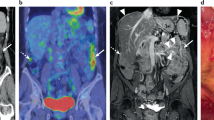
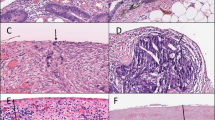
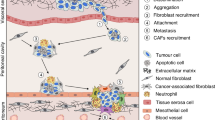
Abstract
Peritoneal surface malignancies comprise a heterogeneous group of primary tumours, including peritoneal mesothelioma, and peritoneal metastases of other tumours, including ovarian, gastric, colorectal, appendicular or pancreatic cancers. The pathophysiology of peritoneal malignancy is complex and not fully understood. The two main hypotheses are the transformation of mesothelial cells (peritoneal primary tumour) and shedding of cells from a primary tumour with implantation of cells in the peritoneal cavity (peritoneal metastasis). Diagnosis is challenging and often requires modern imaging and interventional techniques, including surgical exploration. In the past decade, new treatments and multimodal strategies helped to improve patient survival and quality of life and the premise that peritoneal malignancies are fatal diseases has been dismissed as management strategies, including complete cytoreductive surgery embedded in perioperative systemic chemotherapy, can provide cure in selected patients. Furthermore, intraperitoneal chemotherapy has become an important part of combination treatments. Improving locoregional treatment delivery to enhance penetration to tumour nodules and reduce systemic uptake is one of the most active research areas. The current main challenges involve not only offering the best treatment option and developing intraperitoneal therapies that are equivalent to current systemic therapies but also defining the optimal treatment sequence according to primary tumour, disease extent and patient preferences. New imaging modalities, less invasive surgery, nanomedicines and targeted therapies are the basis for a new era of intraperitoneal therapy and are beginning to show encouraging outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sadeghi, B. et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88, 358–363 (2000).
Sugarbaker, P. H. Peritonectomy procedures. Ann. Surg. 221, 29–42 (1995).
Alyami, M. et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 20, e368–e377 (2019). Comprehensive evidence-based overview on the PIPAC technique for PSM of various origins and its feasibility, safety and efficacy, including a summary of current indications for PIPAC and HIPEC treatment.
Glehen, O. et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 116, 5608–5618 (2010). French multicentre study that gives an excellent overview on outcomes of CRS and HIPEC for patients with PSM of gastrointestinal origin. In addition, risk factors for poor outcomes are presented.
Van der Speeten, K., Lemoine, L. & Sugarbaker, P. Overview of the optimal perioperative intraperitoneal chemotherapy regimens used in current clinical practice. Pleura Peritoneum. 2, 63–72 (2017).
Foster, J. M. et al. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high-risk surgical oncology procedures. JAMA Netw. Open 2, e186847 (2019).
Hübner, M. et al. Guidelines for perioperative care in cytoreductive surgery (CRS) with or without hyperthermic intraperitoneal chemotherapy (HIPEC): Enhanced recovery after surgery (ERAS®) Society Recommendations — part I: preoperative and intraoperative management. Eur. J. Surg. Oncol. 46, 2292–2310 (2020). Evidence-based Delphi consensus for optimal perioperative care for patients undergoing CRS ± HIPEC: ERAS guidelines.
Cortés-Guiral, D., Mohamed, F., Glehen, O. & Passot, G. Prehabilitation of patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal malignancy. Eur. J. Surg. Oncol. 47, 60–64 (2021).
Hübner, M. et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced Recovery After Surgery (ERAS®) Society Recommendations — part II: postoperative management and special considerations. Eur. J. Surg. Oncol. 46, 2311–2323 (2020).
Coleridge, S. L. et al. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2019, CD005343 (2019).
Ruscito, I. et al. Incorporating parp-inhibitors in primary and recurrent ovarian cancer: a meta-analysis of 12 phase II/III randomized controlled trials. Cancer Treat. Rev. 87, 102040 (2020).
Goere, D. et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann. Surg. 257, 1065–1071 (2013).
Jaime, P. FIGO Committee on Gynecologic Oncology FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J. Gynecol. Oncol. 26, 87–89 (2015).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Bloemendaal, A., Verwaal, V., Van Ruth, S., Boot, H. & Zoetmulder, F. Conventional surgery and systemic chemotherapy for peritoneal carcinomatosis of colorectal origin: a prospective study. Eur. J. Surg. Oncol. 31, 1145–1151 (2005).
Chu, D. Z., Lang, N. P., Thompson, C., Osteen, P. K. & Westbrook, K. C. Peritioneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 63, 364–367 (1989).
Klos, D. et al. Trends in peritoneal surface malignancies: evidence from a Czech nationwide population-based study. World J. Surg. Oncol. 17, 182 (2019). Epidemiology of PM of ovarian origin as the most frequently concerned tumour entity.
Zhang, Y. et al. Global patterns and trends in ovarian cancer incidence: age, period and birth cohort analysis. BMC Cancer 19, 984 (2019).
Torre, L. A. et al. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 68, 284–296 (2018).
Burg, L. et al. Incidence and predictors of peritoneal metastases of gynecological origin: a population-based study in the Netherlands. J. Gynecol. Oncol. 31, e58 (2020).
Bray, F., Loos, A. H., Tognazzo, S. & La Vecchia, C. Ovarian cancer in Europe: cross-sectional trends in incidence and mortality in 28 countries, 1953–2000. Int. J. Cancer 113, 977–990 (2005).
National Academies of Sciences, Engineering, and Medicine. Ovarian Cancers: Evolving Paradigms in Research and Care (National Academies Press; 2016).
Abbasi, S. Y., El Taani, H., Saad, A. & Badheeb, A. Advanced gastric cancer in Jordan from 2004 to 2008: a study of epidemiology and outcomes. Gastrointest. Cancer Res. 4, 122 (2011).
Thomassen, I. et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int. J. Cancer 134, 622–628 (2014).
Rijken, A. et al. The burden of peritoneal metastases from gastric cancer: a systematic review on the incidence, risk factors and survival. J. Clin. Med. 10, 4882 (2021).
Segelman, J. et al. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 99, 699–705 (2012).
Jayne, D., Fook, S., Loi, C. & Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. J. Br. Surg. 89, 1545–1550 (2002).
Van den Heuvel, M., Lemmens, V., Verhoeven, R. & de Hingh, I. The incidence of mucinous appendiceal malignancies: a population-based study. Int. J. Colorectal Dis. 28, 1307–1310 (2013).
Flanagan, M. et al. Peritoneal metastases from extra-abdominal cancer — a population-based study. Eur. J. Surg. Oncol. 44, 1811–1817 (2018).
Satoh, H. et al. Peritoneal carcinomatosis in lung cancer patients. Oncol. Rep. 8, 1305–1307 (2001).
Bertozzi, S. et al. Prevalence, risk factors, and prognosis of peritoneal metastasis from breast cancer. SpringerPlus 4, 1–8 (2015).
Cashin, P. H., Jansson Palmer, G., Asplund, D., Graf, W. & Syk, I. Peritoneal mesothelioma in Sweden: a population-based study. Cancer Med. 8, 6468–6475 (2019).
Consonni, D. et al. Peritoneal mesothelioma and asbestos exposure: a population-based case–control study in Lombardy, Italy. Occup. Environ. Med. 76, 545–553 (2019).
Alpert, N., van Gerwen, M. & Taioli, E. Epidemiology of mesothelioma in the 21st century in Europe and the United States, 40 years after restricted/banned asbestos use. Transl. Lung Cancer Res. 9 (Suppl. 1), S28 (2020).
Chua, T. C. et al. Early-and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 30, 2449–2456 (2012).
Quénet, F. et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 22, 256–266 (2021). Pivotal RCT comparing the benefit of short duration oxaliplatin-based HIPEC in addition to CRS and perioperative chemotherapy for patients with colorectal PM.
Yan, T. D. et al. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database. Cancer 117, 1855–1863 (2011).
Ishigami, H. et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J. Clin. Oncol. 36, 1922–1929 (2018). Landmark randomized controlled trial evaluating the impact of combined intravenous and intraperitoneal chemotherapy (neoadjuvant intraperitoneal and systemic chemotherapy) on the prognosis of patients with gastric PM.
Kitayama, J. et al. ASO author reflections: repeated intraperitoneal paclitaxel with systemic chemotherapy as the first-line treatment for peritoneal malignancy. Ann. Surg. Oncol. 28, 3871–3872 (2021).
Ishigami, H., Kitayama, J., Yamaguchi, H., Emoto, S. & Watanabe, T. Phase II study of intravenous and intraperitoneal paclitaxel combined with S-1 for gastric cancer with metastases to the distant peritoneum. Ann. Oncol. 23, ix233 (2012).
Verwaal, V. J. et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 21, 3737–3743 (2003).
Noiret, B. et al. Centralization and oncologic training reduce postoperative morbidity and failure-to-rescue rates after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies: study on a 10-year national French practice. Ann. Surg. 272, 847–854 (2020).
Schuitevoerder, D., Sherman, S. K., Izquierdo, F. J., Eng, O. S. & Turaga, K. K. Assessment of the surgical workforce pertaining to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the United States. Ann. Surg. Oncol. 27, 3097–3102 (2020).
Gopinath, K. & Bhatt, A. Preface ‘Changing trend in peritoneal surface oncology in Asian countries’. Indian J. Surg. Oncol. 10, 1–2 (2019).
van Driel, W. J. et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 378, 230–240 (2018). Dutch multicentre randomized controlled trial showing large survival benefit with no increase of morbidity in favour of adding cisplatin-based HIPEC to CRS for patients with ovarian cancer undergoing interval surgery after neoadjuvant chemotherapy.
Low, R. N., Barone, R. M. & Rousset, P. Peritoneal MRI in patients undergoing cytoreductive surgery and HIPEC: history, clinical applications, and implementation. Eur. J. Surg. Oncol. 47, 65–74 (2021).
Franko, J. Therapeutic efficacy of systemic therapy for colorectal peritoneal carcinomatosis: surgeon’s perspective. Pleura Peritoneum 3, 20180102 (2018).
Müller, H., Hotopp, T., Tofeili, A. & Wutke, K. Systemic chemotherapy using FLOT-regimen combined with cytoreductive surgery plus HIPEC for treatment of peritoneal metastasized gastric cancer. Hepatogastroenterology 61, 703–706 (2014).
Becker, O., Beaulaton, C., Masliah-Planchon, J., Servois, V. & Watson, S. Nivolumab activity in advanced refractory malignant peritoneal mesothelioma. Eur. J. Cancer 2020, S0959–S8049 (1990).
Glehen, O. et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer 14, 183 (2014).
Honoré, C. et al. Ninety percent of the adverse outcomes occur in 10% of patients: can we identify the populations at high risk of developing peritoneal metastases after curative surgery for colorectal cancer? Int. J. Hyperth. 33, 505–510 (2017).
Smeenk, R., Van Velthuysen, M., Verwaal, V. & Zoetmulder, F. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur. J. Surg. Oncol. 34, 196–201 (2008).
Yurgelun, M. B. et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J. Clin. Oncol. 35, 1086–1095 (2017).
Pietragalla, A., Arcieri, M., Marchetti, C., Scambia, G. & Fagotti, A. Ovarian cancer predisposition beyond BRCA1 and BRCA2 genes. Int. J. Gynecol. Cancer 30, 1803–1810 (2020).
Win, A. K. et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol. Biomark. Prev. 26, 404–412 (2017).
Corso, G. et al. Geographical distribution of E-cadherin germline mutations in the context of diffuse gastric cancer: a systematic review. Cancers 13, 1267 (2021).
Testa, J. R. et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 43, 1022–1025 (2011).
Foulkes, W. D. Inherited susceptibility to common cancers. N. Engl. J. Med. 359, 2143–2153 (2008).
Sekine, M., Nishino, K. & Enomoto, T. BRCA genetic test and risk-reducing salpingo-oophorectomy for hereditary breast and ovarian cancer: state-of-the-art. Cancers 13, 2562 (2021).
Sinicrope, F. A. Lynch syndrome-associated colorectal cancer. N. Engl. J. Med. 379, 764–773 (2018).
Rustgi, S. D., Ching, C. K. & Kastrinos, F. Inherited predisposition to gastric cancer. Gastrointest. Endosc. Clin. 31, 467–487 (2021).
Albanese, A. M. et al. Peritoneal surface area: measurements of 40 structures covered by peritoneum: correlation between total peritoneal surface area and the surface calculated by formulas. Surg. Radiol. Anat. 31, 369–377 (2009).
Flessner, M. F. Peritoneal transport physiology: insights from basic research. J. Am. Soc. Nephrol. 2, 122–135 (1991).
Azuar, A. S. et al. Impact of surgical peritoneal environment on postoperative tumor growth and dissemination in a preimplanted tumor model. Surg. Endosc. 23, 1733–1739 (2009).
Binda, M. M., Molinas, C. R., Hansen, P. & Koninckx, P. R. Effect of desiccation and temperature during laparoscopy on adhesion formation in mice. Fertil. Steril. 86, 166–175 (2006).
Carpinteri, S. et al. Peritoneal tumorigenesis and inflammation are ameliorated by humidified-warm carbon dioxide insufflation in the mouse. Ann. Surg. Oncol. 22 (Suppl. 3), S1540–S1547 (2015).
Mutsaers, S. E., Whitaker, D. & Papadimitriou, J. M. Changes in the concentration of microvilli on the free surface of healing mesothelium are associated with alterations in surface membrane charge. J. Pathol. 180, 333–339 (1996).
Sampurno, S. et al. Modes of carbon dioxide delivery during laparoscopy generate distinct differences in peritoneal damage and hypoxia in a porcine model. Surg. Endosc. 34, 4395–4402 (2020).
Volz, J., Koster, S., Spacek, Z. & Paweletz, N. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum. Surg. Endosc. 13, 611–614 (1999).
Mutsaers, S. E., Prele, C. M., Pengelly, S. & Herrick, S. E. Mesothelial cells and peritoneal homeostasis. Fertil. Steril. 106, 1018–1024 (2016).
LaRocca, P. J. & Rheinwald, J. G. Coexpression of simple epithelial keratins and vimentin by human mesothelium and mesothelioma in vivo and in culture. Cancer Res. 44, 2991–2999 (1984).
Dobbie, J. W., Pavlina, T., Lloyd, J. & Johnson, R. C. Phosphatidylcholine synthesis by peritoneal mesothelium: its implications for peritoneal dialysis. Am. J. Kidney Dis. 12, 31–36 (1988). Comprehensive science-based overview on the physiology of the normal and diseased peritoneum: an orphan organ.
Grupp, A. et al. The expression patterns of peritoneal defensins. Perit. Dial. Int. 27, 654–662 (2007).
Tang, S. et al. Regulation of complement C3 and C4 synthesis in human peritoneal mesothelial cells by peritoneal dialysis fluid. Clin. Exp. Immunol. 136, 85–94 (2004).
Zarrinkalam, K. H., Leavesley, D. I., Stanley, J. M., Atkins, G. J. & Faull, R. J. Expression of defensin antimicrobial peptides in the peritoneal cavity of patients on peritoneal dialysis. Perit. Dial. Int. 21, 501–508 (2001).
van Baal, J. O. et al. The histophysiology and pathophysiology of the peritoneum. Tissue Cell 49, 95–105 (2017).
Yamaji, K. et al. Interleukin-6 production by peritoneal mesothelial cells and its regulation by inflammatory factors in rats administered carbon tetrachloride intraperitoneally. Toxicol. Appl. Pharmacol. 226, 38–45 (2008).
Jonjic, N. et al. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J. Exp. Med. 176, 1165–1174 (1992).
Yao, V., Platell, C. & Hall, J. C. Peritoneal mesothelial cells produce inflammatory related cytokines. Anz. J. Surg. 74, 997–1002 (2004).
Colmont, C. S. et al. Human peritoneal mesothelial cells respond to bacterial ligands through a specific subset of Toll-like receptors. Nephrol. Dial. Transplant. 26, 4079–4090 (2011).
Hausmann, M. J., Rogachev, B., Weiler, M., Chaimovitz, C. & Douvdevani, A. Accessory role of human peritoneal mesothelial cells in antigen presentation and T-cell growth. Kidney Int. 57, 476–486 (2000).
Park, J. H. et al. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J. Immunol. 179, 514–521 (2007).
Foussat, A. et al. Production of stromal cell-derived factor 1 by mesothelial cells and effects of this chemokine on peritoneal B lymphocytes. Eur. J. Immunol. 31, 350–359 (2001).
Kato, S. et al. Endotoxin-induced chemokine expression in murine peritoneal mesothelial cells: the role of toll-like receptor 4. J. Am. Soc. Nephrol. 15, 1289–1299 (2004).
Boulanger, E. et al. AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int. 61, 148–156 (2002).
Cui, L. et al. Biodefense function of omental milky spots through cell adhesion molecules and leukocyte proliferation. Cell Tissue Res. 310, 321–330 (2002).
Liang, Y. & Sasaki, K. Expression of adhesion molecules relevant to leukocyte migration on the microvilli of liver peritoneal mesothelial cells. Anat. Rec. 258, 39–46 (2000).
Valle, M. T. et al. Antigen-presenting function of human peritoneum mesothelial cells. Clin. Exp. Immunol. 101, 172–176 (1995).
Shaw, T. J. et al. Human peritoneal mesothelial cells display phagocytic and antigen-presenting functions to contribute to intraperitoneal immunity. Int. J. Gynecol. Cancer 26, 833–838 (2016).
Bellingan, G. J. et al. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J. Exp. Med. 196, 1515–1521 (2002).
Zsiros, V. & Kiss, A. L. Cellular and molecular events of inflammation induced transdifferentiation (EMT) and regeneration (MET) in mesenteric mesothelial cells. Inflamm. Res. 69, 1173–1179 (2020).
Wilson, R. B., Archid, R. & Reymond, M. A. Reprogramming of mesothelial-mesenchymal transition in chronic peritoneal diseases by estrogen receptor modulation and TGF-beta1 inhibition. Int. J. Mol. Sci. 21, 4158 (2020).
Pronk, A. et al. Thrombogenicity and procoagulant activity of human mesothelial cells. Arterioscler. Thromb. 12, 1428–1436 (1992).
Iakhiaev, A. & Idell, S. Activation and degradation of protein C by primary rabbit pleural mesothelial cells. Lung 184, 81–88 (2006).
Schaefer, B. et al. Quantitative histomorphometry of the healthy peritoneum. Sci. Rep. 6, 21344 (2016).
Owens, M. W. & Grimes, S. R. Pleural mesothelial cell response to inflammation: tumor necrosis factor-induced mitogenesis and collagen synthesis. Am. J. Physiol. 265, L382–L388 (1993).
Yang, W. S., Kim, B. S., Lee, S. K., Park, J. S. & Kim, S. B. Interleukin-1beta stimulates the production of extracellular matrix in cultured human peritoneal mesothelial cells. Perit. Dial. Int. 19, 211–220 (1999).
Owens, M. W. & Milligan, S. A. Growth factor modulation of rat pleural mesothelial cell mitogenesis and collagen synthesis. Effects of epidermal growth factor and platelet-derived factoR. Inflammation 18, 77–87 (1994).
Saed, G. M., Zhang, W., Chegini, N., Holmdahl, L. & Diamond, M. P. Alteration of type I and III collagen expression in human peritoneal mesothelial cells in response to hypoxia and transforming growth factor-beta1. Wound Repair. Regen. 7, 504–510 (1999).
Rennard, S. I. et al. Role of pleural mesothelial cells in the production of the submesothelial connective tissue matrix of lung. Am. Rev. Respir. Dis. 130, 267–274 (1984).
Torres, K. et al. TGF-beta and inflammatory blood markers in prediction of intraperitoneal adhesions. Adv. Med. Sci. 63, 220–223 (2018).
Selgas, R. et al. Epithelial-to-mesenchymal transition of the mesothelial cell–its role in the response of the peritoneum to dialysis. Nephrol. Dial. Transplant. 21 (Suppl. 2), ii2–ii7 (2006).
Saed, G. M., Zhang, W., Chegini, N., Holmdahl, L. & Diamond, M. P. Transforming growth factor beta isoforms production by human peritoneal mesothelial cells after exposure to hypoxia. Am. J. Reprod. Immunol. 43, 285–291 (2000).
Xiao, L., Sun, L., Liu, F. Y., Peng, Y. M. & Duan, S. B. Connective tissue growth factor knockdown attenuated matrix protein production and vascular endothelial growth factor expression induced by transforming growth factor-beta1 in cultured human peritoneal mesothelial cells. Ther. Apher. Dial. 14, 27–34 (2010).
McArdle, C. S., McMillan, D. C. & Hole, D. J. The impact of blood loss, obstruction and perforation on survival in patients undergoing curative resection for colon cancer. Br. J. Surg. 93, 483–488 (2006).
Komatsu, S. et al. Prognostic factors and scoring system for survival in colonic perforation. Hepatogastroenterology 52, 761–764 (2005).
Chen, H. S. & Sheen-Chen, S. M. Obstruction and perforation in colorectal adenocarcinoma: an analysis of prognosis and current trends. Surgery 127, 370–376 (2000).
Cheynel, N. et al. Incidence, patterns of failure, and prognosis of perforated colorectal cancers in a well-defined population. Dis. Colon. Rectum 52, 406–411 (2009).
Terauchi, M. et al. Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin. Exp. Metastasis 24, 329–339 (2007).
Kokenyesi, R., Murray, K. P., Benshushan, A., Huntley, E. D. & Kao, M. S. Invasion of interstitial matrix by a novel cell line from primary peritoneal carcinosarcoma, and by established ovarian carcinoma cell lines: role of cell-matrix adhesion molecules, proteinases, and E-cadherin expression. Gynecol. Oncol. 89, 60–72 (2003).
Christou, N. et al. E-cadherin: a potential biomarker of colorectal cancer prognosis. Oncol. Lett. 13, 4571–4576 (2017).
Hayashi, K. et al. Real-time Imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 67, 8223–8228 (2007).
Rutz, H. P. Hydrodynamic consequences of glycolysis — thermodynamic basis and clinical relevance. Cancer Biol. Ther. 3, 812–815 (2004).
Heldin, C. H., Rubin, K., Pietras, K. & Ostman, A. High interstitial fluid pressure — an obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813 (2004).
Hansen, E. et al. Tumor-cells in blood shed from the surgical field. Arch. Surg. 130, 387–393 (1995).
Tanaka, N., Nobori, M. & Suzuki, Y. Does bile spillage during an operation present a risk for peritoneal metastasis in bile duct carcinoma? Surg. Today 27, 1010–1014 (1997).
Meyers, M. A. Distribution of intraabdominal malignant seeding — dependency on dynamics of flow of ascitic fluid. Am. J. Roentgenol. 119, 198–206 (1973).
Lindberg, U., Karlsson, R., Lassing, I., Schutt, C. E. & Hoglund, A. S. The microfilament system and malignancy. Semin. Cancer Biol. 18, 2–11 (2008).
Moffitt, L., Karimnia, N., Stephens, A. & Bilandzic, M. Therapeutic targeting of collective invasion in ovarian cancer. Int. J. Mol. Sci. 20, 1466 (2019).
Zajac, O. et al. Tumour spheres with inverted polarity drive the formation of peritoneal metastases in patients with hypermethylated colorectal carcinomas. Nat. Cell Biol. 20, 296–306 (2018).
Bittinger, F. et al. PECAM-1 expression in human mesothelial cells: an in vitro study. Pathobiology 64, 320–327 (1996).
Ziprin, P., Alkhamesi, N. A., Ridgway, P. F., Peck, D. H. & Darzi, A. W. Tumour-expressed CD43 (sialophorin) mediates tumour-mesothelial cell adhesion. Biol. Chem. 385, 755–761 (2004).
Hassan, A. A., Artemenko, M., Tang, M. K. S. & Wong, A. S. T. Selectins: an important family of glycan-binding cell adhesion molecules in ovarian cancer. Cancers 12, 2238 (2020).
Gebauer, F. et al. Selectin binding is essential for peritoneal carcinomatosis in a xenograft model of human pancreatic adenocarcinoma in pfp–/rag2– mice. Gut 62, 741–750 (2013).
Borsig, L. et al. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber. Effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 282, 14984–14991 (2007).
Eustache, J. H. et al. Wide net on surgery: the central role of neutrophil extracellular traps. Ann. Surg. 272, 277–283 (2020).
Oosterling, S. J. et al. Anti-beta 1 integrin antibody reduces surgery-induced adhesion of colon carcinoma cells to traumatized peritoneal surfaces. Ann. Surg. 247, 85–94 (2008).
Ween, M. P., Oehler, M. K. & Ricciardelli, C. Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. Int. J. Mol. Sci. 12, 1009–1029 (2011).
Hilliard, T. S. The impact of mesothelin in the ovarian cancer tumor microenvironment. Cancers 10, 277 (2018).
Koppe, M. J., Nagtegaal, I. D., de Wilt, J. H. & Ceelen, W. P. Recent insights into the pathophysiology of omental metastases. J. Surg. Oncol. 110, 670–675 (2014).
Gerber, S. A. et al. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am. J. Pathol. 169, 1739–1752 (2006).
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 (2011).
Meza-Perez, S. & Randall, T. D. Immunological functions of the omentum. Trends Immunol. 38, 526–536 (2017).
Sorensen, E. W. et al. Omental immune aggregates and tumor metastasis within the peritoneal cavity. Immunol. Res. 45, 185–194 (2009).
Zulfiqar, M. et al. Krukenberg tumors: update on imaging and clinical features. Am. J. Roentgenol. 215, 1020–1029 (2020).
van der Bij, G. J. et al. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann. Surg. 249, 727–734 (2009).
Klein, C. L. et al. Effects of cytokines on the expression of cell adhesion molecules by cultured human omental mesothelial cells. Pathobiology 63, 204–212 (1995).
Ikari, Y., Yee, K. O. & Schwartz, S. M. Role of alpha5beta1 and alphavbeta3 integrins on smooth muscle cell spreading and migration in fibrin gels. Thromb. Haemost. 84, 701–705 (2000).
Basson, M. D. et al. Effects of increased ambient pressure on colon cancer cell adhesion. J. Cell. Biochem. 78, 47–61 (2000).
Ridgway, P. F. et al. Pneumoperitoneum augmented tumor invasiveness is abolished by matrix metalloproteinase blockade. Surg. Endosc. 16, 533–536 (2002).
Jacobi, C. A. et al. Pneumoperitoneum with carbon dioxide stimulates growth of malignant colonic cells. Surgery 121, 72–78 (1997).
Heath, R. M., Jayne, D. G., O’Leary, R., Morrison, E. E. & Guillou, P. J. Tumour-induced apoptosis in human mesothelial cells: a mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br. J. Cancer 90, 1437–1442 (2004).
Carreiras, F. et al. Migration properties of the human ovarian adenocarcinoma cell line IGROV1: importance of alpha v beta 3 integrins and vitronectin. Int. J. Cancer 80, 285–294 (1999).
Lubbe, W. J. et al. Guanylyl cyclase c prevents colon cancer metastasis by regulating tumor epithelial cell matrix metalloproteinase-9. Cancer Res. 69, 3529–3536 (2009).
Narasimhan, V. et al. Medium-throughput drug screening of patient-derived organoids from colorectal peritoneal metastases to direct personalized therapy. Clin. Cancer Res. 26, 3662–3670 (2020).
Wang, Z. B., Li, M. & Li, J. C. Recent advances in the research of lymphatic stomata. Anat. Rec. 293, 754–761 (2020).
Li, J. C. & Yu, S. M. Study on the ultrastructure of the peritoneal stomata in humans. Acta Anatomica. 141, 26–30 (1991).
Namba, Y. An electron microscopic demonstration of the invasion of tumor cells into the diaphragm [Japanese]. Nippon Geka Gakkai Zasshi. 90, 1915–1921 (1989).
Yonemura, Y. et al. Trans-lymphatic metastasis in peritoneal dissemination. J. Gastrointest. Dig. Syst. S12, 007 (2013).
Li, Y. Y. & Li, J. C. Cell signal transduction mechanism for nitric oxide regulating lymphatic stomata and its draining capability. Anat. Rec. 291, 216–223 (2008).
Nagy, J. A., Herzberg, K. T., Dvorak, J. M. & Dvorak, H. F. Pathogenesis of malignant ascites formation: initiating events that lead to fluid accumulation. Cancer Res. 53, 2631–2643 (1993).
Anwar, A. & Kasi, A. Peritoneal Cancer (StatPearls Publishing LLC, 2021).
Sugarbaker, P. H. Pictorial essays on peritoneal metastases imaging: CT, MRI and PET-CT (Nova Science Publishers, 2020).
Warde, P. et al. Computed tomography in advanced ovarian cancer: an evaluation of diagnostic accuracy. Am. J. Med. Sci. 293, 94–98 (1987).
Bhatt, A. et al. Prospective correlation of the radiological, surgical and pathological findings in patients undergoing cytoreductive surgery for colorectal peritoneal metastases: implications for the preoperative estimation of the peritoneal cancer index. Colorectal Dis. 22, 2123–2132 (2020).
Ahmed, S. A. et al. The accuracy of multi-detector computed tomography and laparoscopy in the prediction of peritoneal carcinomatosis index score in primary ovarian cancer. Acad. Radiol. 26, 1650–1658 (2019).
Borley, J. et al. Radiological predictors of cytoreductive outcomes in patients with advanced ovarian cancer. BJOG 122, 843–849 (2015).
Sugarbaker, P. H. et al. Concerning CT features used to select patients for treatment of peritoneal metastases, a pictoral essay. Int. J. Hyperth. 33, 497–504 (2017).
Gonzalez-Moreno, S., Gonzalez-Bayon, L., Ortega-Perez, G. & Gonzalez-Hernando, C. Imaging of peritoneal carcinomatosis. Cancer J. 15, 184–189 (2009).
van ‘t Sant, I. et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur. Radiol. 30, 3101–3112 (2020).
Koh, J. L., Yan, T. D., Glenn, D. & Morris, D. L. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 16, 327–333 (2009).
Dirisamer, A. et al. Detection of histologically proven peritoneal carcinomatosis with fused 18F-FDG-PET/MDCT. Eur. J. Radiol. 69, 536–541 (2009).
Chandramohan, A., Thrower, A., Smith, S., Shah, N. & Moran, B. “PAUSE”: a method for communicating radiological extent of peritoneal malignancy. Clin. Radiol. 72, 972–980 (2017). Overview and comparison of available imaging modalities for PSM.
Li, J., Yan, R., Lei, J. & Jiang, C. Comparison of PET with PET/CT in detecting peritoneal carcinomatosis: a meta-analysis. Abdom. Imaging 40, 2660–2666 (2015).
Wang, W. et al. Are positron emission tomography-computed tomography (PET-CT) scans useful in preoperative assessment of patients with peritoneal disease before cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC)? Int. J. Hyperth. 34, 524–531 (2018).
Dewulf, J., Adhikari, K., Vangestel, C., Wyngaert, T. V. D. & Elvas, F. Development of antibody immuno-PET/SPECT radiopharmaceuticals for imaging of oncological disorders-an update. Cancers 12, 1868 (2020).
Guzik, P. et al. Identification of a PET radiotracer for imaging of the folate receptor-α: a potential tool to select patients for targeted tumor therapy. J. Nucl. Med. 62, 1475 (2021).
Low, R. N. & Barone, R. M. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann. Surg. Oncol. 19, 1394–1401 (2012).
Dohan, A. et al. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br. J. Surg. 104, 1244–1249 (2017).
Menassel, B. et al. Preoperative CT and MRI prediction of non-resectability in patients treated for pseudomyxoma peritonei from mucinous appendiceal neoplasms. Eur. J. Surg. Oncol. 42, 558–566 (2016).
Nougaret, S. et al. Radiomics and radiogenomics in ovarian cancer: a literature review. Abdom. Radiol. 46, 2308–2322 (2021).
Rodríguez-Enríquez, S. et al. Multi-biomarker pattern for tumor identification and prognosis. J. Cell. Biochem. 112, 2703–2715 (2011).
Carpelan-Holmström, M., Louhimo, J., Stenman, U. H., Alfthan, H. & Haglund, C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 22, 2311–2316 (2002).
Sturgeon, C. Practice guidelines for tumor marker use in the clinic. Clin. Chem. 48, 1151–1159 (2002).
Gebauer, G. & Müller-Ruchholtz, W. Tumor marker concentrations in normal and malignant tissues of colorectal cancer patients and their prognostic relevance. Anticancer Res. 17, 2939–2942 (1997).
Emoto, S. et al. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination. Gastric Cancer 15, 154–161 (2012).
Lai, I. R., Lee, W. J., Huang, M. T. & Lin, H. H. Comparison of serum CA72-4, CEA, TPA, CA19-9 and CA125 levels in gastric cancer patients and correlation with recurrence. Hepatogastroenterology 49, 1157–1160 (2002).
Yamao, T. et al. Tumor markers CEA, CA19-9 and CA125 in monitoring of response to systemic chemotherapy in patients with advanced gastric cancer. Jpn. J. Clin. Oncol. 29, 550–555 (1999).
Moayyedi, P. et al. ACG and CAG clinical guideline: management of dyspepsia. Am. J. Gastroenterol. 112, 988–1013 (2017).
Shaukat, A. et al. ACG clinical guidelines: colorectal cancer screening 2021. Am. J. Gastroenterol. 116, 458–479 (2021).
Montminy, E. M., Jang, A., Conner, M. & Karlitz, J. J. Screening for colorectal cancer. Med. Clin. North. Am. 104, 1023–1036 (2020).
Zullo, A. et al. Diagnostic yield of upper endoscopy according to appropriateness: a systematic review. Dig. Liver Dis. 51, 335–339 (2019).
Frazzoni, L. et al. Systematic review with meta-analysis: the appropriateness of colonoscopy increases the probability of relevant findings and cancer while reducing unnecessary exams. Aliment. Pharmacol. Ther. 53, 22–32 (2021).
Raś, R. et al. Preoperative colonoscopy in patients with a supposed primary ovarian cancer. Medicine 98, e14929 (2019).
Valle, M., Federici, O. & Garofalo, A. Patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, and role of laparoscopy in diagnosis, staging, and treatment. Surg. Oncol. Clin. North. Am. 21, 515–531 (2012).
Esquivel, J. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann. Surg. Oncol. 14, 128–133 (2007).
Jacquet P., Sugarbaker P. In Peritoneal Carcinomatosis: Principles of Management. Cancer Treatment and Research (ed. Sugarbaker P.) Vol. 82, 359-374 (Springer US, 1996).
Patel, C. M., Sahdev, A. & Reznek, R. H. CT, MRI and PET imaging in peritoneal malignancy. Cancer Imaging 11, 123–139 (2011).
Marmor, R. A., Kelly, K. J., Lowy, A. M. & Baumgartner, J. M. Laparoscopy is safe and accurate to evaluate peritoneal surface metastasis prior to cytoreductive surgery. Ann. Surg. Oncol. 23, 1461–1467 (2016).
Pomel, C., Appleyard, T. L., Gouy, S., Rouzier, R. & Elias, D. The role of laparoscopy to evaluate candidates for complete cytoreduction of peritoneal carcinomatosis and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 31, 540–543 (2005).
von Breitenbuch, P., Boerner, T., Jeiter, T., Piso, P. & Schlitt, H. J. Laparoscopy as a useful selection tool for patients with prior surgery and peritoneal metastases suitable for multimodality treatment strategies. Surg. Endosc. 32, 2288–2294 (2018).
Jayakrishnan, T. T. et al. Role of laparoscopy in patients with peritoneal metastases considered for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). World J. Surg. Oncol. 12, 270 (2014).
Iversen, L. H., Rasmussen, P. C. & Laurberg, S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br. J. Surg. 100, 285–292 (2013).
van Oudheusden, T. R. et al. Peritoneal cancer patients not suitable for cytoreductive surgery and HIPEC during explorative surgery: risk factors, treatment options, and prognosis. Ann. Surg. Oncol. 22, 1236–1242 (2015).
Passot, G. et al. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br. J. Surg. 105, 663–667 (2018).
von Renteln, D. et al. Standard diagnostic laparoscopy is superior to NOTES approaches: results of a blinded, randomized controlled porcine study. Endoscopy 44, 596–604 (2012).
Benzerdjeb, N. et al. Prognostic impact of combined progression index based on peritoneal grading regression score and peritoneal cytology in peritoneal metastasis. Histopathology 77, 548–559 (2020).
Delhorme, J. B. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendicular and extra-appendicular origin. Br. J. Surg. 105, 668–676 (2018).
Carr, N. J. et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the peritoneal surface Oncology Group International (PSOGI) Modified Delphi Process. Am. J. Surg. Pathol. 40, 14–26 (2016).
Ahadi, M., Sokolova, A., Brown, I., Chou, A. & Gill, A. J. The 2019 World Health Organization Classification of appendiceal, colorectal and anal canal tumours: an update and critical assessment. Pathology 53, 454–461 (2021).
Kusamura, S. et al. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. 47, 36–59 (2021). Comprehensive evidence-based guidelines and Delphi consensus on all aspects of care for patients having peritoneal mesothelioma.
Galateau Salle, F. et al. New insights on diagnostic reproducibility of biphasic mesotheliomas: a multi-institutional evaluation by the international mesothelioma panel from the MESOPATH Reference Center. J. Thorac. Oncol. 13, 1189–1203 (2018).
Boeckx, N. et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann. Oncol. 28, 1862–1868 (2017).
Petrelli, F. et al. Prognostic role of primary tumor location in non-metastatic gastric cancer: a systematic review and meta-analysis of 50 studies. Ann. Surg. Oncol. 24, 2655–2668 (2017).
Felismino, T. C. et al. Primary tumor location is a predictor of poor prognosis in patients with locally advanced esophagogastric cancer treated with perioperative chemotherapy. J. Gastrointest. Cancer 51, 484–490 (2020).
Péron, J. et al. The location of the primary colon cancer has no impact on outcomes in patients undergoing cytoreductive surgery for peritoneal metastasis. Surgery 165, 476–484 (2019).
Baratti, D. et al. Prognostic impact of primary side and RAS/RAF mutations in a surgical series of colorectal cancer with peritoneal metastases. Ann. Surg. Oncol. 28, 3332–3342 (2021).
Chang, L., Chang, M., Chang, H. M. & Chang, F. Microsatellite instability: a predictive biomarker for cancer immunotherapy. Appl. Immunohistochem. Mol. Morphol. 26, E15–E21 (2018).
Dienstmann, R. & Tabernero, J. Spectrum of gene mutations in colorectal cancer: implications for treatment. Cancer J. 22, 149–155 (2016).
Grieb, B. C. & Agarwal, R. HER2-directed therapy in advanced gastric and gastroesophageal adenocarcinoma: triumphs and troubles. Curr. Treat. Opt. Oncol. 22, 88 (2021).
Greally, M., Kelly, C. M. & Cercek, A. HER2: an emerging target in colorectal cancer. Curr. Probl. Cancer 42, 560–571 (2018).
Robson, M. E. et al. American society of clinical oncology policy statement update: genetic and genomic testing for cancer susceptibility. J. Clin. Oncol. 33, 3660–3667 (2015).
Lheureux, S., Braunstein, M. & Oza, A. M. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J. Clin. 69, 280–304 (2019).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Sohn, B. H. et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clin. Cancer Res. 23, 4441–4449 (2017).
Ubink, I. et al. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases. Br. J. Surg. 105, e204–e211 (2018).
Körfer, J., Lordick, F. & Hacker, U. T. Molecular targets for gastric cancer treatment and future perspectives from a clinical and translational point of view. Cancers 13, 5216 (2021).
Rubbia-Brandt, L. et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann. Oncol. 18, 299–304 (2007).
Solass, W., Sempoux, C., Detlefsen, S., Carr, N. J. & Bibeau, F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum. 1, 99–107 (2016).
Solass, W. et al. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology 74, 1014–1024 (2019).
Böhm, S. et al. Chemotherapy response score: development and validation of a system to quantify histopathologic response to neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J. Clin. Oncol. 33, 2457–2463 (2015).
Böhm, S. et al. Histopathologic response to neoadjuvant chemotherapy as a prognostic biomarker in tubo-ovarian high-grade serous carcinoma: updated Chemotherapy Response Score (CRS) results. Int. J. Gynecol. Cancer 29, 353–356 (2019).
Pinto, D., Chandra, A., Crothers, B. A., Kurtycz, D. F. I. & Schmitt, F. The international system for reporting serous fluid cytopathology-diagnostic categories and clinical management. J. Am. Soc. Cytopathol. 9, 469–477 (2020).
Virgilio, E. et al. Gastric cancer cells in peritoneal lavage fluid: a systematic review comparing cytological with molecular detection for diagnosis of peritoneal metastases and prediction of peritoneal recurrences. Anticancer. Res. 38, 1255 (2018).
Honore, C., Goere, D., Souadka, A., Dumont, F. & Elias, D. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann. Surg. Oncol. 20, 183–192 (2013).
Segelman, J., Akre, O., Gustafsson, U. O., Bottai, M. & Martling, A. Individualized prediction of risk of metachronous peritoneal carcinomatosis from colorectal cancer. Colorectal Dis. 16, 359–367 (2014).
Elias, D. et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann. Surg. 254, 289–293 (2011).
Tanaka, M. et al. Meta-analysis of recurrence pattern after resection for pancreatic cancer. Br. J. Surg. 106, 1590–1601 (2019).
Yepuri, N., Bahary, N., Jain, A. & Dhir, M. Review and update on the role of peritoneal cytology in the treatment of gastric cancer. J. Surg. Res. 235, 607–614 (2019).
Yang, H. K. et al. Extensive peritoneal lavage with saline after curative gastrectomy for gastric cancer (EXPEL): a multicentre randomised controlled trial. Lancet Gastroenterol. Hepatol. 6, 120–127 (2021).
Klaver, C. E. L. et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 4, 761–770 (2019).
Goere, D. et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 21, 1147–1154 (2020).
Ceelen, W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: the end of the road? Eur. J. Surg. Oncol. 45, 400–402 (2019).
Arjona-Sanchez, A. et al. HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer 18, 183 (2018).
Koga, S. et al. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer 61, 232–237 (1988).
Kaibara, N., Hamazoe, R., Iitsuka, Y., Maeta, M. & Koga, S. Hyperthermic peritoneal perfusion combined with anticancer chemotherapy as prophylactic treatment of peritoneal recurrence of gastric cancer. Hepatogastroenterology 36, 75–78 (1989).
Yonemura, Y. et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology 48, 1776–1782 (2001).
Brenkman, H. J. F., Paeva, M., van Hillegersberg, R., Ruurda, J. P. & Haj Mohammad, N. Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer — a systematic review. J. Clin. Med. 8, 1685 (2019).
Cuzick, J. Preventive therapy for cancer. Lancet Oncol. 18, e472–e482 (2017).
Kastrinos, F., Samadder, N. J. & Burt, R. W. Use of family history and genetic testing to determine risk of colorectal cancer. Gastroenterology 158, 389–403 (2020).
Hinchcliff, E. M., Bednar, E. M., Lu, K. H. & Rauh-Hain, J. A. Disparities in gynecologic cancer genetics evaluation. Gynecol. Oncol. 153, 184–191 (2019).
Zauber, A. G. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig. Dis. Sci. 60, 681–691 (2015).
Kim, J.-H. et al. Association between the National Cancer Screening Programme (NSCP) for gastric cancer and oesophageal cancer mortality. Br. J. Cancer 123, 480–486 (2020).
Kauff, N. D. et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J. Clin. Oncol. 26, 1331–1337 (2008).
Rebbeck, T. R. et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N. Engl. J. Med. 346, 1616–1622 (2002).
Guillem, J. G. et al. ASCO/SSO review of current role of risk-reducing surgery in common hereditary cancer syndromes. J. Clin. Oncol. 24, 4642–4660 (2006).
Seevaratnam, R. et al. A systematic review of the indications for genetic testing and prophylactic gastrectomy among patients with hereditary diffuse gastric cancer. Gastric Cancer 15 (Suppl. 1), S153–S163 (2012).
El Rami, F. E., Barsoumian, H. B. & Khneizer, G. W. Hereditary diffuse gastric cancer therapeutic roadmap: current and novel approaches in a nutshell. Ther. Adv. Med. Oncol. 12, 1758835920967238 (2020).
Pandalai, P. K., Lauwers, G. Y., Chung, D. C., Patel, D. & Yoon, S. S. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery 149, 347–355 (2011).
Bleicher, J. & Lambert, L. A. A palliative approach to management of peritoneal carcinomatosis and malignant ascites. Surg. Oncol. Clin. North. Am. 30, 475–490 (2021).
Franko, J. et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J. Clin. Oncol. 30, 263–267 (2012).
Solass, W. et al. Morphology of the peritoneal cavity and pathophysiological consequences. Pleura Peritoneum. 1, 193–201 (2016).
Masoumi Moghaddam, S., Amini, A., Morris, D. L. & Pourgholami, M. H. Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer Metastasis Rev. 31, 143–162 (2012).
Im, S. A. et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med. 381, 307–316 (2019).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372, 724–734 (2015).
Golan, T. et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N. Engl. J. Med. 381, 317–327 (2019).
Moore, K. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 379, 2495–2505 (2018).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
André, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Lemery, S., Keegan, P. & Pazdur, R. First FDA approval agnostic of cancer site — when a biomarker defines the indication. N. Engl. J. Med. 377, 1409–1412 (2017).
Lordick, F. & Janjigian, Y. Y. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat. Rev. Clin. Oncol. 13, 348–360 (2016).
Mateo, J. et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 29, 1895–1902 (2018).
Sugarbaker, P. H. Peritonectomy procedures. Cancer Treat. Res. 134, 247–264 (2007).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240, 205–213 (2004).
National Cancer Institute. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v4.0 (CTCAE). National Cancer Institute http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (2020).
Arjona-Sanchez, A. et al. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for limited peritoneal metastasis. The PSOGI international collaborative registry. Eur. J. Surg. Oncol. 47, 1420–1426 (2020).
Ansari, N. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumours. Eur. J. Surg. Oncol. 42, 1035–1041 (2016).
Passot, G. et al. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J. Surg. Oncol. 113, 796–803 (2016).
Glehen, O., Mohamed, F. & Gilly, F. N. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 5, 219–228 (2004).
Armstrong, D. K. et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 354, 34–43 (2006).
Soucisse, M. L., Liauw, W., Hicks, G. & Morris, D. L. Early postoperative intraperitoneal chemotherapy for lower gastrointestinal neoplasms with peritoneal metastasis: a systematic review and critical analysis. Pleura Peritoneum. 4, 20190007 (2019).
Canbay, E. et al. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann. Surg. Oncol. 21, 1147–1152 (2014).
Sugarbaker, P. H. & Chang, D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. Eur. J. Surg. Oncol. 43, 1228–1235 (2017).
Charrier, T. et al. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy with oxaliplatin increases the risk of postoperative hemorrhagic complications: analysis of predictive factors. Ann. Surg. Oncol. 23, 2315–2322 (2016).
Siebert, M. et al. Severe hypersensitivity reactions to platinum compounds post-pressurized intraperitoneal aerosol chemotherapy (PIPAC): first literature report. Cancer Chemother. Pharmacol. 83, 425–2322 (2018).
Tan, G. H. C., Shannon, N. B., Chia, C. S., Soo, K. C. & Teo, M. C. C. Platinum agents and mitomycin C-specific complications in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Int. J. Hyperth. 34, 595–600 (2018).
Alyami, M. et al. Ninety-day post-operative morbidity and mortality using the National Cancer Institute’s common terminology criteria for adverse events better describe post-operative outcome after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int. J. Hyperth. 34, 532–537 (2017).
Lurvink, R. J. et al. The Delphi and GRADE methodology used in the PSOGI 2018 consensus statement on pseudomyxoma peritonei and peritoneal mesothelioma. Eur. J. Surg. Oncol. 47, 4–10 (2019).
Sugarbaker, P. H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 7, 69–76 (2006).
Govaerts, K. et al. Appendiceal tumours and pseudomyxoma peritonei: literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 47, 11–35 (2021).
Kusamura, S. et al. Multicentre study of the learning curve and surgical performance of cytoreductive surgery with intraperitoneal chemotherapy for pseudomyxoma peritonei. Br. J. Surg. 101, 1758–1765 (2014).
Kusamura, S. et al. The role of hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei after cytoreductive surgery. JAMA Surg. 156, e206363 (2021).
Reddy, S. et al. Extending the indications of intestinal transplantation — modified multi visceral transplantation for end-stage pseudomyxoma peritoneii. Transplantation 101, S89 (2017).
Pillai, K., Akhter, J., Chua, T. C. & Morris, D. L. Anticancer property of bromelain with therapeutic potential in malignant peritoneal mesothelioma. Cancer Invest. 31, 241–250 (2013).
Pillai, K., Ehteda, A., Akhter, J., Chua, T. C. & Morris, D. L. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anticancer Drugs 25, 150–160 (2014).
Valle, S. J. et al. A novel treatment of bromelain and acetylcysteine (BromAc) in patients with peritoneal mucinous tumours: a phase I first in man study. Eur. J. Surg. Oncol. 47, 115–122 (2021).
Sgarbura, O. et al. Complete pathologic response after two-stage cytoreductive surgery with HIPEC for bulky pseudomyxoma peritonei: proof of concept. Int. J. Hyperth. 37, 585–591 (2020).
Malgras, B. et al. Impact of combination chemotherapy in peritoneal mesothelioma hyperthermic intraperitoneal chemotherapy (HIPEC): the RENAPE study. Ann. Surg. Oncol. 25, 3271–3279 (2018).
Simon, G. R. et al. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J. Clin. Oncol. 26, 3567–3572 (2008).
Zalcman, G. et al. Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma avastin cisplatin pemetrexed study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 387, 1405–1414 (2016).
Baas, P. et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397, 375–386 (2021).
Greenbaum, A. & Alexander, H. R. Peritoneal mesothelioma. Transl. Lung Cancer Res. 9 (Suppl. 1), S120–S132 (2020).
Yan, T. D. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J. Clin. Oncol. 27, 6237–6242 (2009).
Kusamura, S., Torres Mesa, P. A., Cabras, A., Baratti, D. & Deraco, M. The role of Ki-67 and pre-cytoreduction parameters in selecting diffuse malignant peritoneal mesothelioma (DMPM) patients for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann. Surg. Oncol. 23, 1468–1473 (2016).
Alexander, H. R. et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 153, 779–786 (2013).
Le Roy, F. et al. Conversion to complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma after bidirectional chemotherapy. Ann. Surg. Oncol. 24, 3640–3646 (2017).
Alyami, M. et al. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 47, 128–133 (2021).
Sgarbura, O. et al. MESOTIP: phase II multicenter randomized trial evaluating the association of PIPAC and systemic chemotherapy vs. systemic chemotherapy alone as 1st-line treatment of malignant peritoneal mesothelioma. Pleura Peritoneum. 4, 20190010 (2019).
Elias, D. et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 27, 681–685 (2009).
Franko, J. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 116, 3756–3762 (2010).
Esquivel, J. et al. The American Society of Peritoneal Surface Malignancies (ASPSM) multiinstitution evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 patients with colorectal cancer with peritoneal carcinomatosis. Ann. Surg. Oncol. 21, 4195–4201 (2014).
Cashin, P. H. et al. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: a randomised trial. Eur. J. Cancer 53, 155–162 (2016).
Goere, D. et al. Results of a randomized phase 3 study evaluating the potential benefit of a second-look surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP- NTC01226394). J. Clin. Oncol. 36 (Suppl. 15), 3531 (2018).
Abboud, K. et al. Management of colorectal peritoneal metastases: expert opinion. J. Visc. Surg. 156, 377–379 (2019).
Al-Batran, S. E. et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 Trial. JAMA Oncol. 3, 1237–1244 (2017).
Bonnot, P. E. et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J. Clin. Oncol. 37, 2028–2040 (2019).
Chia, C. S. et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann. Surg. Oncol. 23, 1971–1979 (2016).
Brandl, A., Yonemura, Y., Glehen, O., Sugarbaker, P. & Rau, B. Long term survival in patients with peritoneal metastasised gastric cancer treated with cytoreductive surgery and HIPEC: a multi-institutional cohort from PSOGI. Eur. J. Surg. Oncol. 47, 172–180 (2021).
Badgwell, B. et al. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann. Surg. Oncol. 24, 3338–3344 (2017).
Beeharry, M. K. et al. Study protocol of a multicenter phase III randomized controlled trial investigating the efficiency of the combination of neoadjuvant chemotherapy (NAC) and neoadjuvant laparoscopic intraperitoneal hyperthermic chemotherapy (NLHIPEC) followed by R0 gastrectomy with intraoperative HIPEC for advanced gastric cancer (AGC): dragon II trial. BMC Cancer 20, 224 (2020).
Koemans, W. J. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer 19, 420 (2019).
Alyami, M. et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur. J. Surg. Oncol. 47, 123–127 (2021).
Coccolini, F. et al. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur. J. Surg. Oncol. 41, 911–919 (2015).
Coccolini, F. et al. Effect of intraperitoneal chemotherapy and peritoneal lavage in positive peritoneal cytology in gastric cancer. Systematic review and meta-analysis. Eur. J. Surg. Oncol. 42, 1261–1267 (2016).
Yonemura, Y. et al. Effects of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy and neoadjuvant intraperitoneal/systemic chemotherapy on peritoneal metastases from gastric cancer. Ann. Surg. Oncol. 24, 478–485 (2017).
Bristow, R. E., Tomacruz, R. S., Armstrong, D. K., Trimble, E. L. & Montz, F. J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol. 20, 1248–1259 (2002).
Vergote, I., Chiva, L. & du Bois, A. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 378, 1362–1363 (2018).
Kehoe, S. et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 386, 249–257 (2015).
Colombo, N. et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann. Oncol. 30, 672–705 (2019).
Harter, P. et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N. Engl. J. Med. 380, 822–832 (2019).
Huo, Y. R., Richards, A., Liauw, W. & Morris, D. L. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: a systematic review and meta-analysis. Eur. J. Surg. Oncol. 41, 1578–1589 (2015).
Harter, P. et al. Surgery for recurrent ovarian cancer: role of peritoneal carcinomatosis: exploratory analysis of the DESKTOP I Trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann. Surg. Oncol. 16, 1324–1330 (2009).
Harter, P. et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann. Surg. Oncol. 13, 1702–1710 (2006).
Fanfani, F. et al. Is there a role for tertiary (TCR) and quaternary (QCR) cytoreduction in recurrent ovarian cancer? Anticancer. Res. 35, 6951–6955 (2015).
Shi, T. et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(21)00006-1 (2021).
Spiliotis, J. et al. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of recurrent advanced ovarian cancer: a prospective study. J. Buon. 16, 74–79 (2011).
Bakrin, N. et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur. J. Surg. Oncol. 39, 1435–1443 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03220932?term=NCT03220932&draw=2&rank=1 (2019).
Tempfer, C. B. et al. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol. Oncol. 150, 23–30 (2018).
Bakrin, N. et al. PIPAC-OV3: a multicenter, open-label, randomized, two-arm phase III trial of the effect on progression-free survival of cisplatin and doxorubicin as pressurized intra-peritoneal aerosol chemotherapy (PIPAC) vs. chemotherapy alone in patients with platinum-resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer. Pleura Peritoneum. 3, 20180114 (2018).
Tempfer, C. B., Hilal, Z., Dogan, A., Petersen, M. & Rezniczek, G. A. Concentrations of cisplatin and doxorubicin in ascites and peritoneal tumor nodules before and after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with peritoneal metastasis. Eur. J. Surg. Oncol. 44, 1112–1117 (2018).
Goéré, D. et al. Complete cytoreductive surgery plus HIPEC for peritoneal metastases from unusual cancer sites of origin: results from a worldwide analysis issue of the Peritoneal Surface Oncology Group International (PSOGI). Int. J. Hyperth. 33, 520–527 (2017).
Amblard, I. et al. Cytoreductive surgery and HIPEC improve survival compared to palliative chemotherapy for biliary carcinoma with peritoneal metastasis: a multi-institutional cohort from PSOGI and BIG RENAPE groups. Eur. J. Surg. Oncol. 44, 1378–1383 (2018).
Mehta, S. et al. Is there an oncological interest in the combination of CRS/HIPEC for peritoneal carcinomatosis of HCC? Results of a multicenter international study. Eur. J. Surg. Oncol. 44, 1786–1792 (2018).
Farma, J. M. et al. Limited survival in patients with carcinomatosis from foregut malignancies after cytoreduction and continuous hyperthermic peritoneal perfusion. J. Gastrointest. Surg. 9, 1346–1353 (2005).
Cardi, M. et al. Treatment of peritoneal carcinomatosis from breast cancer by maximal cytoreduction and HIPEC: a preliminary report on 5 cases. Breast 22, 845–849 (2013).
McLemore, E. C. et al. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann. Surg. Oncol. 12, 886–894 (2005).
Rossi, C. R. et al. Hyperthermic intraperitoneal intraoperative chemotherapy after cytoreductive surgery for the treatment of abdominal sarcomatosis: clinical outcome and prognostic factors in 60 consecutive patients. Cancer 100, 1943–1950 (2004).
Baratti, D. et al. Peritoneal sarcomatosis: is there a subset of patients who may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Ann. Surg. Oncol. 17, 3220–3228 (2010).
Salti, G. I., Ailabouni, L. & Undevia, S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal sarcomatosis. Ann. Surg. Oncol. 19, 1410–1415 (2012).
Baumgartner, J. M. et al. Aggressive locoregional management of recurrent peritoneal sarcomatosis. J. Surg. Oncol. 107, 329–334 (2013).
Sommariva, A. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal sarcomatosis: long-term outcome from a single institution experience. Anticancer. Res. 33, 3989–3994 (2013).
Olesiński, T. Cytoreductive surgery and HIPEC in the treatment of peritoneal metastases of sarcomas and other rare malignancies. Pol. Prz. Chirurgiczny. 89, 31–36 (2017).
Norlén, O. et al. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J. Surg. 36, 1419–1431 (2012).
Norlén, O. et al. Peritoneal carcinomatosis from small intestinal neuroendocrine tumors: clinical course and genetic profiling. Surgery 156, 1512–1521 (2014).
Pavel, M. et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 103, 172–185 (2016).
Elias, D. et al. Neuroendocrine carcinomas: optimal surgery of peritoneal metastases (and associated intra-abdominal metastases). Surgery 155, 5–12 (2014).
Mayrbaurl, B. et al. Quality of life across chemotherapy lines in patients with advanced colorectal cancer: a prospective single-center observational study. Support Care Cancer 24, 667–674 (2016).
Corn, B. W., Feldman, D. B. & Wexler, I. The science of hope. Lancet Oncol. 21, e452–e459 (2020).
Kaasa, S. & Loge, J. H. Quality-of-life assessment in palliative care. Lancet Oncol. 3, 175–182 (2002).
Lambert, L. A. & Wiseman, J. Palliative management of peritoneal metastases. Ann. Surg. Oncol. 25, 2165–2171 (2018).
Odendahl, K. et al. Quality of life of patients with end-stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Eur. J. Surg. Oncol. 41, 1379–1385 (2015).
Franko, J. et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 17, 1709–1719 (2016).
Prigerson, H. G. et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 1, 778–784 (2015).
Enzinger, A. C., Zhang, B., Schrag, D. & Prigerson, H. G. Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J. Clin. Oncol. 33, 3809–3816 (2015).
Weeks, J. C. et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N. Engl. J. Med. 367, 1616–1625 (2012).
Francescutti, V. A. et al. Characterizing the patient experience of CS/HIPEC through in-depth interviews with patients: identification of key concepts in the development of a patient-centered program. Ann. Surg. Oncol. 26, 1063–1070 (2019).
Tan, W. J. et al. Quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: an Asian perspective. Ann. Surg. Oncol. 20, 4219–4223 (2013).
Roudijk, B., Donders, R. & Stalmeier, P. Cultural values: can they explain self-reported health? Qual. Life Res. 26, 1531–1539 (2017).
Cashin, P. H. et al. Quality of life and cost effectiveness in a randomized trial of patients with colorectal cancer and peritoneal metastases. Eur. J. Surg. Oncol. 44, 983–990 (2018).
Stearns, A. T. et al. Long-term quality of life after cytoreductive surgery and heated intraperitoneal chemotherapy for pseudomyxoma peritonei: a prospective longitudinal study. Ann. Surg. Oncol. 25, 965–973 (2018).
Koole, S. N. et al. Health-related quality of life after interval cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with stage III ovarian cancer. Eur. J. Surg. Oncol. 47, 101–107 (2021).
Dodson, R. M. et al. Quality-of-life evaluation after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 23 (Suppl. 5), 772–783 (2016).
Zhu, Y., Hanna, N., Boutros, C. & Alexander, H. R. Jr. Assessment of clinical benefit and quality of life in patients undergoing cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) for management of peritoneal metastases. J. Gastrointest. Oncol. 4, 62–71 (2013).
Shan, L. L., Saxena, A., Shan, B. L. & Morris, D. L. Quality of life after cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy for peritoneal carcinomatosis: a systematic review and meta-analysis. Surg. Oncol. 23, 199–210 (2014).
Verduin, M., Hoeben, A., De Ruysscher, D. & Vooijs, M. Patient-derived cancer organoids as predictors of treatment response. Front. Oncol. 11, 820 (2021).
Schutgens, F. & Clevers, H. Human organoids: tools for understanding biology and treating diseases. Annu. Rev. Pathol. 15, 211–234 (2020).
Fujii, M. & Sato, T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat. Mater. 20, 156–169 (2020).
Brodeur, M. N. et al. Carboplatin response in preclinical models for ovarian cancer: comparison of 2D monolayers, spheroids, ex vivo tumors and in vivo models. Sci. Rep. 11, 1–12 (2021).
Ceelen, W., Braet, H., Van Ramshorst, G., Willaert, W. & Remaut, K. Intraperitoneal chemotherapy for peritoneal metastases: an expert opinion. Expert Opin. Drug Deliv. 17, 511–522 (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Sugarbaker, P. H. Intraperitoneal paclitaxel: pharmacology, clinical results and future prospects. J. Gastrointest. Oncol. 12 (Suppl. 1), S231 (2021).
Dakwar, G. R. et al. Nanomedicine-based intraperitoneal therapy for the treatment of peritoneal carcinomatosis — mission possible? Adv. Drug Deliv. Rev. 108, 13–24 (2017).
Levine E. & Ceelen W. P. In Biomaterials and Drug Delivery Systems for Intraperitoneal Chemotherapy Ch. 32 (Taylor & Francis Group, 2016).
Dakwar G. & Remaut, K. In Intraperitoneal Nonviral Nucleic Acid Delivery in the Treatment of Peritoneal Cancer Ch. 32 (Taylor & Francis Group, 2016).
Shariati, M., Willaert, W., Ceelen, W., De Smedt, S. C. & Remaut, K. Aerosolization of nanotherapeutics as a newly emerging treatment regimen for peritoneal carcinomatosis. Cancers 11, 906 (2019).
Shariati, M. et al. Synergy between intraperitoneal aerosolization (PIPAC) and cancer nanomedicine: cisplatin-loaded polyarginine-hyaluronic acid nanocarriers efficiently eradicate peritoneal metastasis of advanced human ovarian cancer. ACS Appl. Mater. Interfaces 12, 29024–29036 (2020).
Shariati, M. et al. High pressure nebulization (PIPAC) versus injection for the intraperitoneal administration of mRNA complexes. Pharm. Res. 36, 1–13 (2019).
Chen, C.-H. et al. Thermosensitive injectable hydrogel for simultaneous intraperitoneal delivery of doxorubicin and prevention of peritoneal adhesion. Int. J. Mol. Sci. 19, 1373 (2018).
Song, L. et al. Peritoneal adhesion prevention with a biodegradable and injectable N,O-carboxymethyl chitosan-aldehyde hyaluronic acid hydrogel in a rat repeated-injury model. Sci. Rep. 6, 1–13 (2016).
Bhatt A. In Management of Peritoneal Metastases — Cytoreductive Surgery, HIPEC and Beyond 469–506 (Springer, 2018).
Van Oudheusden, T. R. Targeting the peritoneum with novel drug delivery systems — review peritoneal carcinomatosis. Anticancer Res. 35, 627–634 (2015).
Gao, X. et al. Novel thermosensitive hydrogel for preventing formation of abdominal adhesions. Int. J. Nanomed. 8, 2453–2463 (2013).
De Smet, L., Ceelen, W., Remon, J. P. & Vervaet, C. Optimization of drug delivery systems for intraperitoneal therapy to extend the residence time of the chemotherapeutic agent. ScientificWorldJournal 2013, 720858 (2013).
Padmakumar, S., Parayath, N. N., Nair, S. V., Menon, D. & Amiji, M. M. Enhanced anti-tumor efficacy and safety with metronomic intraperitoneal chemotherapy for metastatic ovarian cancer using biodegradable nanotextile implants. J. Controlled Rel. 305, 29–40 (2019).
Bommareddy, P. K., Shettigar, M. & Kaufman, H. L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 18, 498–513 (2018).
Liu, Z., Ravindranathan, R., Kalinski, P., Guo, Z. S. & Bartlett, D. L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 8, 14754 (2017).
Cohn, D. E. et al. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin®) in recurrent ovarian, tubal, or peritoneal cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 146, 477–483 (2017).
Giehl, E. et al. In vivo priming of peritoneal tumor-reactive lymphocytes with a potent oncolytic virus for adoptive cell therapy. Front. Immunol. 12, 610042 (2021).
Lee, Y. S. et al. Oncolytic vaccinia virus reinvigorates peritoneal immunity and cooperates with immune checkpoint inhibitor to suppress peritoneal carcinomatosis in colon cancer. J. Immunother. Cancer 8, e000857 (2020).
Bhatt, A. & Glehen, O. Extent of peritoneal resection for peritoneal metastases: looking beyond a complete cytoreduction. Ann. Surg. Oncol. 27, 1458–1470 (2020).
Dip, F. et al. Consensus conference statement on the general use of near-infrared fluorescence imaging and indocyanine green guided surgery: results of a modified Delphi study. Ann. Surg. https://doi.org/10.1097/SLA.0000000000004412 (2020).
Liberale, G. et al. Fluorescence imaging after indocyanine green injection for detection of peritoneal metastases in patients undergoing cytoreductive surgery for peritoneal carcinomatosis from colorectal cancer. Ann. Surg. 264, 1110–1115 (2016).
Jiao, J. et al. Quicker, deeper and stronger imaging: a review of tumor-targeted, near-infrared fluorescent dyes for fluorescence guided surgery in the preclinical and clinical stages. Eur. J. Pharmaceutics Biopharmaceutics. 152, 123–143 (2020).
Van Dam, G. M. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat. Med. 17, 1315–1319 (2011).
Farghaly, S. A. Single Port Access (SPA) Robot assisted laparoscopic partial parietal peritonectomy in patients with advanced and recurrent ovarian cancer: Farghaly’s technique. J. Minim. Invasive Gynecol. 19, S157 (2012).
Chen, T. C. & Liang, J. T. Robotic low anterior resection with total hysterectomy, bilateral salpingo-oophorectomy and en bloc pelvic peritonectomy followed by hyperthermic intraperitoneal chemotherapy for the multi-modal treatment of rectosigmoid cancer with peritoneal seeding - a video vignette. Colorectal Dis. 23, 1591–1592 (2021).
Reymond, M., Demtroeder, C., Solass, W., Winnekendonk, G. & Tempfer, C. Electrostatic precipitation pressurized intraperitoneal aerosol chemotherapy (ePIPAC): first in-human application. Pleura Peritoneum 1, 109–116 (2016).
Kakchekeeva, T. et al. In vivo feasibility of electrostatic precipitation as an adjunct to pressurized intraperitoneal aerosol chemotherapy (ePIPAC). Ann. Surg. Oncol. 23, 592–598 (2016).
Bachmann, C. et al. Technology development of hyperthermic pressurized intraperitoneal aerosol chemotherapy (hPIPAC). Surgical Endosc. 35, 6358–6365 (2021).
Bushati, M. et al. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: Results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI). Eur. J. Surg. Oncol. 44, 1942–1948 (2018).
Yurttas, C. et al. Systematic review of variations in hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastasis from colorectal cancer. J. Clin. Med. 7, 567 (2018).
Cremolini, C. et al. Surrogate endpoints in second-line trials of targeted agents in metastatic colorectal cancer: a literature-based systematic review and meta-analysis. Cancer Res. Treat. 49, 834–845 (2017).
Kipps, E., Tan, D. & Kaye, S. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat. Rev. Cancer 13, 273–282 (2013).
Acknowledgements
The authors thank Jeanine for their contribution in Box 2.
Author information
Authors and Affiliations
Contributions
Introduction (D.C.-G., M.H. and F.L.); Epidemiology (D.C.-G., M.H., A.B., K.K.T. and M.C.); Mechanisms/Pathophysiology (D.C.-G., M.H., W.C. and R.R.); Diagnosis/Screening/Prevention (D.C.-G., M.H., M.A., O.G., F.L., O.S. and K.V.D.S.); Management (D.C.-G., M.H., M.A., O.G. and F.L.); Quality of life (D.C.-G., M.H., A.B., O.S. and K.V.D.S.); Outlook (D.C.-G., M.H., W.C. and R.R.); Overview of the Primer (D.C.-G.).
Corresponding author
Ethics declarations
Competing interests
R.R. has received group research and engineering support from Fisher and Paykel. K.V.D.S. is a board member of the European Society of Surgical Oncology (ESSO) and an executive committee member of the Peritoneal Surface Oncology Group International (PSOGI). D.C.-G. holds honorary functions within ESSO as member of the Boards of directors, the Spanish Society of Surgical Oncology (SEOQ) as member of the Boards of Directors, the Spanish Federation of Oncologic Societies (FESEO) as secretary and member of the Boards of Directors, the International Society for the Study of Pleura and Peritoneum (ISSPP) as member of the communication committee, and the Enhanced Recovery After Surgery (ERAS) society and is responsible for the ERAS HIPEC website content. A.B. is on the review committee of the Department of Science and Technology of India, and declares no financial or non-financial competing interests. M.A. is a member of the ISSPP Education committee. M.H. has received research funding (institution) from Nestlé health science, speaker honorary (institution) from MSD, Nestlé, Fresenius, Capnomed and Encare, is a board member of the ERAS society and is chair of education of the ISSPP. O.G. is a consultant for GAMIDA (financial competing interest), Director of RENAPE, and President of BIG RENAPE association (non-financial competing interests). O.S. holds honorary functions with ESSO (member of the Communication committee), ISSPP (Public Relations committee), the French Society of Surgical Oncology (executive board), the French National Network for Peritoneum (RENAPE; board member) and the Romanian Students’ Society of Surgery (honorary president). W.C. is Board member of the Belgian Society of Surgical Oncology and Royal Belgian Society of Surgery, and Editor-in-Chief of Acta Chirurgica Belgica, and Associate editor of European Surgical Research and International Journal of Hyperthermia. F.L., K.K.T. and M.C. declares no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks Peter Cashin, Yasuhiro Kodera, Winston Spencer Liauw, Beate Rau and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Melanoma: https://www.sciencedirect.com/topics/medicine-and-dentistry/nodular-melanoma
Supplementary information
Glossary
- Sarcoma
-
A cancer of the connective tissue including bones, nerves, muscles, tendons, cartilage or blood vessels.
- Orphan diseases
-
Diseases that have been ignored, mostly owing to their rarity.
- Metachronous metastases
-
Metachronous metastases are diagnosed at least 3 months after diagnosis of the primary tumour, whereas synchronous metastases are diagnosed with the primary tumour or up to 3 months after its diagnosis.
- Pseudomyxoma peritonei
-
(PMP). This syndrome results from the progressive accumulation of mucin in the peritoneal cavity; perforation of an epithelial neoplasm of the appendix is the most common cause.
- T stage
-
The invasion depth of a primary tumour into the wall of the affected organ.
- Cytology
-
Exam of single cell types in fluid specimens.
- Familial adenomatous polyposis
-
A rare genetic condition resulting from a defect of APC leading to multiple polyps throughout the colon and associated with a near 100% risk of developing bowel cancer.
- Microvilli
-
Small finger-like projections that increase the surface area of some cell types, including mesothelial cells.
- Peritoneal cavity
-
The anatomical space in the abdomen and pelvis lined by peritoneum.
- Mesothelial cells
-
Specialized cells that line certain body cavities or organs; these multifunctional cells have various functions, including the lubrication of surfaces and absorption of excess fluids.
- Adhesions
-
Connective tissue that joins adjacent anatomical structures; adhesions typically form as a result of an inflammatory process or after surgery.
- Paracolic gutter
-
The space lateral to the colon and the abdominal wall.
- Mesentery
-
The connective tissue that supports the intestines and contains blood vessels and draining lymphatics.
- Omentum
-
A fold of peritoneum and fatty tissue connecting the stomach with the transverse colon (greater omentum) and the porta hepatis (lesser omentum).
- Pneumoperitoneum
-
The consequence of air or CO2 filling the peritoneal cavity; the creation of artificial space is required for minimal invasive surgery.
- Spiral CT
-
High-resolution cross-sectional imaging modality on the basis of CT with a faster machine rotating continuously around the body, enabling more rapid acquisition and higher image resolution.
- FOLFOX
-
A systemic chemotherapy regimen including folinic acid (FOL), 5-fluorouracil (F), and oxaliplatin (OX), which can benefit from the addition of a targeted treatment such as an anti-epidermal growth factor receptor or an anti-vascular endothelial growth factor agent; different doses and duration of administration, especially of 5-fluorouracil, are in use.
- FOLFIRI
-
A systemic chemotherapy regimen including folinic acid (FOL), 5-fluorouracil (F) and irinotecan (IRI), which can benefit from the addition of a targeted treatment such as an anti-epidermal growth factor receptor or an anti-vascular endothelial growth factor agent.
Rights and permissions
About this article
Cite this article
Cortés-Guiral, D., Hübner, M., Alyami, M. et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers 7, 91 (2021). https://doi.org/10.1038/s41572-021-00326-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-021-00326-6
This article is cited by
-
Intraperitoneal administration of mRNA encoding interleukin-12 for immunotherapy in peritoneal carcinomatosis
Journal of Nanobiotechnology (2025)
-
Primary and secondary metastatic dissemination: multiple routes to cancer-related death
Molecular Cancer (2025)
-
The EGR1-mediated lncRNA TENM3-AS1 potentiates gastric cancer metastasis via reprogramming fatty acid metabolism
Molecular Cancer (2025)
-
Risk factors for postoperative acute kidney injury after cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: a meta-analysis and systematic review
World Journal of Surgical Oncology (2025)
-
MCT4: a key player influencing gastric cancer metastasis and participating in the regulation of the metastatic immune microenvironment
Journal of Translational Medicine (2025)