the dystrophin gene reading frame and protein structure in DMD myoblasts using the CinDel method. Mol. Ther. Nucleic Acids 5, e283 (2016).
Nicolas, A. et al. Assessment of the structural and functional impact of in-frame mutations of the DMD gene, using the tools included in the eDystrophin online database. Orphanet J. Rare Dis. 7, 45 (2012).
Delalande, O. et al. Dystrophin’s central domain forms a complex filament that becomes disorganized by in-frame deletions. J. Biol. Chem. 293, 6637–6646 (2018).
Goyenvalle, A. et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 306, 1796–1799 (2004).
Wein, N. et al. Translation from a DMD exon 5 IRES results in a functional dystrophin isoform that attenuates dystrophinopathy in humans and mice. Nat. Med. 20, 992–1000 (2014).
Malik, V. et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann. Neurol. 67, 771–780 (2010).
Hirst, R. C., McCullagh, K. J. & Davies, K. E. Utrophin upregulation in Duchenne muscular dystrophy. Acta Myol. 24, 209–216 (2005).
Miura, P. & Jasmin, B. J. Utrophin upregulation for treating Duchenne or Becker muscular dystrophy: how close are we? Trends Mol. Med. 12, 122–129 (2006).
Muntoni, F. et al. A phase 1b trial to assess the pharmacokinetics of ezutromid in pediatric Duchenne muscular dystrophy patients on a balanced diet. Clin. Pharmacol. Drug Dev. 8, 922–933 (2019).
Wilkinson, I. V. L. et al. Chemical proteomics and phenotypic profiling identifies the aryl hydrocarbon receptor as a molecular target of the utrophin modulator ezutromid. Angew. Chem. 59, 2420–2428 (2020).
Heier, C. R. et al. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol. Med. 5, 1569–1585 (2013).
Reeves, E. K. M., Hoffman, E. P., Nagaraju, K., Damsker, J. M. & McCall, J. M. VBP15: preclinical characterization of a novel anti-inflammatory delta 9,11 steroid. Bioorg. Med. Chem. 21, 2241–2249 (2013).
Hoffman, E. P. et al. Vamorolone trial in Duchenne muscular dystrophy shows dose-related improvement of muscle function. Neurology 93, e1312–e1323 (2019).
Finanger, E. et al. Phase 1 study of edasalonexent (CAT-1004), an oral NF-kappaB inhibitor, in pediatric patients with Duchenne muscular dystrophy. J. Neuromuscul. Dis. 6, 43–54 (2019).
Shelton, G. D. & Engvall, E. Gross muscle hypertrophy in whippet dogs is caused by a mutation in the myostatin gene. Neuromuscul. Disord. 17, 721–722 (2007).
Aiello, D., Patel, K. & Lasagna, E. The myostatin gene: an overview of mechanisms of action and its relevance to livestock animals. Anim. Genet. 49, 505–519 (2018).
Campbell, C. et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle Nerve 55, 458–464 (2017).
Wagner, K. R. et al. Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy. Neuromuscul. Disord. 30, 492–502 (2020).
Buyse, G. M. et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): a double-blind randomised placebo-controlled phase 3 trial. Lancet 385, 1748–1757 (2015).
Bettica, P. et al. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 26, 643–649 (2016).
Petrof, B. J. Molecular pathophysiology of myofiber injury in deficiencies of the dystrophin-glycoprotein complex. Am. J. Phys. Med. Rehabil. 81 (Suppl. 11), S162–S174 (2002).
Clerk, A., Strong, P. N. & Sewry, C. A. Characterisation of dystrophin during development of human skeletal muscle. Development 114, 395–402 (1992).
Mokhtarian, A., Lefaucheur, J. P., Even, P. C. & Sebille, A. Hindlimb immobilization applied to 21-day-old mdx mice prevents the occurrence of muscle degeneration. J. Appl. Physiol. 86, 924–931 (1999).
Acknowledgements
The laboratory of A.A.-R. is part of the Duchenne Center Netherlands. Duchenne muscular dystrophy (DMD) research in the laboratory of A.A.-R. is currently supported by Duchenne Parent Project, Duchenne UK, Prinses Beatrix Spierfonds, Spieren voor Spieren and the EU Horizon 2020 project BIND. DMD research in the laboratory of D.D. is currently supported by the National Institutes of Health (NS90634, AR70517, AR69085), Jackson Freel DMD Research Fund, Jesse’s Journey: The Foundation for Gene and Cell Therapy, Parent Project Muscular Dystrophy (USA), Hope for Javier, Michael’s Cause, Ryan’s Quest, Pietro’s Fight, Duchenne UK, Ryan’s Rally, Team Joseph, Charley’s Fund, Cure Duchenne, and Jett Foundation. DMD research in the laboratory of N.G. is currently supported by Duchenne Parent Project, Rondou Fonds and Kan-Go! Fonds. We thank NIH NeuroBioBank for providing patient muscle tissue for histological studies. We thank N. Wasala (University of Missouri) and K. Zhang (University of Missouri) for their help in the preparation of Fig. 2.
Author information
Authors and Affiliations
Contributions
Introduction (A.A.-R.); Epidemiology (S.T. and A.A.-R.); Mechanisms/pathophysiology (D.D. and A.A.-R.); Diagnosis, screening and prevention (A.A.-R. and N.G.); Management (N.G., E.M. and S.T.); Quality of life (N.G. and E.M.); Outlook (A.A.-R. and D.D.); Overview of Primer (A.A.-R.).
Corresponding author
Ethics declarations
Competing interests
D.D. is a member of the scientific advisory board for Solid Biosciences and an equity holder of Solid Biosciences. D.D. is an inventor on patents licensed to various companies. D.D. has served as an ad hoc consultant for 4DMT, Decibel Therapeutics, Evox, Primary Insight, Vida Ventures, Global Guidepoint and GLG consultancy in the past 3 years. The lab of D.D. has received research support from Solid Biosciences and Edgewise Therapeutics in the past 3 years. N.G. has received compensation as member of scientific boards or as speaker at symposia from Sarepta, Pfizer, Italpharmaco and PTC Therapeutics. S.T. has patents on sequences for exon skip by antisense nucleic acids as a member of NCNP together with Nippon Shinyaku. As principal inventor of these patents, S.T. is entitled to a share of royalties. S.T. discloses being an ad hoc consultant for Ono Pharmaceutical, Daiichisankyo, Asahikasei Pharma, Teijin Pharma, AGADA Biosciences, and Wave and being a member of the scientific advisory boards of Nippon Shinyaku, Taiho Pharma and Sarepta therapeutics. S.T. received speaker honoraria from Japan Health Science Foundation and Astellas Pharma and has also received research supports from Taiho Pharma, Daiichisankyo, Nippon Shinyaku, Takeda Pharmaceutical and the Noguchi Institute in the past 3 years. E.M. is a Principal Investigator in clinical trials and advisory board member for Sarepta, Santhera, PTC, Roche, Italfarmaco, NS Pharma and Pfizer. A.A.-R. discloses being employed by Leiden University Medical Center (LUMC), which has patents on exon-skipping technology, some of which has been licensed to BioMarin and subsequently sublicensed to Sarepta. As co-inventor of some of these patents, A.A.-R. is entitled to a share of royalties. A.A.-R. further discloses being an ad hoc consultant for PTC Therapeutics, Sarepta Therapeutics, CRISPR Therapeutics, Summit PLC, Alpha Anomeric, BioMarin Pharmaceuticals Inc., Eisai, Astra Zeneca, Santhera, Audentes, Global Guidepoint and GLG consultancy, Grunenthal, Wave, and BioClinica, having been a member of the Duchenne Network Steering Committee (BioMarin), and being a member of the scientific advisory boards of ProQR, Hybridize Therapeutics, Silence Therapeutics, Sarepta Therapeutics and Philae Pharmaceuticals. Remuneration for these activities is paid to LUMC. LUMC also received speaker honoraria from PTC Therapeutics and BioMarin Pharmaceuticals and funding for contract research from Italpharmaco and Alpha Anomeric. Project funding is received from Sarepta Therapeutics.
Additional information
Peer review information
Nature Reviews Disease Primers thanks J. Novak, who co-reviewed with T. Partridge, P. Clemens, H. Gordish-Dressman, M. Ryan, J. Tremblay, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, D., Goemans, N., Takeda, S. et al. Duchenne muscular dystrophy. Nat Rev Dis Primers 7, 13 (2021). https://doi.org/10.1038/s41572-021-00248-3
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41572-021-00248-3
This article is cited by
-
A type IV spinal muscular atrophy with gastrocnemius pseudohypertrophy caused by SMN1 deletion: a case report and literature review
BMC Neurology (2026)
-
Parkin overexpression attenuates muscle atrophy and improves mitochondrial bioenergetics but not histological features of Duchenne muscular dystrophy in mice
Scientific Reports (2026)
-
Emerging therapeutic strategies in muscular dystrophy: an updated review on pathogenesis and treatment advances
Molecular Biology Reports (2026)
-
Caregiver-reported Patient Experiences with Duchenne Muscular Dystrophy: Qualitative In-trial Interviews 1 Year After Delandistrogene Moxeparvovec in the Pivotal EMBARK Trial
Neurology and Therapy (2026)
-
Two-Year Outcomes Following Delandistrogene Moxeparvovec Treatment in Ambulatory Patients with Duchenne Muscular Dystrophy: Phase 3 EMBARK Trial
Neurology and Therapy (2026)