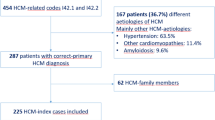
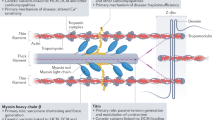
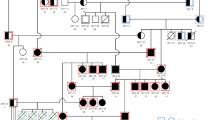
Abstract
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiomyopathy and represents a leading cause of morbidity and mortality. HCM is a sarcomeric disease characterized by genetically determined defects in sarcomere proteins, leading to left ventricular hypertrophy, hypercontractility and diastolic dysfunction. The phenotypic spectrum of the disease is heterogeneous, ranging from mild forms that can remain stable and asymptomatic for many years, through to childhood-onset, severe cases that can result in progressive heart failure and ventricular arrhythmias. Multi-imaging techniques including echocardiography and cardiac magnetic resonance are pivotal for diagnostic and prognostic assessment in HCM. For decades, therapeutic approaches were limited to invasive septal reduction therapies and nonspecific pharmacological treatment for heart failure. In the last 10 years, however, an in-depth understanding of the pathological mechanisms of HCM has led to the development of targeted therapies, such as myosin inhibitors, which have proven to be safe and effective in improving functional capacity and reducing symptoms. Innovative therapeutic approaches, such as gene therapies that aim to target the genetic variants underpinning the condition, are currently under investigation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Semsarian, C., Ingles, J., Maron, M. S. & Maron, B. J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 65, 1249–1254 (2015).
Hespe, S. et al. Genes associated with hypertrophic cardiomyopathy: a reappraisal by the ClinGen Hereditary Cardiovascular Disease Gene Curation Expert Panel. J. Am. Coll. Cardiol. 85, 727–740 (2025).
Ho, C. Y. et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the sarcomeric human cardiomyopathy registry (SHaRe). Circulation 138, 1387–1398 (2018). Seminal work from the SHaRe registry in which the clinical characteristics and prognosis of genotype positive and genotype negative patients are described.
Arbelo, E. et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur. Heart J. 44, 3503–3626 (2023). The ESC guidelines that include the experts’ recommendation for diagnosis and treatment of hypertrophic cardiomyopathy.
Massera, D., Sherrid, M. V., Maron, M. S., Rowin, E. J. & Maron, B. J. How common is hypertrophic cardiomyopathy… really?: disease prevalence revisited 27 years after CARDIA. Int. J. Cardiol. 382, 64–67 (2023).
Massera, D. et al. Prevalence of unexplained left ventricular hypertrophy by cardiac magnetic resonance imaging in MESA. JAHA 8, e012250 (2019).
Butzner, M. et al. Clinical diagnosis of hypertrophic cardiomyopathy over time in the United States (a population-based claims analysis). Am. J. Cardiol. 159, 107–112 (2021).
De Marvao, A. et al. Phenotypic expression and outcomes in individuals with rare genetic variants of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 78, 1097–1110 (2021).
Butters, A., Lakdawala, N. K. & Ingles, J. Sex differences in hypertrophic cardiomyopathy: interaction with genetics and environment. Curr. Heart Fail. Rep. 18, 264–273 (2021).
van Driel, B., Nijenkamp, L., Huurman, R., Michels, M. & van der Velden, J. Sex differences in hypertrophic cardiomyopathy: new insights. Curr. Opin. Cardiol. 34, 254–259 (2019).
Wells, S., Rowin, E. J., Bhatt, V., Maron, M. S. & Maron, B. J. Association between race and clinical profile of patients referred for hypertrophic cardiomyopathy. Circulation 137, 1973–1975 (2018).
Eberly, L. A. et al. Association of race with disease expression and clinical outcomes among patients with hypertrophic cardiomyopathy. JAMA Cardiol. 5, 83–91 (2020).
Tjahjadi, C. et al. Differences in characteristics and outcomes between patients with hypertrophic cardiomyopathy from Asian and European centers. JAHA 11, e023313 (2022).
Arola, A. et al. Epidemiology of idiopathic cardiomyopathies in children and adolescents. A nationwide study in Finland. Am. J. Epidemiol. 146, 385–393 (1997).
Nugent, A. W. et al. The epidemiology of childhood cardiomyopathy in Australia. N. Engl. J. Med. 348, 1639–1646 (2003).
Lipshultz, S. E. et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N. Engl. J. Med. 348, 1647–1655 (2003).
Braunwald, E. Hypertrophic cardiomyopathy: the early years. J. Cardiovasc. Trans. Res. 2, 341–348 (2009).
Braunwald, E. Hypertrophic cardiomyopathy: the first century 1869–1969. Glob. Cardiol. Sci. Pract. 2012, 5 (2012).
Morita, H. et al. Shared genetic causes of cardiac hypertrophy in children and adults. N. Engl. J. Med. 358, 1899–1908 (2008).
Watkins, H. et al. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N. Engl. J. Med. 326, 1108–1114 (1992).
Lehman, S. J., Crocini, C. & Leinwand, L. A. Targeting the sarcomere in inherited cardiomyopathies. Nat. Rev. Cardiol. 19, 353–363 (2022).
Kawana, M., Sarkar, S. S., Sutton, S., Ruppel, K. M. & Spudich, J. A. Biophysical properties of human β-cardiac myosin with converter mutations that cause hypertrophic cardiomyopathy. Sci. Adv. 3, e1601959 (2017).
Helms, A. S. et al. Effects of MYBPC3 loss-of-function mutations preceding hypertrophic cardiomyopathy. JCI Insight 5, e133782 (2020).
Helms, A. S. et al. Spatial and functional distribution of MYBPC3 pathogenic variants and clinical outcomes in patients with hypertrophic cardiomyopathy. Circ: Genom. Precis. Med. 13, 396–405 (2020). Study evaluating the distribution of MYBPC3 variants and clinical outcomes of patients with these variants.
Pettinato, A. M. et al. Development of a cardiac sarcomere functional genomics platform to enable scalable interrogation of human TNNT2 variants. Circulation 142, 2262–2275 (2020).
Madan, A. et al. TNNT2 mutations in the tropomyosin binding region of TNT1 disrupt its role in contractile inhibition and stimulate cardiac dysfunction. Proc. Natl Acad. Sci. USA 117, 18822–18831 (2020).
Teekakirikul, P. et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J. Clin. Invest. 120, 3520–3529 (2010).
Coleman, J. A., Ashkir, Z., Raman, B. & Bueno-Orovio, A. Mechanisms and prognostic impact of myocardial ischaemia in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 39, 1979–1996 (2023).
Aguiar Rosa, S., Rocha Lopes, L., Fiarresga, A., Ferreira, R. C. & Mota Carmo, M. Coronary microvascular dysfunction in hypertrophic cardiomyopathy: pathophysiology, assessment, and clinical impact. Microcirculation 28, e12656 (2021).
Ranjbarvaziri, S. et al. Altered cardiac energetics and mitochondrial dysfunction in hypertrophic cardiomyopathy. Circulation 144, 1714–1731 (2021).
Nollet, E. E. et al. Mitochondrial dysfunction in human hypertrophic cardiomyopathy is linked to cardiomyocyte architecture disruption and corrected by improving NADH-driven mitochondrial respiration. Eur. Heart J. 44, 1170–1185 (2023).
Santini, L., Coppini, R. & Cerbai, E. Ion channel impairment and myofilament Ca2+ sensitization: two parallel mechanisms underlying arrhythmogenesis in hypertrophic cardiomyopathy. Cells 10, 2789 (2021).
Coppini, R. et al. Electrophysiological and contractile effects of disopyramide in patients with obstructive hypertrophic cardiomyopathy. JACC Basic. Transl. Sci. 4, 795–813 (2019).
Ayoub, C. et al. Comparison of valsalva maneuver, amyl nitrite, and exercise echocardiography to demonstrate latent left ventricular outflow obstruction in hypertrophic cardiomyopathy. Am. J. Cardiol. 120, 2265–2271 (2017).
Sherrid, M. V., Gunsburg, D. Z., Moldenhauer, S. & Pearle, G. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 36, 1344–1354 (2000).
Ergi, D. G. et al. Changes in left ventricular-aortic angulation are associated with the development of obstruction in hypertrophic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 170, 190–199 (2025).
Sawma, T. et al. Outcomes of septal myectomy in patients with obstructive hypertrophic cardiomyopathy and minimal septal hypertrophy. J. Thor. Cardiovasc. Surg. https://doi.org/10.1016/j.jtcvs.2025.02.024 (2025).
on behalf of the ACMG Laboratory Quality Assurance Committee. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the Association for molecular pathology. Genet. Med. 17, 405–423 (2015).
Marian, A. J. & Braunwald, E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 121, 749–770 (2017).
Meisner, J. K. et al. Low penetrance sarcomere variants contribute to additive risk in hypertrophic cardiomyopathy. Circulation https://doi.org/10.1161/CIRCULATIONAHA.124.069398 (2024). A study that explains how low penetrance sarcomere variants influence HCM expression.
Harper, A. R. et al. Common genetic variants and modifiable risk factors underpin hypertrophic cardiomyopathy susceptibility and expressivity. Nat. Genet. 53, 135–142 (2021).
Joy, G. et al. Microstructural and microvascular phenotype of sarcomere mutation carriers and overt hypertrophic cardiomyopathy. Circulation 148, 808–818 (2023).
Hughes, R. K. et al. Myocardial perfusion defects in hypertrophic cardiomyopathy mutation carriers. JAHA 10, e020227 (2021).
Captur, G. et al. The embryological basis of subclinical hypertrophic cardiomyopathy. Sci. Rep. 6, 27714 (2016).
Joy, G., Moon, J. C. & Lopes, L. R. Detection of subclinical hypertrophic cardiomyopathy. Nat. Rev. Cardiol. 20, 369–370 (2023).
Joy, G. et al. Electrophysiological characterization of subclinical and overt hypertrophic cardiomyopathy by magnetic resonance imaging-guided electrocardiography. J. Am. Coll. Cardiol. 83, 1042–1055 (2024).
Fourey, D. et al. Prevalence and clinical implication of double mutations in hypertrophic cardiomyopathy: revisiting the gene-dose effect. Circ. Cardiovasc. Genet. 10, e001685 (2017).
Marston, N. A. et al. Clinical characteristics and outcomes in childhood-onset hypertrophic cardiomyopathy. Eur. Heart J. 42, 1988–1996 (2021).
Olivotto, I., Cecchi, F., Poggesi, C. & Yacoub, M. H. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ: Heart Fail. 5, 535–546 (2012).
Topriceanu, C.-C., Pereira, A. C., Moon, J. C., Captur, G. & Ho, C. Y. Meta-analysis of penetrance and systematic review on transition to disease in genetic hypertrophic cardiomyopathy. Circulation https://doi.org/10.1161/CIRCULATIONAHA.123.065987 (2023).
Lorenzini, M. et al. Penetrance of hypertrophic cardiomyopathy in sarcomere protein mutation carriers. J. Am. Coll. Cardiol. 76, 550–559 (2020).
Pelliccia, F. et al. Long-term outcome of nonobstructive versus obstructive hypertrophic cardiomyopathy: a systematic review and meta-analysis. Int. J. Cardiol. 243, 379–384 (2017).
Marstrand, P. et al. Hypertrophic cardiomyopathy with left ventricular systolic dysfunction: insights from the SHaRe registry. Circulation 141, 1371–1383 (2020). Study showing clinical characteristics and outcomes of patients with left ventricular systolic dysfunction.
Fumagalli, C. et al. Incidence of stroke in patients with hypertrophic cardiomyopathy in stable sinus rhythm during long-term monitoring. Int. J. Cardiol. 381, 70–75 (2023).
Coppini, R. et al. Clinical phenotype and outcome of hypertrophic cardiomyopathy associated with thin-filament gene mutations. J. Am. Coll. Cardiol. 64, 2589–2600 (2014).
Kaski, J. P. et al. Indications and management of implantable cardioverter-defibrillator therapy in childhood hypertrophic cardiomyopathy. Cardiol. Young-. 33, 681–698 (2023).
Bos, J. M. et al. Characterization of a phenotype-based genetic test prediction score for unrelated patients with hypertrophic cardiomyopathy. Mayo Clin. Proc. 89, 727–737 (2014).
Shiwani, H. et al. Demographic-based personalized left ventricular hypertrophy thresholds for hypertrophic cardiomyopathy diagnosis. J. Am. Coll. Cardiol. 85, 685–695 (2025).
Biagini, E. et al. Usefulness of electrocardiographic patterns at presentation to predict long-term risk of cardiac death in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 118, 432–439 (2016).
Hughes, R. K. et al. Apical hypertrophic cardiomyopathy: the variant less known. JAHA 9, e015294 (2020).
Delcrè, S. D. L. et al. Relationship of ECG findings to phenotypic expression in patients with hypertrophic cardiomyopathy: a cardiac magnetic resonance study. Int. J. Cardiol. 167, 1038–1045 (2013).
Sangha, V. et al. Identification of hypertrophic cardiomyopathy on electrocardiographic images with deep learning. Preprint at medrXiv https://doi.org/10.1101/2023.12.23.23300490 (2023).
Carrick, R. T. et al. Identification of high-risk imaging features in hypertrophic cardiomyopathy using electrocardiography: a deep-learning approach. Heart Rhythm https://doi.org/10.1016/j.hrthm.2024.01.031 (2024).
Rapezzi, C. et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 34, 1448–1458 (2013).
Bos, J. M., Towbin, J. A. & Ackerman, M. J. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 54, 201–211 (2009).
La Canna, G. et al. Phenotyping left ventricular obstruction with postprandial re-test echocardiography in hypertrophic cardiomyopathy. Am. J. Cardiol. 125, 1688–1693 (2020).
Maron, B. J. et al. Diagnosis and evaluation of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 79, 372–389 (2022).
Pieroni, M. et al. Beyond sarcomeric hypertrophic cardiomyopathy: how to diagnose and manage phenocopies. Curr. Cardiol. Rep. https://doi.org/10.1007/s11886-022-01778-2 (2022).
Wilde, A. A. M. et al. European heart rhythm association (EHRA)/Heart rhythm society (HRS)/Asia pacific heart rhythm society (APHRS)/Latin American heart rhythm society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. J. Arrhythmia 38, 491–553 (2022).
Writing Committee Members. et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 142, e533–e557 (2020).
Perrone-Filardi, P. et al. Non-invasive cardiovascular imaging for evaluating subclinical target organ damage in hypertensive patients. Eur. Heart J. Cardiovasc. Imaging 18, 945–960 (2017).
Cardim, N. et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European association of cardiovascular imaging endorsed by the Saudi heart association. Eur. Heart J. Cardiovasc. Imaging 16, 280–280 (2015).
D’Andrea, A. et al. The role of multimodality imaging in athlete’s heart diagnosis: current status and future directions. JCM 10, 5126 (2021).
Nitsche, C. et al. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J. Am. Coll. Cardiol. 77, 128–139 (2021).
Scully, P. R. et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur. Heart J. 41, 2759–2767 (2020).
Kaski, J. P. et al. Cardiomyopathies in children and adolescents: aetiology, management, and outcomes in the European society of cardiology EURObservational research programme cardiomyopathy and myocarditis registry. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehae109 (2024). A study evaluating clinical charateristics and outcomes of children and adolescents with cardiomyopathies.
Kaski, J. P. et al. Prevalence of sarcomere protein gene mutations in preadolescent children with hypertrophic cardiomyopathy. Circ. Cardiovasc. Genet. 2, 436–441 (2009).
Norrish, G. et al. Clinical presentation and long-term outcomes of infantile hypertrophic cardiomyopathy: a European multicentre study. Esc. Heart Fail. 8, 5057–5067 (2021).
Norrish, G. et al. Yield of clinical screening for hypertrophic cardiomyopathy in child first-degree relatives. Circulation 140, 184–192 (2019).
Moak, J. P. & Kaski, J. P. Hypertrophic cardiomyopathy in children. Heart 98, 1044–1054 (2012).
Norrish, G. et al. Clinical features and natural history of preadolescent nonsyndromic hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 79, 1986–1997 (2022).
McKenna, W. J. & Deanfield, J. E. Hypertrophic cardiomyopathy: an important cause of sudden death. Arch. Dis. Child. 59, 971–975 (1984).
Yetman, A. T., Hamilton, R. M., Benson, L. N. & McCrindle, B. W. Long-term outcome and prognostic determinants in children with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 32, 1943–1950 (1998).
Norrish, G. et al. Clinical presentation and survival of childhood hypertrophic cardiomyopathy: a retrospective study in United Kingdom. Eur. Heart J. 40, 986–993 (2019).
O’Mahony, C. et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur. Heart J. 35, 2010–2020 (2014).
Lynch, A. et al. Risk of sudden death in patients with RASopathy hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 81, 1035–1045 (2023).
Maron, B. J. et al. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 79, 390–414 (2022).
Maron, B. J. et al. Hypertrophic cardiomyopathy in children, adolescents, and young adults associated with low cardiovascular mortality with contemporary management strategies. Circulation 133, 62–73 (2016).
Finocchiaro, G. et al. Sudden cardiac death during exercise in young individuals with hypertrophic cardiomyopathy. JACC Clin. Electrophysiol. 9, 865–867 (2023).
Maron, B. J., Doerer, J. J., Haas, T. S., Tierney, D. M. & Mueller, F. O. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation 119, 1085–1092 (2009).
Finocchiaro, G. et al. Etiology of sudden death in sports: insights from a United Kingdom regional registry. J. Am. Coll. Cardiol. 67, 2108–2115 (2016).
Finocchiaro, G. et al. Sudden cardiac death among adolescents in the United Kingdom. J. Am. Coll. Cardiol. 81, 1007–1017 (2023).
Petek, B. J. et al. Sudden cardiac death in National collegiate athletic association athletes: a 20-year study. Circulation 149, 80–90 (2024).
Elliott, P. M. Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart 92, 785–791 (2005).
Nauffal, V. et al. Worldwide differences in primary prevention implantable cardioverter defibrillator utilization and outcomes in hypertrophic cardiomyopathy. Eur. Heart J. 42, 3932–3944 (2021).
Wang, J. et al. Variable and limited predictive value of the European society of cardiology hypertrophic cardiomyopathy sudden-death risk model: a meta-analysis. Can. J. Cardiol. 35, 1791–1799 (2019).
Amano, M. et al. Validation of guideline recommendation on sudden cardiac death prevention in hypertrophic cardiomyopathy. JACC: Heart Fail. 13, 1014–1026 (2025).
Finocchiaro, G. et al. Sudden cardiac death in cardiomyopathies: acting upon ‘acceptable’ risk in the personalized medicine era. Heart Fail. Rev. 27, 1749–1759 (2022).
Maron, M. S. et al. Evidence that subcutaneous implantable cardioverter-defibrillators are effective and reliable in hypertrophic cardiomyopathy. JACC Clin. Electrophysiol. 6, 1019–1021 (2020).
Norrish, G. et al. Clinical outcomes and programming strategies of implantable cardioverter-defibrillator devices in paediatric hypertrophic cardiomyopathy: a UK national cohort study. EP Europace 23, 400–408 (2021).
Norrish, G. et al. A validation study of the European society of cardiology guidelines for risk stratification of sudden cardiac death in childhood hypertrophic cardiomyopathy. EP Europace 21, 1559–1565 (2019).
Norrish, G. et al. Development of a novel risk prediction model for sudden cardiac death in childhood hypertrophic cardiomyopathy (HCM Risk-Kids). JAMA Cardiol. 4, 918–927 (2019).
Norrish, G. et al. External validation of the HCM Risk-Kids model for predicting sudden cardiac death in childhood hypertrophic cardiomyopathy. Eur. J. Prev. Cardiol. 29, 678–686 (2022).
Miron, A. et al. A validated model for sudden cardiac death risk prediction in pediatric hypertrophic cardiomyopathy. Circulation 142, 217–229 (2020).
Norrish, G. et al. Performance of the PRIMaCY sudden death risk prediction model for childhood hypertrophic cardiomyopathy: implications for implantable cardioverter-defibrillator decision-making. Europace 25, euad330 (2023).
Pelliccia, A. et al. Recommendations for participation in competitive sport and leisure-time physical activity in individuals with cardiomyopathies, myocarditis and pericarditis. Eur. J. Cardiovasc. Prev. Rehabil. 13, 876–885 (2006).
Maron, B. J. et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation 109, 2807–2816 (2004).
Finocchiaro, G., Papadakis, M., Sharma, S. & Sheppard, M. Sudden cardiac death. Eur. Heart J. 38, 1280–1282 (2017).
Corrado, D., Basso, C., Rizzoli, G., Schiavon, M. & Thiene, G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J. Am. Coll. Cardiol. 42, 1959–1963 (2003).
Pelliccia, A. et al. Clinical outcomes in adult athletes with hypertrophic cardiomyopathy: a 7-year follow-up study. Br. J. Sports Med. 54, 1008–1012 (2020).
Lampert, R. et al. Safety of sports for athletes with implantable cardioverter-defibrillators: results of a prospective, multinational registry. Circulation 127, 2021–2030 (2013).
Lampert, R. et al. Vigorous exercise in patients with hypertrophic cardiomyopathy. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2023.1042 (2023). A study showing that vigorous exercise in patients with HCM was not associated with an increased risk of adverse outcomes.
Saberi, S. et al. Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: a randomized clinical trial. JAMA 317, 1349–1357 (2017).
Pelliccia, A. et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 42, 17–96 (2021).
Ommen, S. R. et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol.https://doi.org/10.1016/j.jacc.2024.02.014 (2024). AHA/ACC guidelines for the diagnosis and management of HCM.
Magavern, E. F., Finocchiaro, G., Sharma, S., Papadakis, M. & Borry, P. Time out: ethical reflections on medical disqualification of athletes in the context of mandated pre-participation cardiac screening. Br. J. Sports Med. 52, 1207–1210 (2018).
Goland, S. et al. Pregnancy in women with hypertrophic cardiomyopathy: data from the European society of cardiology initiated registry of pregnancy and cardiac disease (ROPAC). Eur. Heart J. 38, 2683–2690 (2017).
Fumagalli, C. et al. Impact of pregnancy on the natural history of women with hypertrophic cardiomyopathy. Eur. J. Prev. Cardiol. 31, 3–10 (2024).
Moolla, M. et al. Outcomes of pregnancy in women with hypertrophic cardiomyopathy: a systematic review. Int. J. Cardiol. 359, 54–60 (2022).
Musumeci, M. B. et al. Clinical course of pregnancy and long-term follow-up after delivery in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 77, 1262–1264 (2021).
Regitz-Zagrosek, V. et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 39, 3165–3241 (2018).
Ammirati, E. et al. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives: pharmacological treatment of HCM. Eur. J. Heart Fail. 18, 1106–1118 (2016).
Argirò, A. et al. Stage-specific therapy for hypertrophic cardiomyopathy. Eur. Heart J. Suppl. 25, C155–C161 (2023).
Dybro, A. M. et al. Randomized trial of metoprolol in patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 78, 2505–2517 (2021).
Dybro, A. M. et al. Effects of metoprolol on exercise hemodynamics in patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 79, 1565–1575 (2022).
Adler, A. et al. Safety of outpatient initiation of disopyramide for obstructive hypertrophic cardiomyopathy patients. JAHA 6, e005152 (2017).
Bertero, E. et al. Real‐world candidacy to mavacamten in a contemporary hypertrophic obstructive cardiomyopathy population. Eur. J. Heart Fail. 26, 59–64 (2024).
Cui, H. et al. Survival following alcohol septal ablation or septal myectomy for patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 79, 1647–1655 (2022).
Lu, G. et al. Left ventricle myocardial remodeling following septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J. Cardiovasc. Magn. Reson. 27, 101864 (2025).
Maron, M. S. et al. Outcomes over follow-up ≥ 10 years after surgical myectomy for symptomatic obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 163, 91–97 (2022).
Maurizi, N. et al. Long-term outcomes after septal reduction therapies in obstructive hypertrophic cardiomyopathy: insights from the SHARE registry. Circulation https://doi.org/10.1161/CIRCULATIONAHA.124.069378 (2024).
Olivotto, I. et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 396, 759–769 (2020). Randomized clinical trial that showed that the myosin inhibitor mavacamten was safe and effective in the treatment of obstructive HCM.
Maron, M. S. et al. Aficamten for symptomatic obstructive hypertrophic cardiomyopathy. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2401424 (2024). Randomized clinical trial that showed that the myosin inhibitor aficamten was safe and effective in the treatment of obstructive HCM.
Desai, M. Y. et al. Mavacamten in patients with hypertrophic cardiomyopathy referred for septal reduction: week 56 results from the VALOR-HCM randomized clinical trial. JAMA Cardiol. 8, 968 (2023).
Chuang, C. et al. Discovery of aficamten (CK-274), a next-generation cardiac myosin inhibitor for the treatment of hypertrophic cardiomyopathy. J. Med. Chem. 64, 14142–14152 (2021).
Desai, M. Y. et al. Real-world experience with mavacamten in obstructive hypertrophic cardiomyopathy: observations from a tertiary care center. Prog. Cardiovasc. Dis. https://doi.org/10.1016/j.pcad.2024.02.001 (2024).
Ho, C. Y. et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 75, 2649–2660 (2020).
Masri, A. et al. Efficacy and safety of aficamten in symptomatic non-obstructive hypertrophic cardiomyopathy: results from the REDWOOD-HCM trial, cohort 4. J. Card. Fail. https://doi.org/10.1016/j.cardfail.2024.02.020 (2024).
Maron, M. S. et al. Safety and efficacy of metabolic modulation with ninerafaxstat in patients with nonobstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. https://doi.org/10.1016/j.jacc.2024.03.387 (2024).
McDonagh, T. A. et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 44, 3627–3639 (2023).
Lopaschuk, G. D. & Verma, S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors. JACC Basic. Transl. Sci. 5, 632–644 (2020).
Subramanian, M. et al. Efficacy of SGLT2 inhibitors in patients with diabetes and nonobstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 188, 80–86 (2023).
Aglan, A. et al. Impact of sodium-glucose cotransporter 2 inhibitors on mortality in hypertrophic cardiomyopathy. JACC Adv. 3, 100843 (2024).
Schoonvelde, S. A. C., Wiethoff, I., Hiligsmann, M., Evers, S. M. A. A. & Michels, M. Quality of life and societal costs in hypertrophic cardiomyopathy: protocol of the AFFECT-HCM study. Neth. Heart J. 31, 238–243 (2023).
Nassif, M. et al. Validation of the Kansas city cardiomyopathy questionnaire in symptomatic obstructive hypertrophic cardiomyopathy. JACC Heart Fail. 10, 531–539 (2022).
Spertus, J. A. et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 397, 2467–2475 (2021).
Reaney, M. et al. Development of the hypertrophic cardiomyopathy symptom questionnaire (HCMSQ): a new patient-reported outcome (PRO) instrument. PharmacoEconomics Open https://doi.org/10.1007/s41669-022-00335-5 (2022).
Argiro, A. et al. Applications of gene therapy in cardiomyopathies. JACC Heart Fail. https://doi.org/10.1016/j.jchf.2023.09.015 (2023).
Hong, K. N. et al. International consensus on differential diagnosis and management of patients with danon disease. J. Am. Coll. Cardiol. 82, 1628–1647 (2023).
Rossano, J. et al. Safety profile of the first pediatric cardiomyopathy gene therapy trial: RP-A501 (AAV9:LAMP2B) for danon disease. J. Card. Fail. 29, 554 (2023).
Rocket pharmaceuticals announces positive updates from phase 1 clinical trial for RP-A501 in danon disease at the Heart Failure Society of America (HFSA) annual scientific meeting 2022.
Greenberg, B. et al. Phase 1 study of AAV9.LAMP2B gene therapy in danon disease. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2412392 (2024).
Greer-Short, A. et al. AAV9-mediated MYBPC3 gene therapy with optimized expression cassette enhances cardiac function and survival in MYBPC3 cardiomyopathy models. Nat. Commun. 16, 2196 (2025).
Haroldson, J. et al. MyPeak-1: a phase 1b study to evaluate safety and efficacy of TN-201, an adeno-associated virus serotype 9 (AAV9) investigational gene therapy, in adults with MYBPC3-associated hypertrophic cardiomyopathy (HCM). J. Card. Fail. 30, S5 (2024).
Chai, A. C. et al. Base editing correction of hypertrophic cardiomyopathy in human cardiomyocytes and humanized mice. Nat. Med. 29, 401–411 (2023).
Reichart, D. et al. Efficient in vivo genome editing prevents hypertrophic cardiomyopathy in mice. Nat. Med. 29, 412–421 (2023).
Satish, T., Hong, K. N., Kaski, J. P. & Greenberg, B. H. Challenges in cardiomyopathy gene therapy clinical trial design. JACC Heart Fail. https://doi.org/10.1016/j.jchf.2024.08.024 (2024).
Xu, Z. et al. Incremental significance of myocardial oedema for prognosis in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 24, 876–884 (2023).
Wang, J. et al. Assessment of late gadolinium enhancement in hypertrophic cardiomyopathy improves risk stratification based on current guidelines. Eur. Heart J. 44, 4781–4792 (2023).
Groarke, J. D. et al. Intrinsic mitral valve alterations in hypertrophic cardiomyopathy sarcomere mutation carriers. Eur. Heart J. – Cardiovasc. Imaging 19, 1109–1116 (2018).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 29, 277–314 (2016).
Gareri, C. et al. Antisense oligonucleotides and small interfering RNA for the treatment of dyslipidemias. JCM 11, 3884 (2022).
Merino, J. L. et al. Practical compendium of antiarrhythmic drugs: a clinical consensus statement of the European heart rhythm association of the ESC. Europace https://doi.org/10.1093/europace/euaf076 (2025).
Ammirati, E. et al. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur. J. Heart Fail. 18, 1106–1118 (2016).
Author information
Authors and Affiliations
Contributions
Introduction (I.O. and A.A.); Epidemiology (I.O., A.A. and V.P.); Mechanisms/pathophysiology (I.O., A.A. and V.P.); Diagnosis, screening and prevention (I.O., A.A., R.J., G.F., J.P.K. and E.A); Management (I.O., A.A., G.F., J.P.K. and E.A.); Quality of life (I.O. and A.A.); Outlook (I.O. and A.A.); overview of the Primer (I.O.).
Corresponding author
Ethics declarations
Competing interests
A.A. is a consultant for Lexeo Therapeutics. I.O. is a consultant and/or adviser for Amicus Therapeutics, Inc.; Boston Scientific Corporation; Bristol Myers Squibb; Cytokinetics, Inc.; and Tenaya Therapeutics, Inc., and has received grant and/or research support from Amicus Therapeutics, Inc.; Bayer AG; Boston Scientific Corporation; Bristol Myers Squibb; Genzyme Corporation; The Menarini Group; Sanofi; Shire plc; and Takeda Pharmaceuticals International, Inc. E.A. is the Chief Science Officer for Lexeo Therapeutics, a shareholder with Rocket Pharmaceuticals and is a founder of Papillion Therapeutics and a founder, on the scientific board of and a shareholder of Corstasis Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks Yuichi Shimada, who co-reviewed with Lusha Liang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
HCM Risk-Kids: https://hcmriskkids.org
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Argirò, A., Parikh, V., Jurcut, R. et al. Hypertrophic cardiomyopathy. Nat Rev Dis Primers 11, 58 (2025). https://doi.org/10.1038/s41572-025-00643-0
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41572-025-00643-0