Abstract
Mesothelioma is a lethal cancer caused by exposure to asbestos, which arises predominantly in the pleural lining of the thoracic cavity or, less commonly, in the peritoneum, pericardium or tunica vaginalis. The incidence of mesothelioma increased globally during the late twentieth century, correlating with the use of asbestos, and it continues to rise in some regions. Asbestos tumorigenesis involves fibre persistence that leads to DNA damage mediated by chronic inflammation. The genomic landscape of mesothelioma is predominantly characterized by tumour suppressor alterations, most frequently occurring in BAP1, CDKN2A, CDKN2B, MTAP, NF2 and TP53. Patients with mesothelioma commonly present with fatigue, dyspnoea and/or cough caused by pleural effusion, pain and reduced appetite with weight loss. Imaging, cytology, histology and immunohistochemistry are used in diagnosis and support tumour staging. Genetic tests are relevant to reveal disease predispositions. Mesotheliomas are classified on the basis of histology into three distinct subtypes: epithelioid (the most common subtype with the best prognosis), biphasic and sarcomatoid (worst prognosis). Chemotherapy has been the standard of care for the past two decades but immune checkpoint inhibition targeting PD1 and CTLA4 is now considered to be the first-line treatment, showing improvement compared with chemotherapy. Few randomized trials have investigated the role of surgery and radiotherapy and none has found a clear benefit over systemic therapies. Mesothelioma is associated with considerable negative effects on quality of life in physical and emotional domains and also substantially affects patients’ families and caregivers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Plas, E., Riedl, C. R. & Pfluger, H. Malignant mesothelioma of the tunica vaginalis testis: review of the literature and assessment of prognostic parameters. Cancer 83, 2437–2446 (1998).
Hassan, R. et al. Current treatment options and biology of peritoneal mesothelioma: meeting summary of the first NIH peritoneal mesothelioma conference. Ann. Oncol. 17, 1615–1619 (2006).
Sauter, J. L. et al. The 2021 WHO classification of tumors of the pleura: advances since the 2015 classification. J. Thorac. Oncol. 17, 608–622 (2022).
Alpert, N., van Gerwen, M. & Taioli, E. Epidemiology of mesothelioma in the 21(st) century in Europe and the United States, 40 years after restricted/banned asbestos use. Transl. Lung Cancer Res. 9, S28–S38 (2020).
Henley, S. J. et al. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003–2008. Int. J. Occup. Env. Health 19, 1–10 (2013).
LaDou, J. et al. The case for a global ban on asbestos. Env. Health Perspect. 118, 897–901 (2010).
Baumann, F., Ambrosi, J. P. & Carbone, M. Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol. 14, 576–578 (2013).
Ferlay, J. et al. Global Cancer Observatory: Cancer Today (IARC, accessed 23 July 2025); https://gco.iarc.who.int/today.
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Ferlay, J. et al. Mesothelioma. IARC https://gco.iarc.who.int/media/globocan/factsheets/cancers/18-mesothelioma-fact-sheet.pdf (2024).
Huang, J. et al. Global incidence, risk factors, and temporal trends of mesothelioma: a population-based study. J. Thorac. Oncol. 18, 792–802 (2023).
Panou, V. et al. Gender differences in asbestos exposure and disease location in 327 patients with mesothelioma. Eur. Respir. J. 50, PA4294 (2017).
Lin, R. T., Chien, L. C., Jimba, M., Furuya, S. & Takahashi, K. Implementation of national policies for a total asbestos ban: a global comparison. Lancet Planet. Health 3, e341–e348 (2019).
Zhu, W., Liu, J., Li, Y., Shi, Z. & Wei, S. Global, regional, and national trends in mesothelioma burden from 1990 to 2019 and the predictions for the next two decades. SSM Popul. Health 23, 101441 (2023).
Mazurek, J. M., Blackley, D. J. & Weissman, D. N. Malignant mesothelioma mortality in women – United States, 1999–2020. MMWR Morb. Mortal. Wkly Rep. 71, 645–649 (2022).
Wagner, J. C., Sleggs, C. A. & Marchand, P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br. J. Ind. Med. 17, 260–271 (1960).
Selikoff, I. J., Churg, J. & Hammond, E. C. Asbestos exposure and neoplasia. JAMA 252, 91–95 (1984).
Bourdes, V., Boffetta, P. & Pisani, P. Environmental exposure to asbestos and risk of pleural mesothelioma: review and meta-analysis. Eur. J. Epidemiol. 16, 411–417 (2000).
Boffetta, P., Donato, F., Pira, E., Luu, H. N. & La Vecchia, C. Risk of mesothelioma after cessation of asbestos exposure: a systematic review and meta-regression. Int. Arch. Occup. Env. Health 92, 949–957 (2019).
Lacourt, A. et al. Co-exposure to refractory ceramic fibres and asbestos and risk of pleural mesothelioma. Eur. Respir. J. 44, 725–733 (2014).
Visci, G., Rizzello, E., Zunarelli, C., Violante, F. S. & Boffetta, P. Relationship between exposure to ionizing radiation and mesothelioma risk: a systematic review of the scientific literature and meta-analysis. Cancer Med. 11, 778–789 (2022).
Zhai, Z. et al. Assessment of global trends in the diagnosis of mesothelioma from 1990 to 2017. JAMA Netw. Open 4, e2120360 (2021).
Carbone, M. et al. Medical and surgical care of patients with mesothelioma and their relatives carrying germline BAP1 mutations. J. Thorac. Oncol. 17, 873–889 (2022).
Bueno, R. et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 48, 407–416 (2016).
Carbone, M. et al. Eighth international mesothelioma interest group. Oncogene 26, 6959–6967 (2007).
Mutsaers, S. E. et al. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 6, 113 (2015).
Suarez, J. S. et al. HMGB1 released by mesothelial cells drives the development of asbestos-induced mesothelioma. Proc. Natl Acad. Sci. USA 120, e2307999120 (2023).
Jube, S. et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 72, 3290–3301 (2012).
Jiang, L. et al. Iron overload signature in chrysotile-induced malignant mesothelioma. J. Pathol. 228, 366–377 (2012).
Hu, Q. et al. Homozygous deletion of CDKN2A/2B is a hallmark of iron-induced high-grade rat mesothelioma. Lab. Invest. 90, 360–373 (2010).
Hmeljak, J. et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 8, 1548–1565 (2018).
Mangiante, L. et al. Multiomic analysis of malignant pleural mesothelioma identifies molecular axes and specialized tumor profiles driving intertumor heterogeneity. Nat. Genet. 55, 607–618 (2023).
Hiltbrunner, S. et al. Genomic landscape of pleural and peritoneal mesothelioma tumours. Br. J. Cancer 127, 1997–2005 (2022).
Quetel, L. et al. Genetic alterations of malignant pleural mesothelioma: association with tumor heterogeneity and overall survival. Mol. Oncol. 14, 1207–1223 (2020).
Meiller, C. et al. Multi-site tumor sampling highlights molecular intra-tumor heterogeneity in malignant pleural mesothelioma. Genome Med. 13, 113 (2021).
Zhang, M. et al. Clonal architecture in mesothelioma is prognostic and shapes the tumour microenvironment. Nat. Commun. 12, 1751 (2021).
Kukuyan, A. M. et al. Inactivation of Bap1 cooperates with losses of Nf2 and Cdkn2a to drive the development of pleural malignant mesothelioma in conditional mouse models. Cancer Res. 79, 4113–4123 (2019).
Badhai, J. et al. Combined deletion of Bap1, Nf2, and Cdkn2ab causes rapid onset of malignant mesothelioma in mice. J. Exp. Med. 217, e20191257 (2020).
Testa, J. R. & Berns, A. Preclinical models of malignant mesothelioma. Front. Oncol. 10, 101 (2020).
Blum, Y. et al. Dissecting heterogeneity in malignant pleural mesothelioma through histo-molecular gradients for clinical applications. Nat. Commun. 10, 1333 (2019).
Szczepanski, A. P. & Wang, L. Emerging multifaceted roles of BAP1 complexes in biological processes. Cell Death Discov. 7, 20 (2021).
Mo, J. et al. DDX3X: structure, physiologic functions and cancer. Mol. Cancer 20, 38 (2021).
Carbone, M. et al. Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Cancer Discov. 10, 1103–1120 (2020).
Masclef, L. et al. Roles and mechanisms of BAP1 deubiquitinase in tumor suppression. Cell Death Differ. 28, 606–625 (2021).
Ismail, I. H. et al. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res. 74, 4282–4294 (2014).
Lee, H. S., Lee, S. A., Hur, S. K., Seo, J. W. & Kwon, J. Stabilization and targeting of INO80 to replication forks by BAP1 during normal DNA synthesis. Nat. Commun. 5, 5128 (2014).
Singh, A. et al. BAP1 loss induces mitotic defects in mesothelioma cells through BRCA1-dependent and independent mechanisms. Oncogene 42, 572–585 (2023).
Bononi, A. et al. BAP1 is a novel regulator of HIF-1α. Proc. Natl Acad. Sci. USA 120, e2217840120 (2023).
Galani, V. et al. The role of apoptosis defects in malignant mesothelioma pathogenesis with an impact on prognosis and treatment. Cancer Chemother. Pharmacol. 84, 241–253 (2019).
Bononi, A. et al. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature 546, 549–553 (2017).
Zhang, Y. et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 20, 1181–1192 (2018).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015).
Fassl, A., Geng, Y. & Sicinski, P. CDK4 and CDK6 kinases: from basic science to cancer therapy. Science 375, eabc1495 (2022).
Levine, A. J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 20, 471–480 (2020).
Sekido, Y. & Sato, T. NF2 alteration in mesothelioma. Front. Toxicol. 5, 1161995 (2023).
Wu, J. et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572, 402–406 (2019).
Brevet, M. et al. Coactivation of receptor tyrosine kinases in malignant mesothelioma as a rationale for combination targeted therapy. J. Thorac. Oncol. 6, 864–874 (2011).
Bhadresha, K., Mirza, S., Penny, C. & Mughal, M. J. Targeting AXL in mesothelioma: from functional characterization to clinical implication. Crit. Rev. Oncol. Hematol. 188, 104043 (2023).
Quispel-Janssen, J. M. et al. Comprehensive pharmacogenomic profiling of malignant pleural mesothelioma identifies a subgroup sensitive to FGFR inhibition. Clin. Cancer Res. 24, 84–94 (2018).
Offin, M. et al. Genomic and transcriptomic analysis of a diffuse pleural mesothelioma patient-derived xenograft library. Genome Med. 14, 127 (2022).
Kryukov, G. V. et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science 351, 1214–1218 (2016).
Engstrom, L. D. et al. MRTX1719 is an MTA-cooperative PRMT5 inhibitor that exhibits synthetic lethality in preclinical models and patients with MTAP-deleted cancer. Cancer Discov. 13, 2412–2431 (2023). This phase I clinical trial shows early, promising signal of activity in patients with MTAP-deleted mesothelioma, highlighting the potential for emerging precision therapeutics.
Delage, B. et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer 126, 2762–2772 (2010).
Bononi, A. et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ. 24, 1694–1704 (2017).
Fan, J. et al. Clinical significance of FBXW7 loss of function in human cancers. Mol. Cancer 21, 87 (2022).
Ramundo, V., Zanirato, G. & Aldieri, E. The epithelial-to-mesenchymal transition (EMT) in the development and metastasis of malignant pleural mesothelioma. Int. J. Mol. Sci. 22, 12216 (2021).
Yang, J. et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 (2020).
Zolondick, A. A. et al. Asbestos-induced chronic inflammation in malignant pleural mesothelioma and related therapeutic approaches-a narrative review. Precis. Cancer Med. 4, 27 (2021).
Xu, A., Wu, L. J., Santella, R. M. & Hei, T. K. Role of oxyradicals in mutagenicity and DNA damage induced by crocidolite asbestos in mammalian cells. Cancer Res. 59, 5922–5926 (1999).
Harber, J., Kamata, T., Pritchard, C. & Fennell, D. Matter of TIME: the tumor-immune microenvironment of mesothelioma and implications for checkpoint blockade efficacy. J. Immunother. Cancer 9, e003032 (2021).
Chéné, A. L. et al. Pleural effusions from patients with mesothelioma induce recruitment of monocytes and their differentiation into M2 macrophages. J. Thorac. Oncol. 11, 1765–1773 (2016).
Zhang, F. et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 7, 52294–52306 (2016).
Gratchev, A. TGF-β signalling in tumour associated macrophages. Immunobiology 222, 75–81 (2017).
Gschwandtner, M., Derler, R. & Midwood, K. S. More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 10, 2759 (2019).
Horio, D. et al. Tumor-associated macrophage-derived inflammatory cytokine enhances malignant potential of malignant pleural mesothelioma. Cancer Sci. 111, 2895–2906 (2020).
Ohara, Y. et al. Connective tissue growth factor produced by cancer-associated fibroblasts correlates with poor prognosis in epithelioid malignant pleural mesothelioma. Oncol. Rep. 44, 838–848 (2020).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005).
Li, Q. et al. Pleural mesothelioma instigates tumor-associated fibroblasts to promote progression via a malignant cytokine network. Am. J. Pathol. 179, 1483–1493 (2011).
Bianco, A., Valente, T., De Rimini, M. L., Sica, G. & Fiorelli, A. Clinical diagnosis of malignant pleural mesothelioma. J. Thorac. Dis. 10, S253–s261 (2018).
Hollen, P. J., Gralla, R. J., Liepa, A. M., Symanowski, J. T. & Rusthoven, J. J. Adapting the lung cancer symptom scale (LCSS) to mesothelioma: using the LCSS-Meso conceptual model for validation. Cancer 101, 587–595 (2004).
Brims, F. et al. Early specialist palliative care on quality of life for malignant pleural mesothelioma: a randomised controlled trial. Thorax 74, 354–361 (2019).
Hoon, S. N. et al. Symptom burden and unmet needs in malignant pleural mesothelioma: exploratory analyses from the RESPECT-meso study. J. Palliat. Care 36, 113–120 (2021).
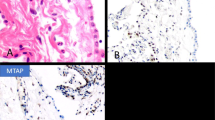
Seker, N. S. et al. Investigation of MTAP and BAP1 staining loss and P16/CDKN2A deletion in pleural cytology specimens and its role in the diagnosis of mesothelioma. Diagn. Cytopathol. 52, 211–216 (2024).
Michael, C. W., Bedrossian, C., Sadri, N. & Klebe, S. The cytological features of effusions with mesothelioma in situ: a report of 9 cases. Diagn. Cytopathol. 51, 374–388 (2023).
Klebe, S. et al. The concept of mesothelioma in situ, with consideration of its potential impact on cytology diagnosis. Pathology 53, 446–453 (2021).
Li, Y. et al. Reliability of assessing morphologic features with prognostic significance in cytology specimens of epithelioid diffuse pleural mesothelioma and implications for cytopathology reporting. Cancer Cytopathol. 131, 495–506 (2023).
Kindler, H. L. et al. Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 36, 1343–1373 (2018).
Pezzuto, F. et al. Evaluation of prognostic histological parameters proposed for pleural mesothelioma in diffuse malignant peritoneal mesothelioma. A short report. Diagn. Pathol. 16, 64 (2021).
Husain, A. N. et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 142, 89–108 (2018).
Marchevsky, A. M. Application of immunohistochemistry to the diagnosis of malignant mesothelioma. Arch. Pathol. Lab. Med. 132, 397–401 (2008).
Churg, A. Mesothelioma: morphologic and immunohistochemical findings. Pathologie 45, 309–315 (2024).
Churg, A. New developments in mesothelial pathology. Histopathology 84, 136–152 (2024).
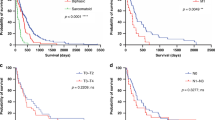
Rosen, L. E. et al. Nuclear grade and necrosis predict prognosis in malignant epithelioid pleural mesothelioma: a multi-institutional study. Mod. Pathol. 31, 598–606 (2018).
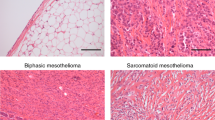
Nicholson, A. G. et al. EURACAN/IASLC proposals for updating the histologic classification of pleural mesothelioma: towards a more multidisciplinary approach. J. Thorac. Oncol. 15, 29–49 (2020).
Schulte, J. J. & Husain, A. N. Updates on grading mesothelioma. Histopathology 84, 153–162 (2024).
WHO Classification of Tumours Editorial Board (eds) WHO Classification of Tumours: Thoracic Tumours Vol. 5 (WHO, 2021).
Schulte, J. J. et al. Comparison of nuclear grade, necrosis, and histologic subtype between biopsy and resection in pleural malignant mesothelioma: an international multi-institutional analysis. Am. J. Clin. Pathol. 156, 989–999 (2021).
Verma, V. et al. Survival by histologic subtype of malignant pleural mesothelioma and the impact of surgical resection on overall survival. Clin. Lung Cancer 19, e901–e912 (2018).
Danuzzo, F. et al. Pleural mesothelioma in situ: a comprehensive review. Eur. J. Cancer Prev. 33, 545–551 (2024).
Churg, A. & Naso, J. R. The separation of benign and malignant mesothelial proliferations: new markers and how to use them. Am. J. Surg. Pathol. 44, e100–e112 (2020).
Hung, Y. P. & Chirieac, L. R. Molecular and immunohistochemical testing in mesothelioma and other mesothelial lesions. Arch. Pathol. Lab. Med. 148, e77–e89 (2024).
Opitz, I. et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 58, 1–24 (2020).
Zahid, I., Sharif, S., Routledge, T. & Scarci, M. What is the best way to diagnose and stage malignant pleural mesothelioma? Interact. Cardiovasc. Thorac. Surg. 12, 254–259 (2011).
Erasmus, J. J. et al. Integrated computed tomography-positron emission tomography in patients with potentially resectable malignant pleural mesothelioma: staging implications. J. Thorac. Cardiovasc. Surg. 129, 1364–1370 (2005).
Berzenji, L., Van Schil, P. E. & Carp, L. The eighth TNM classification for malignant pleural mesothelioma. Transl. Lung Cancer Res. 7, 543–549 (2018).
Wolf, A. S. et al. The International Association for the Study of Lung Cancer pleural mesothelioma staging project: expanded database to inform revisions in the ninth edition of the TNM classification of pleural mesothelioma. J. Thorac. Oncol. 19, 1242–1252 (2024).
Gill, R. R. et al. The International Association for the Study of Lung Cancer mesothelioma staging project: proposals for revisions of the ‘T’ descriptors in the forthcoming 9(th) edition of the TNM classification for pleural mesothelioma. J. Thorac. Oncol. 19, 1310–1325 (2024).
Nowak, A. K. et al. The International Association for the Study of Lung Cancer pleural mesothelioma staging project: proposal for revision of the TNM stage groupings in the forthcoming (ninth) edition of the TNM classification for pleural mesothelioma. J. Thorac. Oncol. 19, 1339–1351 (2024).
Bille, A. et al. The International Sssociation for the Study of Lung Cancer mesothelioma staging project: proposals for the “N” descriptors in the forthcoming ninth edition of the TNM classification for pleural mesothelioma. J. Thorac. Oncol. 19, 1326–1338 (2024).
Testa, J. R. et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 43, 1022–1025 (2011).
Baumann, F. et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 36, 76–81 (2015).
Hathaway, F. et al. Family matters: germline testing in thoracic cancers. Am. Soc. Clin. Oncol. Educ. Book 43, e389956 (2023).
Pastorino, S. et al. A subset of mesotheliomas with improved survival occurring in carriers of BAP1 and other germline mutations. J. Clin. Oncol. 36, 3485–3494 (2018).
Hassan, R. et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc. Natl Acad. Sci. USA 116, 9008–9013 (2019).
Zauderer, M. G. et al. Prevalence and preliminary validation of screening criteria to identify carriers of germline BAP1 mutations. J. Thorac. Oncol. 14, 1989–1994 (2019).
Pass, H. I. et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N. Engl. J. Med. 353, 1564–1573 (2005).
Hollevoet, K. et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta-analysis. J. Clin. Oncol. 30, 1541–1549 (2012).
Pass, H. I. et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N. Engl. J. Med. 367, 1417–1427 (2012).
Roberts, H. C. et al. Screening for malignant pleural mesothelioma and lung cancer in individuals with a history of asbestos exposure. J. Thorac. Oncol. 4, 620–628 (2009).
Woolhouse, I. et al. British thoracic society guideline for the investigation and management of malignant pleural mesothelioma. Thorax 73, i1–i30 (2018).
Vogelzang, N. J. et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 21, 2636–2644 (2003). This pivotal study establishes a new global standard of care in the first-line treatment setting for patients with malignant pleural mesothelioma.
van Meerbeeck, J. P. et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J. Clin. Oncol. 23, 6881–6889 (2005).
Dudek, A. Z. et al. Randomized study of maintenance pemetrexed versus observation for treatment of malignant pleural mesothelioma: CALGB 30901. Clin. Lung Cancer 21, 553–561 e551 (2020).
de Gooijer, C. J. et al. Switch-maintenance gemcitabine after first-line chemotherapy in patients with malignant mesothelioma (NVALT19): an investigator-initiated, randomised, open-label, phase 2 trial. Lancet Respir. Med. 9, 585–592 (2021).
Karam, A. S., Abdelwahab, S., Ezz El Din, M. M. A. & Alorabi, M. O. A randomized comparative study on maintenance gemcitabine versus supportive care in pleural mesothelioma. Future Oncol. 21, 2203–2213 (2025).
Zalcman, G. et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 387, 1405–1414 (2016).
Szlosarek, P. W. et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin. Cancer Res. 12, 7126–7131 (2006).
Szlosarek, P. W. et al. Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1-deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol. 3, 58–66 (2017).
Szlosarek, P. W. et al. Pegargiminase plus first-line chemotherapy in patients with nonepithelioid pleural mesothelioma: the ATOMIC-Meso randomized clinical trial. JAMA Oncol. 10, 475–483 (2024). A positive phase III clinical trial that uses a prospective, rational patient stratification based on susceptibility to arginine deprivation (in non-epithelioid mesotheliomas).
Ceresoli, G. L. et al. Tumour treating fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol. 20, 1702–1709 (2019).
Baas, P. et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397, 375–386 (2021). This pivotal trial led to approval of immunotherapy as a standard of care for the treatment of malignant pleural mesothelioma.
Bylicki, O. et al. Real-world efficacy and safety of combination nivolumab plus ipilimumab for untreated, unresectable, pleural mesothelioma: the meso-immune (GFPC 04-2021) trial. Lung Cancer 194, 107866 (2024).
Dumoulin, D. W. et al. Nivolumab and ipilimumab in the real-world setting in patients with mesothelioma. Lung Cancer 187, 107440 (2024).
Enrico, D. et al. Efficacy of first-line nivolumab plus ipilimumab in unresectable pleural mesothelioma: a multicenter real-world study (ImmunoMeso LATAM). Clin. Lung Cancer 25, 723–731 e722 (2024).
Schmid, S. et al. Real-world outcomes of patients with malignant pleural mesothelioma receiving a combination of ipilimumab and nivolumab as first- or later-line treatment. JTO Clin. Res. Rep. 5, 100735 (2024).
McNamee, N. et al. Brief report: real-world toxicity and survival of combination immunotherapy in pleural mesothelioma-RIOMeso. J. Thorac. Oncol. 19, 636–642 (2024).
Santoro, A. et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the international expanded access program. J. Thorac. Oncol. 3, 756–763 (2008).
Yap, T. A. et al. Development of immunotherapy combination strategies in cancer. Cancer Discov. 11, 1368–1397 (2021).
Horn, L. et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 379, 2220–2229 (2018).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Chu, Q. et al. Pembrolizumab plus chemotherapy versus chemotherapy in untreated advanced pleural mesothelioma in Canada, Italy, and France: a phase 3, open-label, randomised controlled trial. Lancet 402, 2295–2306 (2023). This trial reports demonstrated superiority of chemoimmnunotherapy versus chemotherapy alone for malignant pleural mesothelioma.
Popat, S. et al. BEAT-meso: a randomized phase III study of bevacizumab (B) and standard chemotherapy (C) with or without atezolizumab (A), as first-line treatment (TX) for advanced pleural mesothelioma (PM)—results from the ETOP 13-18 trial. J. Clin. Oncol. 42, LBA8002 (2024).
Aerts, J. G. et al. Dendritic cells loaded with allogeneic tumour cell lysate plus best supportive care versus best supportive care alone in patients with pleural mesothelioma as maintenance therapy after chemotherapy (DENIM): a multicentre, open-label, randomised, phase 2/3 study. Lancet Oncol. 25, 865–878 (2024).
Fennell, D. A. et al. Active symptom control with or without oral vinorelbine in patients with relapsed malignant pleural mesothelioma (VIM): a randomised, phase 2 trial. eClinicalMedicine 48, 101432 (2022).
Fennell, D. A. et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 22, 1530–1540 (2021).
Maio, M. et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 18, 1261–1273 (2017).
Popat, S. et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann. Oncol. 31, 1734–1745 (2020).
Scherpereel, A. et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 20, 239–253 (2019).
Disselhorst, M. J. et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir. Med. 7, 260–270 (2019).
Alley, E. W. et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 18, 623–630 (2017).
Douma, L. H. et al. Pembrolizumab plus lenvatinib in second-line and third-line patients with pleural mesothelioma (PEMMELA): a single-arm phase 2 study. Lancet Oncol. 24, 1219–1228 (2023).
Homicsko, K. et al. PD-1-expressing macrophages and CD8 T cells are independent predictors of clinical benefit from PD-1 inhibition in advanced mesothelioma. J. Immunother. Cancer 11, e007585 (2023).
Khanna, S. et al. Tumor-derived GM-CSF promotes granulocyte immunosuppression in mesothelioma patients. Clin. Cancer Res. 24, 2859–2872 (2018).
Rikimaru, T. et al. Production of granulocyte colony-stimulating factor by malignant mesothelioma. Eur. Respir. J. 8, 183–184 (1995).
Hegmans, J. P. et al. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur. Respir. J. 27, 1086–1095 (2006).
Fujiwara, A. et al. Granulocyte-colony stimulating factor (G-CSF) producing malignant pleural mesothelioma: report of a case. Thorac. Cancer 6, 105–109 (2015).
Chee, S. J. et al. Evaluating the effect of immune cells on the outcome of patients with mesothelioma. Br. J. Cancer 117, 1341–1348 (2017).
Pasello, G. et al. Malignant pleural mesothelioma immune microenvironment and checkpoint expression: correlation with clinical-pathological features and intratumor heterogeneity over time. Ann. Oncol. 29, 1258–1265 (2018).
Mannarino, L. et al. Epithelioid pleural mesothelioma is characterized by tertiary lymphoid structures in long survivors: results from the MATCH study. Int. J. Mol. Sci. 23, 5786 (2022).
Fennell, D. A. et al. Constitutive inflammation and epithelial mesenchymal transition dictate responsiveness to nivolumab: CONFIRM a randomised phase III trial. Nat. Commun. https://doi.org/10.1038/s41467-025-61691-4 (2025).
Homicsko, K. et al. Association of tertiary lymphoid structures with outcomes from PD-1 inhibition in advanced malignant pleural mesothelioma. J. Clin. Oncol. 41, e20545 (2023).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Fridman, W. H. et al. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 19, 441–457 (2022).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Zhang, M. et al. A gut microbiota rheostat forecasts responsiveness to PD-L1 and VEGF blockade in mesothelioma. Nat. Commun. 15, 7187 (2024).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632–1640 (2021).
Zauderer, M. G. et al. EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: a multicentre, open-label, phase 2 study. Lancet Oncol. 23, 758–767 (2022).
Fennell, D. A. et al. Rucaparib in patients with BAP1-deficient or BRCA1-deficient mesothelioma (MiST1): an open-label, single-arm, phase 2a clinical trial. Lancet Respir. Med. 9, 593–600 (2021).
Fennell, D. et al. Evaluating niraparib versus active symptom control in patients with previously treated mesothelioma (NERO): a study protocol for a multicentre, randomised, two-arm, open-label phase II trial in UK secondary care centres. BMJ Open 13, e073120 (2023).
Fennell, D. A. et al. Abemaciclib in patients with p16ink4A-deficient mesothelioma (MiST2): a single-arm, open-label, phase 2 trial. Lancet Oncol. 23, 374–381 (2022).
Fennell, D. A. et al. Maintenance defactinib versus placebo after first-line chemotherapy in patients with merlin-stratified pleural mesothelioma: COMMAND-a double-blind, randomized, phase II study. J. Clin. Oncol. 37, 790–798 (2019).
Yap, T. A. et al. Abstract CT006: first-in-class, first-in-human phase 1 trial of VT3989, an inhibitor of yes-associated protein (YAP)/transcriptional enhancer activator domain (TEAD), in patients (pts) with advanced solid tumors enriched for malignant mesothelioma and other tumors with neurofibromatosis 2 (NF2) mutations. Cancer Res. 83, CT006–CT006 (2023).
Bayman, N. et al. Prophylactic irradiation of tracts in patients with malignant pleural mesothelioma: an open-label, multicenter, phase III randomized trial. J. Clin. Oncol. 37, 1200–1208 (2019).
Clive, A. O. et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): a multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol. 17, 1094–1104 (2016).
Gomez, D. R. et al. The use of radiation therapy for the treatment of malignant pleural mesothelioma: expert opinion from the national cancer institute thoracic malignancy steering committee, international association for the study of lung cancer, and mesothelioma applied research foundation. J. Thorac. Oncol. 14, 1172–1183 (2019).
Tsao, A. S. et al. Current and future management of malignant mesothelioma: a consensus report from the national cancer institute thoracic malignancy steering committee, international association for the study of lung cancer, and mesothelioma applied research foundation. J. Thorac. Oncol. 13, 1655–1667 (2018).
MacRae, R. M. et al. The role of radiation treatment in pleural mesothelioma: highlights of the 14th international conference of the international mesothelioma interest group. Lung Cancer 132, 24–27 (2019).
Krug, L. M. et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J. Clin. Oncol. 27, 3007–3013 (2009).
Cho, B. C. J. et al. Surgery for malignant pleural mesothelioma after radiotherapy (SMART): final results from a single-centre, phase 2 trial. Lancet Oncol. 22, 190–197 (2021).
de Perrot, M. et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 151, 468–473 (2016).
Shaaban, S. G. et al. Utilization of intensity-modulated radiation therapy for malignant pleural mesothelioma in the United States. Clin. Lung Cancer 19, e685–e692 (2018).
Patel, R. et al. Disease-related outcomes and toxicities of intensity modulated radiation therapy after lung-sparing pleurectomy for malignant pleural mesothelioma: a systematic review. Pract. Radiat. Oncol. 10, 423–433 (2020).
Lazarev, S. et al. Where are we with proton beam therapy for thoracic malignancies? Current status and future perspectives. Lung Cancer 152, 157–164 (2021).
Zeng, J. et al. Consensus statement on proton therapy in mesothelioma. Pract. Radiat. Oncol. 11, 119–133 (2021).
Badiyan, S. N. et al. Proton beam therapy for malignant pleural mesothelioma. Transl. Lung Cancer Res. 7, 189–198 (2018).
Rice, S. R. et al. A novel prospective study assessing the combination of photodynamic therapy and proton radiation therapy: safety and outcomes when treating malignant pleural mesothelioma. Photochem. Photobiol. 95, 411–418 (2019).
Simone, C. B. 2nd et al. Stereotactic body radiation therapy for lung cancer. Chest 143, 1784–1790 (2013).
Shin, J. Y. et al. Clinical outcomes of stereotactic body radiation therapy for malignant pleural mesothelioma. Radiother. Oncol. 191, 110057 (2024).
Ghirardelli, P. et al. Salvage radiotherapy for oligo-progressive malignant pleural mesothelioma. Lung Cancer 152, 1–6 (2021).
Videtic, G. M. M. et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract. Radiat. Oncol. 7, 295–301 (2017).
Alley, E. W., Katz, S. I., Cengel, K. A. & Simone, C. B. 2nd Immunotherapy and radiation therapy for malignant pleural mesothelioma. Transl. Lung Cancer Res. 6, 212–219 (2017).
Barsky, A. R., Cengel, K. A., Katz, S. I., Sterman, D. H. & Simone, C. B. 2nd First-ever abscopal effect after palliative radiotherapy and immuno-gene therapy for malignant pleural mesothelioma. Cureus 11, e4102 (2019).
Rimner, A. et al. A phase 1 safety study of avelumab plus stereotactic body radiation therapy in malignant pleural mesothelioma. JTO Clin. Res. Rep. 4, 100440 (2023).
Rimner, A. et al. Randomized phase 2 placebo-controlled trial of nintedanib for the treatment of radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 116, 1091–1099 (2023).
Simone, C. B. 2nd et al. Multicenter phase 1b/2a clinical trial of radioprotectant BIO 300 oral suspension for patients with non-small cell lung cancer receiving concurrent chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 118, 404–414 (2024).
Treasure, T. et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the mesothelioma and radical surgery (MARS) randomised feasibility study. Lancet Oncol. 12, 763–772 (2011).
Lim, E. et al. Extended pleurectomy decortication and chemotherapy versus chemotherapy alone for pleural mesothelioma (MARS 2): a phase 3 randomised controlled trial. Lancet Respir. Med. 12, 457–466 (2024). The findings of this randomized phase III clinical trial challenge the routine use of surgery in resectable mesothelioma.
Lim, E. et al. Further insights into MARS 2 – authors’ reply. Lancet Respir. Med. 12, e55 (2024).
Waller, D. et al. Why the MARS2 trial does not mean the end of all mesothelioma surgery. Cancers 17, 724 (2025).
Kindler, H. L. et al. Treatment of pleural mesothelioma: ASCO guideline update. J. Clin. Oncol. 43, 1006–1038 (2025).
Trovo, M. et al. Radical hemithoracic radiotherapy versus palliative radiotherapy in non-metastatic malignant pleural mesothelioma: results from a phase 3 randomized clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 109, 1368–1376 (2021).
Stahel, R. A. et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol. 16, 1651–1658 (2015).
Riesterer, O. et al. Pattern of failure after adjuvant radiotherapy following extrapleural pneumonectomy of pleural mesothelioma in the SAKK 17/04 trial. Radiother. Oncol. 138, 121–125 (2019).
Gibson, A. E. J. et al. Development of patient and caregiver conceptual models investigating the health-related quality of life impacts of malignant pleural mesothelioma. Patient 17, 551–563 (2024).
Arber, A. & Spencer, L. ‘It’s all bad news’: the first 3 months following a diagnosis of malignant pleural mesothelioma. Psychooncology 22, 1528–1533 (2013).
Hoon, S. N. et al. Randomised placebo-controlled cross-over study examining the role of anamorelin in mesothelioma (the ANTHEM study): rationale and protocol. BMJ Open Respir. Res. 7, e000551 (2020).
Moore, A., Bennett, B., Taylor-Stokes, G. & Daumont, M. J. Caregivers of patients with malignant pleural mesothelioma: who provides care, what care do they provide and what burden do they experience? Qual. Life Res. 32, 2587–2599 (2023).
Bates, G. E. et al. Approach to offering remote support to mesothelioma patients: the mesothelioma survivor project. Transl. Lung Cancer Res. 5, 216–218 (2016).
Buresti, G. et al. Economic impact of malignant mesothelioma in Italy: an estimate of the public and social costs. Med. Lav. 108, 358–366 (2017).
Borrelli, E., Babcock, Z. & Kogut, S. Costs of medical care for mesothelioma. Rare Tumors 11, 2036361319863498 (2019).
Tompa, E. et al. The economic burden of lung cancer and mesothelioma due to occupational and para-occupational asbestos exposure. Occup. Environ. Med. 74, 816–822 (2017).
Maguire, R. et al. Advanced symptom management system for patients with malignant pleural mesothelioma (ASyMSmeso): mixed methods study. J. Med. Internet Res. 22, e19180 (2020).
Jeffery, E. et al. Body composition and nutritional status in malignant pleural mesothelioma: implications for activity levels and quality of life. Eur. J. Clin. Nutr. https://doi.org/10.1038/s41430-019-0418-9 (2019).
Bibby, A. C., Morley, A. J., Keenan, E., Maskell, N. A. & Gooberman-Hill, R. The priorities of people with mesothelioma and their carers: a qualitative interview study of trial participation and treatment decisions. Eur. J. Oncol. Nurs. 57, 102111 (2022).
Breen, L. J., Huseini, T., Same, A., Peddle-McIntyre, C. J. & Lee, Y. C. G. Living with mesothelioma: a systematic review of patient and caregiver psychosocial support needs. Patient Educ. Couns. 105, 1904–1916 (2022).
Nagamatsu, Y. et al. Physician requests by patients with malignant pleural mesothelioma in Japan. BMC Cancer 19, 383 (2019).
Sherborne, V., Seymour, J., Taylor, B. & Tod, A. What are the psychological effects of mesothelioma on patients and their carers? A scoping review. Psychooncology 29, 1464–1473 (2020).
Senek, M., Robertson, S., Darlison, L., Creech, L. & Tod, A. Malignant pleural mesothelioma patients’ experience by gender: findings from a cross-sectional UK-national questionnaire. BMJ Open Respir. Res. 9, e001050 (2022).
Brusselmans, L. et al. Breath analysis as a diagnostic and screening tool for malignant pleural mesothelioma: a systematic review. Transl. Lung Cancer Res. 7, 520–536 (2018).
Hirohashi, T., Igarashi, K., Abe, M., Maeda, M. & Hino, O. Retrospective analysis of large-scale research screening of construction workers for the early diagnosis of mesothelioma. Mol. Clin. Oncol. 2, 26–30 (2014).
Napolitano, A. et al. HMGB1 and its hyperacetylated isoform are sensitive and specific serum biomarkers to detect asbestos exposure and to identify mesothelioma patients. Clin. Cancer Res. 22, 3087–3096 (2016).
Adusumilli, P. S. et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 11, 2748–2763 (2021).
Blyth, K. G. et al. Leveraging the pleural space for anticancer therapies in pleural mesothelioma. Lancet Respir. Med. 12, 476–483 (2024).
Sterman, D. H. et al. A trial of intrapleural adenoviral-mediated Interferon-α2b gene transfer for malignant pleural mesothelioma. Am. J. Respir. Crit. Care Med. 184, 1395–1399 (2011).
Fennell, D. A. et al. First-in-human phase I clinical trial of RSO-021, a first-in class covalent inhibitor of mitochondrial peroxiredoxin 3 (PRX3), in patients with malignant pleural effusion due to mesothelioma and other advanced solid tumors (MITOPE). J. Clin. Oncol. 42, 3019–3019 (2024).
Sahtoe, D. D., van Dijk, W. J., Ekkebus, R., Ovaa, H. & Sixma, T. K. BAP1/ASXL1 recruitment and activation for H2A deubiquitination. Nat. Commun. 7, 10292 (2016).
Yuan, J., Chen, K., Zhang, W. & Chen, Z. Structure of human chromatin-remodelling PBAF complex bound to a nucleosome. Nature 605, 166–171 (2022).
Author information
Authors and Affiliations
Contributions
Introduction (D.A.F.); Epidemiology (M.C.W.); Mechanisms/pathophysiology (Y.S. and A.C.-F.); Diagnosis, screening and prevention (A.N.H. and I.O.); Management (D.A.F., P.B., C.B.S., E.L. and I.O.); Quality of life (F.B.); Outlook (D.A.F.); overview of the Primer (D.A.F.).
Corresponding authors
Ethics declarations
Competing interests
D.A.F.: grants from Aldeyra, Astex Therapeutics, Bayer, BMS, Boehringer Ingelheim, Owkin; non-financial support from BerGenBio, Clovis, Eli Lilly, MSD, Roche and Tesaro GSK; personal fees from Aldeyra, Cambridge Clinical Laboratories, Ikena, Opna Bio, Owkin, RS Oncology, Roche, MSD, during the conduct of the study; I.O.: Roche (institutional grant), AstraZeneca (advisory board), MSD (advisory board), BMS (advisory board), Medtronic (institutional grant and advisory board), Intuitive (proctorship and speaker’s fee), Sanofi (speaker’s fee), Regeneron (advisory board), XVIVO (institutional grant), Siemens (speaker’s fee), Astellas (speaker’s fee). I.O. is the International Director for AATS, a member of the Thoracic Clinical Practice Standards Committee and the Thoracic Education Committee of AATS, an ESTS board member, an iMig board member and The Journal of Thoracic and Cardiovascular Surgery Associate Editor. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks G. Ceresoli; S.-C. Chu; J. Van Meerbeeck, who co-reviewed with J. Raskin; L. Mutti; and A. Scherpereel for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fennell, D.A., Sekido, Y., Baas, P. et al. Pleural mesothelioma. Nat Rev Dis Primers 11, 56 (2025). https://doi.org/10.1038/s41572-025-00640-3
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-025-00640-3