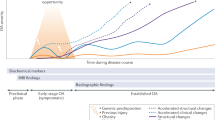
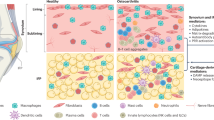
Abstract
Osteoarthritis is a heterogeneous whole-joint disease that can cause pain and is a leading cause of disability and premature work loss. The predominant disease risk factors — obesity and joint injury — are well recognized and modifiable. A greater understanding of the complex mechanisms, including inflammatory, metabolic and post-traumatic processes, that can lead to disease and of the pathophysiology of pain is helping to delineate mechanistic targets. Currently, management is primarily focused on alleviating the main symptoms of pain and obstructed function through lifestyle interventions such as self-management programmes, education, physical activity, exercise and weight management. However, lack of adherence to known effective osteoarthritis therapeutic strategies also contributes to the high global disease burden. For those who have persistent symptoms that are compromising quality of life and have not responded adequately to core treatments, joint replacement is an option to consider. The burden imparted by the disease causes a substantial impact on individuals affected in terms of quality of life. For society, this disease is a substantial driver of increased health-care costs and underemployment. This Primer highlights advances and controversies in osteoarthritis, drawing key insights from the current evidence base.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hunter, D. J., March, L. & Chew, M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet 396, 1711–1712 (2020).
Wieland, H. A., Michaelis, M., Kirschbaum, B. J. & Rudolphi, K. A. Osteoarthritis — an untreatable disease? Nat. Rev. Drug Discov. 4, 331–344 (2005).
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet 393, 1745–1759 (2019).
Kraus, V. B., Blanco, F. J., Englund, M., Karsdal, M. A. & Lohmander, L. S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 23, 1233–1241 (2015).
Whittaker, J. L., Runhaar, J., Bierma-Zeinstra, S. & Roos, E. M. A lifespan approach to osteoarthritis prevention. Osteoarthr. Cartil. 29, 1638–1653 (2021).
Jinks, C. et al. Changing the narrative on osteoarthritis: a call for global action. Osteoarthr. Cartil. 32, 414–420 (2024).
Mahmoudian, A., Lohmander, L. S., Mobasheri, A., Englund, M. & Luyten, F. P. Early-stage symptomatic osteoarthritis of the knee — time for action. Nat. Rev. Rheumatol. 17, 621–632 (2021).
Mahmoudian, A. et al. Timing is everything: towards classification criteria for early-stage symptomatic knee osteoarthritis. Osteoarthr. Cartil. 32, 649–653 (2024).
Hunter, D. J. & Bowden, J. L. Therapy: are you managing osteoarthritis appropriately? Nat. Rev. Rheumatol. 13, 703–704 (2017).
Murphy, L. et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 59, 1207–1213 (2008). A longitudinal study of 3,068 participants in the Johnston County Osteoarthritis Project revealing the lifetime risk of symptomatic OA in at least one knee.
Arslan, I. G. et al. Estimating incidence and prevalence of hip osteoarthritis using electronic health records: a population-based cohort study. Osteoarthr. Cartil. 30, 843–851 (2022).
Haugen, I. K. et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann. Rheum. Dis. 70, 1581–1586 (2011).
Marshall, M., Watt, F. E., Vincent, T. L. & Dziedzic, K. Hand osteoarthritis: clinical phenotypes, molecular mechanisms and disease management. Nat. Rev. Rheumatol. 14, 641–656 (2018). A comprehensive review on the clinical phenotypes, molecular mechanisms and management of hand OA.
Gellhorn, A. C., Katz, J. N. & Suri, P. Osteoarthritis of the spine: the facet joints. Nat. Rev. Rheumatol. 9, 216–224 (2013).
Herrera-Pérez, M. et al. Ankle osteoarthritis aetiology. J. Clin. Med. 10, 4489 (2021).
Stanley, D. Prevalence and etiology of symptomatic elbow osteoarthritis. J. Shoulder Elb. Surg. 3, 386–389 (1994).
Oo, W. M. & Linklater, J. Prevalence of osteoarthritis-related imaging abnormalities in asymptomatic healthy adults. Osteoarthr. Cartil. 32, 1181–1183 (2024).
Kitamura, Y. et al. Prevalence and associated factors for primary osteoarthritis of the scaphotrapeziotrapezoid, radiocarpal, and distal radioulnar joints in the Japanese general elderly population. J. Hand Surg. 50, 103.e1–103.e10 (2023).
Lu, K. et al. Molecular signaling in temporomandibular joint osteoarthritis. J. Orthop. Translat. 32, 21–27 (2022).
GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 5, e508–e522 (2023).
Weng, Q. et al. Global burden of early-onset osteoarthritis, 1990-2019: results from the Global Burden of Disease Study 2019. Ann. Rheum. Dis. 83, 915–925 (2024). Data from the Global Burden of Diseases Study 2019 showed the numbers of incident cases, prevalent cases, YLDs and corresponding age-standardized rates for early-onset OA (diagnosis before age 55 years) from 1990 to 2019.
Maerz, T. & Schiphof, D. From cartilage to culture: opportunities for unraveling the complexities of osteoarthritis through sex and gender. Osteoarthr. Cartil. 32, 1013–1015 (2024).
Loeser, R. F., Collins, J. A. & Diekman, B. O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 412–420 (2016).
Park, D. et al. Association of general and central obesity, and their changes with risk of knee osteoarthritis: a nationwide population-based cohort study. Sci. Rep. 13, 3796 (2023).
Salis, Z., Gallego, B., Nguyen, T. V. & Sainsbury, A. Association of decrease in body mass index with reduced incidence and progression of the structural defects of knee osteoarthritis: a prospective multi-cohort study. Arthritis Rheumatol. 75, 533–543 (2023).
Balogun, S., Scott, D. & Aitken, D. Association between sarcopenic obesity and knee osteoarthritis: a narrative review. Osteoarthr. Cartil. Open 6, 100489 (2024).
Yang, J. et al. Causal relationship between sarcopenia and osteoarthritis: a bi-directional two-sample Mendelian randomized study. Eur. J. Med. Res. 28, 327 (2023).
Szilagyi, I. A., Waarsing, J. H., Schiphof, D., van Meurs, J. B. J. & Bierma-Zeinstra, S. M. A. Towards sex-specific osteoarthritis risk models: evaluation of risk factors for knee osteoarthritis in males and females. Rheumatology 61, 648–657 (2022).
Wang, X. et al. Occupational risk in knee osteoarthritis: a systematic review and meta-analysis of observational studies. Arthritis Care Res. 72, 1213–1223 (2020).
Felson, D. et al. New approach to testing treatments for osteoarthritis: FastOA. Ann. Rheum. Dis. 83, 274–276 (2024).
Brouwer, G. M. et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 56, 1204–1211 (2007).
Li, M. et al. Varus-valgus knee laxity is related to a higher risk of knee osteoarthritis incidence and structural progression: data from the osteoarthritis initiative. Clin. Rheumatol. 41, 1013–1021 (2022).
Thirumaran, A. J., Murphy, N. J., Fu, K. & Hunter, D. J. Femoroacetabular impingement — what the rheumatologist needs to know. Best Pract. Res. Clin. Rheumatol. 38, 101932 (2024).
Silverwood, V. et al. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr. Cartil. 23, 507–515 (2015).
Wei, G. et al. Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms. Bone Res. 11, 63 (2023).
Boer, C. G. et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell 184, 4784–4818.e17 (2021). A genome-wide association study meta-analysis across 826,690 individuals identifying 100 independently associated risk variants across 11 OA phenotypes.
Waheed, A. & Rai, M. F. Osteoarthritis year in review 2023: genetics, genomics, and epigenetics. Osteoarthr. Cartil. 32, 128–137 (2024).
Kehayova, Y. S., Wilkinson, J. M., Rice, S. J. & Loughlin, J. Mediation of the same epigenetic and transcriptional effect by independent osteoarthritis risk-conferring alleles on a shared target gene, COLGALT2. Arthritis Rheumatol. 75, 910–922 (2023).
Xu, X. et al. Identification of KANSL1 as a novel pathogenic gene for developmental dysplasia of the hip. J. Mol. Med. 100, 1159–1168 (2022).
Courties, A. et al. Human-specific duplicate CHRFAM7A gene is associated with more severe osteoarthritis and amplifies pain behaviours. Ann. Rheum. Dis. 82, 710–718 (2023).
Xu, R. et al. Association between single nucleotide variants and severe chronic pain in older adult patients after lower extremity arthroplasty. J. Orthop. Surg. Res. 18, 184 (2023).
Kamps, A. et al. Occurrence of comorbidity following osteoarthritis diagnosis: a cohort study in the Netherlands. Osteoarthr. Cartil. 31, 519–528 (2023).
Dell’Isola, A. et al. Risk of comorbidities following physician-diagnosed knee or hip osteoarthritis: a register-based cohort study. Arthritis Care Res. 74, 1689–1695 (2022).
Swain, S., Sarmanova, A., Coupland, C., Doherty, M. & Zhang, W. Comorbidities in osteoarthritis: a systematic review and meta-analysis of observational studies. Arthritis Care Res. 72, 991–1000 (2020). A recent systematic review highlighted the importance and frequency with which persons with OA experience comorbidities.
Dell’Isola, A., Recenti, F., Englund, M. & Kiadaliri, A. Twenty-year trajectories of morbidity in individuals with and without osteoarthritis. RMD Open 10, e004164 (2024).
The Osteoarthritis Research Society International. Osteoarthritis: A Serious Disease, Submitted to the U.S. Food and Drug Administration December 1, 2016 (OARSI, 2016).
Nüesch, E. et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 342, d1165 (2011).
Wilkie, R. et al. Reasons why osteoarthritis predicts mortality: path analysis within a Cox proportional hazards model. RMD Open 5, e001048 (2019).
Hunter, D. J. & Deveza, L. A. Deconstructing the “types” of osteoarthritis. Osteoarthritis Imaging https://doi.org/10.1016/j.ostima.2024.100257 (2024).
Dell’Isola, A. & Steultjens, M. Classification of patients with knee osteoarthritis in clinical phenotypes: data from the osteoarthritis initiative. PLoS ONE 13, e0191045 (2018).
Mobasheri, A. et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 13, 302–311 (2017).
Huang, C. et al. Development and formulation of the classification criteria for osteoarthritis. Ann. Transl. Med. 8, 1068 (2020).
Angelini, F. et al. Osteoarthritis endotype discovery via clustering of biochemical marker data. Ann. Rheum. Dis. 81, 666–675 (2022).
Vincent, T. L. et al. Osteoarthritis pathophysiology: therapeutic target discovery may require a multifaceted approach. Clin. Geriatr. Med. 38, 193–219 (2022).
Grandi, F. C. et al. Single-cell mass cytometry reveals cross-talk between inflammation-dampening and inflammation-amplifying cells in osteoarthritic cartilage. Sci. Adv. 6, eaay5352 (2020).
Sahu, N., Grandi, F. C. & Bhutani, N. A single-cell mass cytometry platform to map the effects of preclinical drugs on cartilage homeostasis. JCI Insight 7, e160702 (2022).
Fan, Y. et al. Unveiling inflammatory and prehypertrophic cell populations as key contributors to knee cartilage degeneration in osteoarthritis using multi-omics data integration. Ann. Rheum. Dis. 83, 926–944 (2024). Multi-omics data revealing inflammatory and pre-hypertrophic cell populations in OA cartilage degeneration.
Zheng, L., Zhang, Z., Sheng, P. & Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 66, 101249 (2021).
Santos, S., Emery, N., Neu, C. P. & Pierce, D. M. Propagation of microcracks in collagen networks of cartilage under mechanical loads. Osteoarthr. Cartil. 27, 1392–1402 (2019).
Rim, Y. A. & Ju, J. H. The role of fibrosis in osteoarthritis progression. Life 11, 3 (2020).
Deroyer, C. et al. CEMIP (KIAA1199) induces a fibrosis-like process in osteoarthritic chondrocytes. Cell Death Dis. 10, 103 (2019).
Miosge, N., Hartmann, M., Maelicke, C. & Herken, R. Expression of collagen type I and type II in consecutive stages of human osteoarthritis. Histochem. Cell Biol. 122, 229–236 (2004).
Hodgkinson, T., Kelly, D. C., Curtin, C. M. & O’Brien, F. J. Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 18, 67–84 (2022).
Klose-Jensen, R. et al. Subchondral bone turnover, but not bone volume, is increased in early stage osteoarthritic lesions in the human hip joint. Osteoarthr. Cartil. 23, 2167–2173 (2015).
Bettica, P., Cline, G., Hart, D. J., Meyer, J. & Spector, T. D. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum. 46, 3178–3184 (2002).
Botter, S. M. et al. Osteoarthritis induction leads to early and temporal subchondral plate porosity in the tibial plateau of mice: an in vivo microfocal computed tomography study. Arthritis Rheum. 63, 2690–2699 (2011).
Neogi, T. et al. Magnetic resonance imaging-based three-dimensional bone shape of the knee predicts onset of knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Rheum. 65, 2048–2058 (2013).
Burr, D. B. & Gallant, M. A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 8, 665–673 (2012).
Hügle, T. & Geurts, J. What drives osteoarthritis? — Synovial versus subchondral bone pathology. Rheumatology 56, 1461–1471 (2017).
Day, J. S. et al. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J. Orthop. Res. 19, 914–918 (2001).
Burr, D. B. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthr. Cartil. 12, S20–S30 (2004).
Sanchez-Lopez, E., Coras, R., Torres, A., Lane, N. E. & Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 18, 258–275 (2022). An up-to-date comprehensive review on synovitis in OA.
Macchi, V. et al. The infrapatellar fat pad and the synovial membrane: an anatomo-functional unit. J. Anat. 233, 146–154 (2018).
Tang, S. et al. Single-cell atlas of human infrapatellar fat pad and synovium implicates APOE signaling in osteoarthritis pathology. Sci. Transl. Med. 16, eadf4590 (2024). A single-cell study of IPFP and synovium from patients with OA.
Li, J. et al. Synovium and infrapatellar fat pad share common mesenchymal progenitors and undergo coordinated changes in osteoarthritis. J. Bone Miner. Res. 39, 161–176 (2024).
Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J. P. & Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7, 33–42 (2011).
Coryell, P. R., Diekman, B. O. & Loeser, R. F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 17, 47–57 (2021).
Favero, M. et al. Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology 56, 1784–1793 (2017).
Berthiaume, M. J. et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann. Rheum. Dis. 64, 556–563 (2005).
Hunter, D. J. et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 54, 795–801 (2006).
Englund, M., Roemer, F. W., Hayashi, D., Crema, M. D. & Guermazi, A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat. Rev. Rheumatol. 8, 412–419 (2012).
Du, Y. et al. Effects of chronic ankle instability after grade I ankle sprain on the post-traumatic osteoarthritis. Arthritis Res. Ther. 26, 168 (2024).
Mohajer, B. et al. Role of thigh muscle changes in knee osteoarthritis outcomes: osteoarthritis initiative data. Radiology 305, 169–178 (2022).
Siqueira, M. S., Souto, L. R., Martinez, A. F., Serrão, F. V. & de Noronha, M. Muscle activation, strength, and volume in people with patellofemoral osteoarthritis: a systematic review and meta-analysis. Osteoarthr. Cartil. 30, 935–944 (2022).
Johnson, A. J., Barron, S. M., Nichols, J. A. & Cruz-Almeida, Y. Association of muscle quality and pain in adults with symptomatic knee osteoarthritis, independent of muscle strength: findings from a cross-sectional study. Arthritis Rheumatol. 76, 1062–1070 (2024).
Hannan, M. T., Felson, D. T. & Pincus, T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J. Rheumatol. 27, 1513–1517 (2000).
Neogi, T. et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ 339, b2844 (2009).
Neogi, T. Structural correlates of pain in osteoarthritis. Clin. Exp. Rheumatol. 35, 75–78 (2017).
da Costa, B. R. et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ 375, n2321 (2021).
Saltychev, M., Mattie, R., McCormick, Z. & Laimi, K. The magnitude and duration of the effect of intra-articular corticosteroid injections on pain severity in knee osteoarthritis: a systematic review and meta-analysis. Am. J. Phys. Med. Rehabil. 99, 617–625 (2020).
Woller, S. A., Eddinger, K. A., Corr, M. & Yaksh, T. L. An overview of pathways encoding nociception. Clin. Exp. Rheumatol. 35, 40–46 (2017).
Akin-Akinyosoye, K. et al. Traits associated with central pain augmentation in the Knee Pain In the Community (KPIC) cohort. Pain 159, 1035–1044 (2018).
Arant, K. R., Katz, J. N. & Neogi, T. Quantitative sensory testing: identifying pain characteristics in patients with osteoarthritis. Osteoarthr. Cartil. 30, 17–31 (2022).
Miller, R. E., Miller, R. J. & Malfait, A. M. Osteoarthritis joint pain: the cytokine connection. Cytokine 70, 185–193 (2014).
Miller, R. E. et al. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI Insight 3, e95704 (2018).
Malfait, A. M., Miller, R. E. & Block, J. A. Targeting neurotrophic factors: novel approaches to musculoskeletal pain. Pharmacol. Ther. 211, 107553 (2020).
Denk, F., Bennett, D. L. & McMahon, S. B. Nerve growth factor and pain mechanisms. Annu. Rev. Neurosci. 40, 307–325 (2017).
Schmelz, M. et al. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain 160, 2210–2220 (2019).
Wise, B. L., Seidel, M. F. & Lane, N. E. The evolution of nerve growth factor inhibition in clinical medicine. Nat. Rev. Rheumatol. 17, 34–46 (2021). This review recaps the biology of NGF and the studies that have been performed to evaluate the efficacy of NGF inhibition for chronic musculoskeletal pain states.
Heppelmann, B. Anatomy and histology of joint innervation. J. Peripher. Nerv. Syst. 2, 5–16 (1997).
Skoglund, S. Anatomical and physiological studies of knee joint innervation in the cat. Acta Physiol. Scand. Suppl. 36, 1–101 (1956).
Ashraf, S. et al. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann. Rheum. Dis. 70, 523–529 (2011).
Suri, S. et al. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis. 66, 1423–1428 (2007).
Aso, K. et al. Contribution of nerves within osteochondral channels to osteoarthritis knee pain in humans and rats. Osteoarthr. Cartil. 28, 1245–1254 (2020).
Obeidat, A. M. et al. Intra-articular sprouting of nociceptors accompanies progressive osteoarthritis: comparative evidence in four murine models. Front. Neuroanat. 18, 1429124 (2024).
Obeidat, A. M., Miller, R. E., Miller, R. J. & Malfait, A. M. The nociceptive innervation of the normal and osteoarthritic mouse knee. Osteoarthr. Cartil. 27, 1669–1679 (2019).
Aso, K. et al. Associations of symptomatic knee osteoarthritis with histopathologic features in subchondral bone. Arthritis Rheumatol. 71, 916–924 (2019).
Kuttapitiya, A. et al. Microarray analysis of bone marrow lesions in osteoarthritis demonstrates upregulation of genes implicated in osteochondral turnover, neurogenesis and inflammation. Ann. Rheum. Dis. 76, 1764–1773 (2017).
Nanus, D. E. et al. Synovial tissue from sites of joint pain in knee osteoarthritis patients exhibits a differential phenotype with distinct fibroblast subsets. eBioMedicine 72, 103618 (2021).
Blackler, G. et al. Targeting STAT6-mediated synovial macrophage activation improves pain in experimental knee osteoarthritis. Arthritis Res. Ther. 26, 73 (2024).
Kraus, V. B. et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 24, 1613–1621 (2016).
Geraghty, T., Winter, D. R., Miller, R. J., Miller, R. E. & Malfait, A. M. Neuroimmune interactions and osteoarthritis pain: focus on macrophages. Pain Rep. 6, e892 (2021).
Raoof, R. et al. Dorsal root ganglia macrophages maintain osteoarthritis pain. J. Neurosci. 41, 8249–8261 (2021).
Rankin, J. et al. Quantitative sensory testing protocols to evaluate central and peripheral sensitization in knee OA: a scoping review. Pain Med. 23, 526–557 (2022).
Pedersini, P., Gobbo, M., Bishop, M. D., Arendt-Nielsen, L. & Villafañe, J. H. Functional and structural neuroplastic changes related to sensitization proxies in patients with osteoarthritis: a systematic review. Pain Med. 23, 488–498 (2022).
Case, R., Thomas, E., Clarke, E. & Peat, G. Prodromal symptoms in knee osteoarthritis: a nested case–control study using data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 23, 1083–1089 (2015).
Altman, R. et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 29, 1039–1049 (1986).
Altman, R. et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 34, 505–514 (1991).
Hunter, D. J., McDougall, J. J. & Keefe, F. J. The symptoms of osteoarthritis and the genesis of pain. Rheum. Dis. Clin. North Am. 34, 623–643 (2008).
Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 21, 1145–1153 (2013).
De Baets, L. et al. The interplay between symptoms of insomnia and pain in people with osteoarthritis: a narrative review of the current evidence. Sleep Med. Rev. 70, 101793 (2023).
van Berkel, A. C., Ringelenberg, R., Bindels, P. J. E., Bierma-Zeinstra, S. M. A. & Schiphof, D. Nocturnal pain, is the pain different compared with pain during the day? An exploratory cross-sectional study in patients with hip and knee osteoarthritis. Family Pract. 40, 75–82 (2023).
Woolhead, G., Gooberman‐Hill, R., Dieppe, P. & Hawker, G. Night pain in hip and knee osteoarthritis: a focus group study. Arthritis Care Res. 62, 944–949 (2010).
Hawker, G. A. et al. Understanding the pain experience in hip and knee osteoarthritis — an OARSI/OMERACT initiative. Osteoarthr. Cartil. 16, 415–422 (2008).
Kloppenburg, M. & Kwok, W.-Y. Hand osteoarthritis — a heterogeneous disorder. Nat. Rev. Rheumatol. 8, 22–31 (2012).
Maricar, N., Callaghan, M. J., Parkes, M. J., Felson, D. T. & O’Neill, T. W. Interobserver and intraobserver reliability of clinical assessments in knee osteoarthritis. J. Rheumatol. 43, 2171–2178 (2016).
Slemenda, C. et al. Quadriceps weakness and osteoarthritis of the knee. Ann. Intern. Med. 127, 97–104 (1997).
Matullo, K. S., Ilyas, A. & Thoder, J. J. CMC arthroplasty of the thumb: a review. Hand 2, 232–239 (2007).
Metcalfe, D. et al. Does this patient have hip osteoarthritis?: the rational clinical examination systematic review. JAMA 322, 2323–2333 (2019).
Wang, X. et al. Associations between knee effusion-synovitis and joint structural changes in patients with knee osteoarthritis. J. Rheumatol. 44, 1644–1651 (2017).
Bahl, J. S. et al. Biomechanical changes and recovery of gait function after total hip arthroplasty for osteoarthritis: a systematic review and meta-analysis. Osteoarthr. Cartil. 26, 847–863 (2018).
Sakellariou, G. et al. EULAR recommendations for the use of imaging in the clinical management of peripheral joint osteoarthritis. Ann. Rheum. Dis. 76, 1484–1494 (2017).
Altman, R. et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 33, 1601–1610 (1990).
Haugen, I. K. et al. 2023 EULAR classification criteria for hand osteoarthritis. Ann. Rheum. Dis. 83, 1428–1435 (2024).
Haugen, I. K. et al. Development of classification criteria for hand osteoarthritis: comparative analyses of persons with and without hand osteoarthritis. RMD Open 6, e001265 (2020).
Haugen, I. K. et al. Development of radiographic classification criteria for hand osteoarthritis: a methodological report (phase 2). RMD Open 8, e002024 (2022).
Zhang, W. et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 69, 483–489 (2010).
National Institute for Health and Care Excellence. Osteoarthritis in over 16s: diagnosis and management. NICE https://www.nice.org.uk/guidance/ng226 (2022).
Runhaar, J. et al. Towards developing diagnostic criteria for early knee osteoarthritis: data from the CHECK study. Rheumatology 60, 2448–2455 (2021).
Wang, Q. et al. Diagnosis for early stage knee osteoarthritis: probability stratification, internal and external validation; data from the CHECK and OAI cohorts. Semin. Arthritis Rheum. 55, 152007 (2022).
Gaitonde, D. Y., Ericksen, A. & Robbins, R. C. Patellofemoral pain syndrome. Am. Fam. Physician 99, 88–94 (2019).
Runhaar, J. & Bierma-Zeinstra, S. M. A. The challenges in the primary prevention of osteoarthritis. Clin. Geriatr. Med. 38, 259–271 (2022).
Roos, E. M., Risberg, M. A. & Little, C. B. Prevention and early treatment, a future focus for OA research. Osteoarthr. Cartil. 29, 1627–1629 (2021).
Whittaker, J. L. et al. Risk factors for knee osteoarthritis after traumatic knee injury: a systematic review and meta-analysis of randomised controlled trials and cohort studies for the OPTIKNEE Consensus. Br. J. Sports Med. 56, 1406–1421 (2022).
Huang, Y. L., Jung, J., Mulligan, C. M. S., Oh, J. & Norcross, M. F. A majority of anterior cruciate ligament injuries can be prevented by injury prevention programs: a systematic review of randomized controlled trials and cluster-randomized controlled trials with meta-analysis. Am. J. Sports Med. 48, 1505–1515 (2020).
Øiestad, B. E., Juhl, C. B., Culvenor, A. G., Berg, B. & Thorlund, J. B. Knee extensor muscle weakness is a risk factor for the development of knee osteoarthritis: an updated systematic review and meta-analysis including 46 819 men and women. Br. J. Sports Med. 56, 349–355 (2022).
Barrow, D. R. et al. Exercise prescription for weight management in obese adults at risk for osteoarthritis: synthesis from a systematic review. BMC Musculoskelet. Disord. 20, 610 (2019).
Halvorsen, K. C. et al. Higher adherence to anterior cruciate ligament injury prevention programs is associated with lower injury rates: a meta-analysis and meta-regression. HSS J. 19, 154–162 (2023).
Hall, M. et al. How does hip osteoarthritis differ from knee osteoarthritis? Osteoarthr. Cartil. 30, 32–41 (2022).
Oppert, J. M. et al. Exercise training in the management of overweight and obesity in adults: synthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes. Rev. 22, e13273 (2021).
Muntefering, C., Fitzpatrick, M., Johnson, K. & Fields, B. Primary prevention interventions for adults at-risk of obesity: an international scoping review. Prev. Med. 171, 107498 (2023).
Roos, E. M. & Arden, N. K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 12, 92–101 (2016).
Arundale, A. J. H., Silvers-Granelli, H. J. & Myklebust, G. ACL injury prevention: where have we come from and where are we going? J. Orthop. Res. 40, 43–54 (2021).
Thompson-Kolesar, J. A. et al. Age influences biomechanical changes after participation in an anterior cruciate ligament injury prevention program. Am. J. Sports Med. 46, 598–606 (2018).
Mohr, M. et al. An 8-week injury prevention exercise program combined with change-of-direction technique training limits movement patterns associated with anterior cruciate ligament injury risk. Sci. Rep. 14, 3115 (2024).
Andriacchi, T. P., Favre, J., Erhart-Hledik, J. C. & Chu, C. R. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann. Biomed. Eng. 43, 376–387 (2015).
Gersing, A. S. et al. Weight loss regimen in obese and overweight individuals is associated with reduced cartilage degeneration: 96-month data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 27, 863–870 (2019).
Messier, S. P. et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 310, 1263–1273 (2013).
Loeser, R. F. et al. Effects of dietary weight loss with and without exercise on interstitial matrix turnover and tissue inflammation biomarkers in adults with knee osteoarthritis: the Intensive Diet and Exercise for Arthritis trial (IDEA). Osteoarthr. Cartil. 25, 1822–1828 (2017).
Jurado-Castro, J. M., Muñoz-López, M., Ledesma, A. S.-T. & Ranchal-Sanchez, A. Effectiveness of exercise in patients with overweight or obesity suffering from knee osteoarthritis: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health 19, 10510 (2022).
Misra, D. et al. Risk of knee osteoarthritis with obesity, sarcopenic obesity, and sarcopenia. Arthritis Rheumatol. 71, 232–237 (2019).
Godziuk, K., Woodhouse, L. J., Prado, C. M. & Forhan, M. Clinical screening and identification of sarcopenic obesity in adults with advanced knee osteoarthritis. Clin. Nutr. ESPEN 40, 340–348 (2020).
Godziuk, K., Prado, C. M., Woodhouse, L. J. & Forhan, M. The impact of sarcopenic obesity on knee and hip osteoarthritis: a scoping review. BMC Musculoskelet. Disord. 19, 271 (2018).
Zhu, H. et al. Glucagon-like peptide-1 receptor agonists as a disease-modifying therapy for knee osteoarthritis mediated by weight loss: findings from the Shanghai Osteoarthritis Cohort. Ann. Rheum. Dis. 82, 1218–1226 (2023). A large observational cohort study highlighting the effect of GLP1 receptor agonists in reducing body weight and having fewer knee surgeries.
Bliddal, H. et al. Once-weekly semaglutide in persons with obesity and knee osteoarthritis. N. Engl. J. Med. 391, 1573–1583 (2024). A multicentre, placebo-controlled trial confirming the role of GLP1 receptor agonist semaglutide in patients with knee OA obesity.
Zmerly, H. et al. Personalized physical activity programs for the management of knee osteoarthritis in individuals with obesity: a patient-centered approach. Diseases 11, 182 (2023).
Lie, M. M., Risberg, M. A., Storheim, K., Engebretsen, L. & Øiestad, B. E. What’s the rate of knee osteoarthritis 10 years after anterior cruciate ligament injury? An updated systematic review. Br. J. Sports Med. 53, 1162–1167 (2019).
Lien-Iversen, T. et al. Does surgery reduce knee osteoarthritis, meniscal injury and subsequent complications compared with non-surgery after ACL rupture with at least 10 years follow-up? A systematic review and meta-analysis. Br. J. Sports Med. 54, 592–598 (2020).
Montalvo, A. M. et al. Anterior cruciate ligament injury risk in sport: a systematic review and meta-analysis of injury incidence by sex and sport classification. J. Athl. Train. 54, 472–482 (2019).
Poulsen, E. et al. Knee osteoarthritis risk is increased 4-6 fold after knee injury — a systematic review and meta-analysis. Br. J. Sports Med. 53, 1454–1463 (2019).
Grindem, H., Snyder-Mackler, L., Moksnes, H., Engebretsen, L. & Risberg, M. A. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br. J. Sports Med. 50, 804–808 (2016).
Pedersen, M. et al. Meniscus or cartilage injury at the time of anterior cruciate ligament tear is associated with worse prognosis for patient-reported outcome 2 to 10 years after anterior cruciate ligament injury: a systematic review. J. Orthop. Sports Phys. Ther. 50, 490–502 (2020).
Tayfur, B., Charuphongsa, C., Morrissey, D. & Miller, S. C. Neuromuscular function of the knee joint following knee injuries: does it ever get back to normal? A systematic review with meta-analyses. Sports Med. 51, 321–338 (2021).
Garriga, C. et al. Clinical and molecular associations with outcomes at 2 years after acute knee injury: a longitudinal study in the Knee Injury Cohort at the Kennedy (KICK). Lancet Rheumatol. 3, e648–e658 (2021).
Grindem, H., Engebretsen, L., Axe, M., Snyder-Mackler, L. & Risberg, M. A. Activity and functional readiness, not age, are the critical factors for second anterior cruciate ligament injury — the Delaware-Oslo ACL cohort study. Br. J. Sports Med. 54, 1099–1102 (2020).
Felson, D. T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 21, 10–15 (2013).
Pietrosimone, B. et al. Gait biomechanics in individuals meeting sufficient quadriceps strength cutoffs after anterior cruciate ligament reconstruction. J. Athl. Train. 56, 960–966 (2021).
Buck, A. N. et al. Biomechanical threshold values for identifying clinically significant knee-related symptoms six months following anterior cruciate ligament reconstruction. J. Athl. Train. https://doi.org/10.4085/1062-6050-0562.23 (2024).
Risberg, M. A., Grindem, H. & Øiestad, B. E. We need to implement current evidence in early rehabilitation programs to improve long-term outcome after anterior cruciate ligament injury. J. Orthop. Sports Phys. Ther. 46, 710–713 (2016).
Losciale, J. M. et al. Assessing the efficacy of the Stop OsteoARthritis (SOAR) program: a randomized delayed-controlled trial in persons at increased risk of early onset post-traumatic knee osteoarthritis. Osteoarthr. Cartil. 32, 1001–1012 (2024).
Bannuru, R. R. et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 27, 1578–1589 (2019).
Bowden, J. L. et al. Core and adjunctive interventions for osteoarthritis: efficacy and models for implementation. Nat. Rev. Rheumatol. 16, 434–447 (2020).
Kolasinski, S. L. et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 72, 149–162 (2020).
Brophy, R. H. & Fillingham, Y. A. AAOS clinical practice guideline summary: management of osteoarthritis of the knee (nonarthroplasty), third edition. J. Am. Acad. Orthopaedic Surg. 30, e721–e729 (2022).
Conley, B. et al. Core recommendations for osteoarthritis care: a systematic review of clinical practice guidelines. Arthritis Care Res. 75, 1897–1907 (2023).
Moseng, T. et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 83, 730–740 (2024).
Østerås, N. et al. Longer-term quality of care, effectiveness, and cost-effectiveness of implementing a model of care for osteoarthritis: a cluster-randomized controlled trial. Osteoarthritis Cartilage 32, 108–119 (2023).
Mazzei, D. R. et al. Are education, exercise and diet interventions a cost-effective treatment to manage hip and knee osteoarthritis? A systematic review. Osteoarthr. Cartil. 29, 456–470 (2021).
Skou, S. T. et al. Cost-effectiveness of 12 weeks of supervised treatment compared to written advice in patients with knee osteoarthritis: a secondary analysis of the 2-year outcome from a randomized trial. Osteoarthr. Cartil. 28, 907–916 (2020).
Fransen, M. et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br. J. Sports Med. 49, 1554–1557 (2015).
Long, H. et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease study 2019. Arthritis Rheumatol. 74, 1172–1183 (2022). Data from the Global Burden of Disease Study 2019 estimating site-specific age-standardized prevalence rates and analyzing the secular trends of OA at global, regional and national levels.
Mazzei, D. R. et al. Do people with knee osteoarthritis use guideline-consistent treatments after an orthopaedic surgeon recommends nonsurgical care? A cross-sectional survey with long-term follow-up. Osteoarthr. Cartil. Open 4, 100256 (2022).
Bartholdy, C. et al. Poor replicability of recommended exercise interventions for knee osteoarthritis: a descriptive analysis of evidence informing current guidelines and recommendations. Osteoarthr. Cartil. 27, 3–22 (2019).
Weng, Q. et al. Comparative efficacy of exercise therapy and oral non-steroidal anti-inflammatory drugs and paracetamol for knee or hip osteoarthritis: a network meta-analysis of randomised controlled trials. Br. J. Sports Med. 57, 990–996 (2023).
Moseng, T., Dagfinrud, H. & Østerås, N. Implementing international osteoarthritis guidelines in primary care: uptake and fidelity among health professionals and patients. Osteoarthr. Cartil. 27, 1138–1147 (2019).
Bruhn, S. M. et al. Usage of guideline-adherent core treatments for knee osteoarthritis before and after consulting an orthopaedic surgeon: a prospective cohort study. Osteoarthr. Cartil. Open 5, 100411 (2023).
Hofstede, S. N. et al. Variation in use of non-surgical treatments among osteoarthritis patients in orthopaedic practice in the Netherlands. BMJ Open 5, e009117 (2015).
Østerås, N. et al. Improving osteoarthritis management in primary healthcare: results from a quasi-experimental study. BMC Musculoskelet. Disord. 22, 79 (2021).
Østerås, N. et al. Implementing a structured model for osteoarthritis care in primary healthcare: a stepped-wedge cluster-randomised trial. PLoS Med. 16, e1002949 (2019).
Hagen, K. B., Smedslund, G., Østerås, N. & Jamtvedt, G. Quality of community-based osteoarthritis care: a systematic review and meta-analysis. Arthritis Care Res. 68, 1443–1452 (2016).
Cronström, A., Nero, H., Lohmander, L. S. & Dahlberg, L. E. On the waiting list for joint replacement for knee osteoarthritis: are first-line treatment recommendations implemented? Osteoarthr. Cartil. Open 2, 100056 (2020).
King, L. K. et al. Use of recommended non-surgical knee osteoarthritis management in patients prior to total knee arthroplasty: a cross-sectional study. J. Rheumatol. 47, 1253–1260 (2020).
Gibbs, A. J., Wallis, J. A., Taylor, N. F., Kemp, J. L. & Barton, C. J. Osteoarthritis management care pathways are complex and inefficient: a qualitative study of physiotherapist perspectives from specialised osteoarthritis services. Musculoskelet. Care 20, 860–872 (2022).
Cutolo, M., Berenbaum, F., Hochberg, M., Punzi, L. & Reginster, J. Y. Commentary on recent therapeutic guidelines for osteoarthritis. Semin. Arthritis Rheum. 44, 611–617 (2015).
Smith, K. M., Massey, B. J., Young, J. L. & Rhon, D. I. What are the unsupervised exercise adherence rates in clinical trials for knee osteoarthritis? A systematic review. Braz. J. Phys. Ther. 27, 100533 (2023).
Cinthuja, P., Krishnamoorthy, N. & Shivapatham, G. Effective interventions to improve long-term physiotherapy exercise adherence among patients with lower limb osteoarthritis. A systematic review. BMC Musculoskelet. Disord. 23, 147 (2022).
Tan, B. Y. et al. Complex lifestyle and psychological intervention in knee osteoarthritis: scoping review of randomized controlled trials. Int. J. Environ. Res. Public Health 18, 12757 (2021).
Goff, A. J. et al. Patient education improves pain and function in people with knee osteoarthritis with better effects when combined with exercise therapy: a systematic review. J. Physiother. 67, 177–189 (2021).
Wallis, J. A., Webster, K. E., Levinger, P. & Taylor, N. F. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthr. Cartil. 21, 1648–1659 (2013).
Verhagen, A. P. et al. Do we need another trial on exercise in patients with knee osteoarthritis?: no new trials on exercise in knee OA. Osteoarthr. Cartil. 27, 1266–1269 (2019).
Skou, S. T. & Roos, E. M. Physical therapy for patients with knee and hip osteoarthritis: supervised, active treatment is current best practice. Clin. Exp. Rheumatol. 37, 112–117 (2019).
Chu, I. J. H., Lim, A. Y. T. & Ng, C. L. W. Effects of meaningful weight loss beyond symptomatic relief in adults with knee osteoarthritis and obesity: a systematic review and meta-analysis. Obes. Rev. 19, 1597–1607 (2018).
Oo, W. M., Mobasheri, A. & Hunter, D. J. A narrative review of anti-obesity medications for obese patients with osteoarthritis. Expert Opin. Pharmacother. 23, 1381–1395 (2022).
Couldrick, J. M. et al. Evidence for key individual characteristics associated with outcomes following combined first-line interventions for knee osteoarthritis: a systematic review. PLoS ONE 18, e0284249 (2023).
Duong, V., Oo, W. M., Ding, C., Culvenor, A. G. & Hunter, D. J. Evaluation and treatment of knee pain: a review. JAMA 330, 1568–1580 (2023). An up-to-date comprehensive review on knee pain.
Pitsillides, A., Stasinopoulos, D. & Giannakou, K. The effects of cognitive behavioural therapy delivered by physical therapists in knee osteoarthritis pain: a systematic review and meta-analysis of randomized controlled trials. J. Bodyw. Mov. Ther. 25, 157–164 (2021).
Bennell, K. L. et al. Physical therapist-delivered pain coping skills training and exercise for knee osteoarthritis: randomized controlled trial. Arthritis Care Res. 68, 590–602 (2016).
Cudejko, T. et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch. Phys. Med. Rehabil. 99, 153–163 (2018).
Zeng, C. et al. Comparative efficacy and safety of acetaminophen, topical and oral non-steroidal anti-inflammatory drugs for knee osteoarthritis: evidence from a network meta-analysis of randomized controlled trials and real-world data. Osteoarthr. Cartil. 29, 1242–1251 (2021).
Osani, M. C., Vaysbrot, E. E., Zhou, M., McAlindon, T. E. & Bannuru, R. R. Duration of symptom relief and early trajectory of adverse events for oral nonsteroidal antiinflammatory drugs in knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res. 72, 641–651 (2020).
Najm, A., Alunno, A., Gwinnutt, J. M., Weill, C. & Berenbaum, F. Efficacy of intra-articular corticosteroid injections in knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Jt Bone Spine 88, 105198 (2021).
McAlindon, T. E. et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA 317, 1967–1975 (2017).
Concoff, A., Sancheti, P., Niazi, F., Shaw, P. & Rosen, J. The efficacy of multiple versus single hyaluronic acid injections: a systematic review and meta-analysis. BMC Musculoskelet. Disord. 18, 542 (2017).
Oo, W. M. & Hunter, J. Intra-articular therapies for knee osteoarthritis: current update. Curr. Treat. Options Rheumatol. 9, 99–119 (2023).
Machado, G. C. et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ 350, h1225 (2015).
Sveaas, S. H., Smedslund, G., Walsh, D. A. & Dagfinrud, H. Effects of analgesics on self-reported physical function and walking ability in people with hip or knee osteoarthritis: a systematic review and meta-analysis. Phys. Ther. 104, pzad160 (2024).
Chen, B. et al. An updated systematic review and meta-analysis of duloxetine for knee osteoarthritis pain. Clin. J. Pain 37, 852–862 (2021).
Zhang, X. et al. Efficacy and safety of tramadol for knee or hip osteoarthritis: a systematic review and network meta-analysis of randomized controlled trials. Arthritis Care Res. 75, 158–165 (2023).
Jin, X. et al. Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA 315, 1005–1013 (2016).
Beswick, A. D., Wylde, V., Gooberman-Hill, R., Blom, A. & Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2, e000435 (2012).
Wylde, V. et al. Chronic pain after total knee arthroplasty. EFORT Open Rev. 3, 461–470 (2018).
Sayah, S. M. et al. Clinical course of pain and function following total knee arthroplasty: a systematic review and meta-regression. J. Arthroplasty 36, 3993–4002 (2021).
Young, J. J. et al. Total hip arthroplasty versus education and exercise: a propensity-matched analysis of 266 patients who have hip osteoarthritis. J. Arthroplasty 39, S261–S269 (2024).
Leifer, V. P., Katz, J. N. & Losina, E. The burden of OA-health services and economics. Osteoarthr. Cartil. 30, 10–16 (2022).
Sethi, V., Anand, C. & Della Pasqua, O. Clinical assessment of osteoarthritis pain: contemporary scenario, challenges, and future perspectives. Pain Ther. 13, 391–408 (2024).
Guccione, A. A. et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am. J. Public Health 84, 351–358 (1994).
Gikaro, J. M., Xiong, H. & Lin, F. Activity limitation and participation restriction in Osteoarthritis and Rheumatoid arthritis: findings based on the National Health and Nutritional Examination Survey. BMC Musculoskelet. Disord. 23, 647 (2022).
Perruccio, A. V., Yip, C., Power, J. D., Canizares, M. & Badley, E. M. Examining the longitudinal associations between activity limitations, instrumental supports and social participation in osteoarthritis: a CLSA population-based study. PLoS ONE 19, e0299894 (2024).
Yan, H., Guo, J., Zhou, W., Dong, C. & Liu, J. Health-related quality of life in osteoarthritis patients: a systematic review and meta-analysis. Psychol. Health Med. 27, 1859–1874 (2022).
Hawker, G. A. & King, L. K. The burden of osteoarthritis in older adults. Clin. Geriatr. Med. 38, 181–192 (2022).
Losina, E. et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res. 67, 203–215 (2015).
Swain, S. et al. Healthcare utilisation and mortality in people with osteoarthritis in the UK: findings from a national primary care database. Br. J. Gen. Pract. 73, e615–e622 (2023).
Kiadaliri, A. & Englund, M. Osteoarthritis and risk of hospitalization for ambulatory care-sensitive conditions: a general population-based cohort study. Rheumatology 60, 4340–4347 (2021).
Wise, A. et al. Racial and ethnic differences in the prevalence of patients with arthritis and severe joint pain and who received provider counseling about physical activity for arthritis among adults aged 18 years or older — United States 2019. Arthritis Care Res. 76, 1028–1036 (2024).
March, L. M. & Bachmeier, C. J. Economics of osteoarthritis: a global perspective. Baillieres Clin. Rheumatol. 11, 817–834 (1997).
Ackerman, I. N., Pratt, C., Gorelik, A. & Liew, D. Projected burden of osteoarthritis and rheumatoid arthritis in Australia: a population-level analysis. Arthritis Care Res. 70, 877–883 (2018).
Cost of osteoarthritis. Osteoarthritis Action Alliance https://oaaction.unc.edu/policy/cost-of-osteoarthritis/ (2025).
Gandhi, N. et al. Costs and models used in the economic analysis of total knee replacement (TKR): a systematic review. PLoS ONE 18, e0280371 (2023).
Ching, A., Prior, Y., Parker, J. & Hammond, A. Biopsychosocial, work-related, and environmental factors affecting work participation in people with osteoarthritis: a systematic review. BMC Musculoskelet. Disord. 24, 485 (2023).
Thomas, M. J., Guillemin, F., & Neogi, T. Osteoarthritis flares. Clin. Geriatr. Med. 38, 239–257 (2022).
Lo, G. H. et al. Symptom assessment in knee osteoarthritis needs to account for physical activity level. Arthritis Rheumatol. 67, 2897–2904 (2015).
Collins, J. E., Katz, J. N., Dervan, E. E. & Losina, E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthr. Cartil. 22, 622–630 (2014).
Wieczorek, M. et al. Trajectory analysis combining pain and physical function in individuals with knee and hip osteoarthritis: results from the French KHOALA cohort. Rheumatology 59, 3488–3498 (2020).
Wieczorek, M., Rotonda, C., Guillemin, F. & Rat, A. C. What have we learned from trajectory analysis of clinical outcomes in knee and hip osteoarthritis before surgery? Arthritis Care Res. 72, 1693–1702 (2020).
Weinstein, A. M. et al. Estimating the burden of total knee replacement in the United States. J. Bone Jt Surg. Am. 95, 385–392 (2013).
Culliford, D. J. et al. The lifetime risk of total hip and knee arthroplasty: results from the UK general practice research database. Osteoarthr. Cartil. 20, 519–524 (2012).
Ackerman, I. N. et al. Substantial rise in the lifetime risk of primary total knee replacement surgery for osteoarthritis from 2003 to 2013: an international, population-level analysis. Osteoarthr. Cartil. 25, 455–461 (2017).
Burn, E., Murray, D. W., Hawker, G. A., Pinedo-Villanueva, R. & Prieto-Alhambra, D. Lifetime risk of knee and hip replacement following a GP diagnosis of osteoarthritis: a real-world cohort study. Osteoarthr. Cartil. 27, 1627–1635 (2019).
Mobasheri, A. & Loeser, R. Clinical phenotypes, molecular endotypes and theratypes in OA therapeutic development. Nature Rev. Rheumatol. 20, 525–526 (2024).
Ahmed, U., Anwar, A., Savage, R. S., Thornalley, P. J. & Rabbani, N. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res. Ther. 18, 250 (2016).
Latourte, A., Kloppenburg, M. & Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 16, 673–688 (2020). An up-to-date comprehensive review of drug therapy for OA.
Zhang, Y., Yang, H., He, F. & Zhu, X. Intra-articular injection choice for osteoarthritis: making sense of cell source — an updated systematic review and dual network meta-analysis. Arthritis Res. Ther. 24, 260 (2022).
Xiang, X. N. et al. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res. Ther. 13, 14 (2022).
Maheshwer, B. et al. Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and chondral defects: a systematic review and meta-analysis. Arthroscopy 37, 362–378 (2021).
Chen, M. et al. Injectable microgels with hybrid exosomes of chondrocyte-targeted FGF18 gene-editing and self-renewable lubrication for osteoarthritis therapy. Adv. Mater. 36, e2312559 (2024).
Varela-Eirín, M. et al. Targeting of chondrocyte plasticity via connexin43 modulation attenuates cellular senescence and fosters a pro-regenerative environment in osteoarthritis. Cell Death Dis. 9, 1166 (2018).
Fu, L. et al. Up-regulation of FOXD1 by YAP alleviates senescence and osteoarthritis. PLoS Biol. 17, e3000201 (2019).
Ren, X. et al. Maintenance of nucleolar homeostasis by CBX4 alleviates senescence and osteoarthritis. Cell Rep. 26, 3643–3656.e7 (2019).
Li, J. et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann. Rheum. Dis. 79, 635–645 (2020).
Zhu, Z. et al. Metformin use and associated risk of total joint replacement in patients with type 2 diabetes: a population-based matched cohort study. CMAJ 194, E1672–E1684 (2022). A population-based matched cohort study demonstrating that metformin use in patients with type 2 diabetes mellitus was associated with a significantly reduced risk of total joint replacement.
Ruan, G. et al. Can metformin relieve tibiofemoral cartilage volume loss and knee symptoms in overweight knee osteoarthritis patients? Study protocol for a randomized, double-blind, and placebo-controlled trial. BMC Musculoskelet. Disord. 23, 486 (2022).
Lim, Y. Z. et al. Metformin for knee osteoarthritis with obesity: study protocol for a randomised, double-blind, placebo-controlled trial. BMJ Open 13, e079489 (2023).
Boer, C. G. et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 10, 4881 (2019).
Wei, J. et al. Association between gut microbiota and symptomatic hand osteoarthritis: data from the Xiangya Osteoarthritis Study. Arthritis Rheumatol. 73, 1656–1662 (2021).
Chen, J., Wang, A. & Wang, Q. Dysbiosis of the gut microbiome is a risk factor for osteoarthritis in older female adults: a case control study. BMC Bioinformatics 22, 299 (2021).
Dunn, C. M. & Jeffries, M. A. The microbiome in osteoarthritis: a narrative review of recent human and animal model literature. Curr. Rheumatol. Rep. 24, 139–148 (2022).
Mendez, M. E. et al. LPS-induced inflammation prior to injury exacerbates the development of post-traumatic osteoarthritis in mice. J. Bone Miner. Res. 35, 2229–2241 (2020).
Prinz, E. et al. OA susceptibility in mice is partially mediated by the gut microbiome, is transferrable via microbiome transplantation and is associated with immunophenotype changes. Ann. Rheum. Dis. 83, 382–393 (2024).
Fortuna, R. et al. Effect of prebiotic fiber on physical function and gut microbiota in adults, mostly women, with knee osteoarthritis and obesity: a randomized controlled trial. Eur. J. Nutr. 63, 2149–2161 (2024).
Fu, W. et al. Nav1.7 as a chondrocyte regulator and therapeutic target for osteoarthritis. Nature 625, 557–565 (2024). A basic study demonstrating Nav1.7 as a novel chondrocyte-expressed, OA-associated channel.
Jansen, M. P. & Mastbergen, S. C. Joint distraction for osteoarthritis: clinical evidence and molecular mechanisms. Nat. Rev. Rheumatol. 18, 35–46 (2022).
DeJulius, C. R. et al. Engineering approaches for RNA-based and cell-based osteoarthritis therapies. Nat. Rev. Rheumatol. 20, 81–100 (2024). This Review highlights emerging classes of RNA-based technologies that hold potential for OA therapies.
Acknowledgements
D.J.H. is supported by an NHMRC Investigator Grant Leadership 2. C.D. is supported by the National Key Research and Development Program of China. S.T. is supported by the National Natural Science Foundation of China. A.-M.M. is supported by the National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). T.N. is supported by the NIH.
Author information
Authors and Affiliations
Contributions
Introduction (all authors); Epidemiology (all authors); Mechanisms/pathophysiology (all authors); Diagnosis, screening and prevention (all authors); Management (all authors); Quality of life (all authors); Outlook (all authors); overview of the Primer (D.J.H.).
Corresponding authors
Ethics declarations
Competing interests
D.J.H. is the editor of the osteoarthritis section for UpToDate and co-Editor in Chief of Osteoarthritis and Cartilage. D.J.H. provides consulting advice on scientific advisory boards for Haleon, TLCBio, Novartis, Tissuegene, Sanofi and Enlivex. A.-M.M. is co-Editor in Chief of Osteoarthritis and Cartilage. A.-M.M. provides consulting services or serves on advisory boards for Averitas, Orion, LG, Novartis and Eli Lilly. T.N. is a deputy editor of Osteoarthritis and Cartilage and provides consulting services for Novartis and Eli Lilly. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks L. Macfarlane, L. Mandl, H. Gudbergsen, M. Goldring and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, S., Zhang, C., Oo, W.M. et al. Osteoarthritis. Nat Rev Dis Primers 11, 10 (2025). https://doi.org/10.1038/s41572-025-00594-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-025-00594-6
This article is cited by
-
Quercetin-primed MSC exosomes synergistically attenuate osteoarthritis progression
Journal of Orthopaedic Surgery and Research (2025)
-
Subchondral injection of human umbilical cord mesenchymal stem cells ameliorates knee osteoarthritis by inhibiting osteoblast apoptosis and TGF-beta activity
Stem Cell Research & Therapy (2025)
-
Exosomes derived from hypoxia-preconditioned M2 macrophages alleviate degeneration in knee osteoarthritis through the miR‑124‑3p/STAT3 axis
Journal of Translational Medicine (2025)
-
Age-specific comorbidity risks in osteoarthritis: implications for healthy aging across diverse populations
Archives of Public Health (2025)
-
Crosstalk between lymphatic system and synovial inflammatory cells in osteoarthritis: molecular mechanisms and potential cell-based therapies
Journal of Translational Medicine (2025)