Abstract
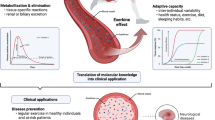
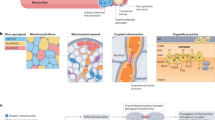
The health benefits of exercise are well-recognized and are observed across multiple organ systems. These beneficial effects enhance overall resilience, healthspan and longevity. The molecular mechanisms that underlie the beneficial effects of exercise, however, remain poorly understood. Since the discovery in 2000 that muscle contraction releases IL-6, the number of exercise-associated signalling molecules that have been identified has multiplied. Exerkines are defined as signalling moieties released in response to acute and/or chronic exercise, which exert their effects through endocrine, paracrine and/or autocrine pathways. A multitude of organs, cells and tissues release these factors, including skeletal muscle (myokines), the heart (cardiokines), liver (hepatokines), white adipose tissue (adipokines), brown adipose tissue (baptokines) and neurons (neurokines). Exerkines have potential roles in improving cardiovascular, metabolic, immune and neurological health. As such, exerkines have potential for the treatment of cardiovascular disease, type 2 diabetes mellitus and obesity, and possibly in the facilitation of healthy ageing. This Review summarizes the importance and current state of exerkine research, prevailing challenges and future directions.
Key points
-
Although the benefits of exercise in enhancing health and treating disease are well-acknowledged, the molecular mechanisms underlying exercise-associated benefits remain ill-defined and are actively being investigated.
-
‘Exerkines’ encompass a broad variety of signalling moieties released in response to acute and/or chronic exercise that exert their effects through endocrine, paracrine and/or autocrine pathways.
-
Exerkines can come in many forms, such as hormones, metabolites, proteins and nucleic acids; interest is increasing in moving beyond singular changes of specific factors to profiling exerkine alterations using ‘omics’ platforms.
-
There is burgeoning interest in the role of extracellular vesicles, which are membranous structures released from cells, in serving as important carriers of molecular signals and drivers of inter-organ crosstalk related to exercise.
-
Multiple organ systems, including the cardiometabolic system, nervous system and immune system, produce exerkines and are influenced by exerkines, which probably contributes to the pleiotropic and variable response to exercise.
-
Emerging research on exerkines suggests multiple promising avenues for translational research and therapeutic modulation to capture exercise-associated benefits; enhanced rigour in experimental design will facilitate comparison between studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Piercy, K. L. et al. The physical activity guidelines for Americans. J. Am. Med. Assoc. 320, 2020–2028 (2018).
Katzmarzyk, P. T., Church, T. S., Craig, C. L. & Bouchard, C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc. 41, 998–1005 (2009).
Ding, D. et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet 388, 1311–1324 (2016).
Trabelsi, K. et al. Globally altered sleep patterns and physical activity levels by confinement in 5056 individuals: ECLB COVID-19 international online survey. Biol. Sport. 38, 495–506 (2021).
Sallis, R. et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br. J. Sports Med. 55, 1099–1105 (2021).
Cullen, T., Thomas, A. W., Webb, R. & Hughes, M. G. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: the effect of exercise intensity and volume. Appl. Physiol. Nutr. Metab. 41, 803–808 (2016).
Nicolini, C. et al. A single bout of high-intensity interval exercise increases corticospinal excitability, brain-derived neurotrophic factor, and uncarboxylated osteolcalcin in sedentary, healthy males. Neuroscience 437, 242–255 (2020).
Pate, R. R. et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. J. Am. Med. Assoc. 273, 402–407 (1995).
Bull, F. C. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54, 1451–1462 (2020).
Troiano, R. P. et al. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 40, 181–188 (2008).
Safdar, A., Saleem, A. & Tarnopolsky, M. A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 12, 504–517 (2016). This paper coined the concept of ‘exerkines’, molecules that are altered in response to acute and chronic exercise, and mediate the systemic adaptations to exercise.
Ransom, F. A contribution to the study of muscle-enzymes. J. Physiol. 40, 1–16 (1910).
Goldstein, M. S. Humoral nature of the hypoglycemic factor of muscular work. Diabetes 10, 232–234 (1961).
Janssen, I., Heymsfield, S. B., Wang, Z. & Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 89, 81–88 (2000).
Pedersen, B. K. Muscle as a secretory organ. Compr. Physiol. 3, 1337–1362 (2013).
Steensberg, A. et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 529, 237–242 (2000). A key paper demonstrating that IL-6 is secreted by contracting muscle, identifying IL-6 as the first ‘myokine’.
Baggish, A. L. et al. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J. Physiol. 589, 3983–3994 (2011).
Vechetti, I. J. Jr, Valentino, T., Mobley, C. B. & McCarthy, J. J. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. J. Physiol. 599, 845–861 (2021).
Sanford, J. A. et al. Molecular Transducers of Physical Activity Consortium (MoTrPAC): mapping the dynamic responses to exercise. Cell 181, 1464–1474 (2020).
National Heart, Lung, and Blood Institute. NHLBI and NIDDK Workshop: exerkines in health, resilience, and diseases executive summary, https://www.nhlbi.nih.gov/events/2020/nhlbi-and-niddk-workshop-exerkines-health-resilience-and-diseases-executive-summary (2020).
Gabriel, B. M. & Zierath, J. R. Circadian rhythms and exercise–re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 15, 197–206 (2019).
Parr, E. B., Heilbronn, L. K. & Hawley, J. A. A time to eat and a time to exercise. Exerc. Sport. Sci. Rev. 48, 4–10 (2020).
van Loon, L. J. et al. Influence of prolonged endurance cycling and recovery diet on intramuscular triglyceride content in trained males. Am. J. Physiol. Endocrinol. Metab. 285, E804–E811 (2003).
Sato, S. et al. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 34, 329–345.e8 (2022). Using a mouse model, this paper presents a detailed atlas of the response to a single bout of exercise at different times, highlighting the influence of exercise timing on tissue changes and exerkine secretion.
Bouchard, C. et al. Familial aggregation of V˙O2max response to exercise training: results from the HERITAGE Family Study. J. Appl. Physiol. 87, 1003–1008 (1999).
Bouchard, C. et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS ONE 7, e37887 (2012).
Leuchtmann, A. B., Adak, V., Dilbaz, S. & Handschin, C. The role of the skeletal muscle secretome in mediating endurance and resistance training adaptations. Front. Physiol. 12, 709807 (2021).
Wahren, J., Felig, P., Ahlborg, G. & Jorfeldt, L. Glucose metabolism during leg exercise in man. J. Clin. Invest. 50, 2715–2725 (1971).
Horowitz, J. F. & Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 72, 558s–563s (2000).
Ostrowski, K., Rohde, T., Asp, S., Schjerling, P. & Pedersen, B. K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 515, 287–291 (1999). One of the earliest papers to examine the effect of acute strenuous exercise, marathon running, on plasma concentrations of pro-inflammatory and anti-inflammatory cytokines.
Contrepois, K. et al. Molecular choreography of acute exercise. Cell 181, 1112–1130.e16 (2020).
Keller, C. et al. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J. Appl. Physiol. 99, 2075–2079 (2005).
Reuter, J. A., Spacek, D. V. & Snyder, M. P. High-throughput sequencing technologies. Mol. Cell 58, 586–597 (2015).
Safdar, A. & Tarnopolsky, M. A. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harb. Perspect. Med. 8, a029827 (2018).
Liangsupree, T., Multia, E. & Riekkola, M.-L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 1636, 461773 (2021).
Artoni, A. et al. Residual platelets are the main determinants of microparticles count in frozen-thawed plasma. Thrombosis Res. 130, 561–562 (2012).
Vanderboom, P. M. et al. A size-exclusion-based approach for purifying extracellular vesicles from human plasma. Cell Rep. Methods 1, 100055 (2021).
Giudice, J. & Taylor, J. M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 34, 49–55 (2017).
Watt, M. J., Miotto, P. M., De Nardo, W. & Montgomery, M. K. The liver as an endocrine organ–linking NAFLD and insulin resistance. Endocr. Rev. 40, 1367–1393 (2019).
Kjær, M., Secher, N. H. & Galbo, H. Physical stress and catecholamine release. Baillières Clin. Endocrinol. Metab. 1, 279–298 (1987).
Nalbandian, M. & Takeda, M. Lactate as a signaling molecule that regulates exercise-induced adaptations. Biology 5, 38 (2016).
Takahashi, H. et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 1, 291–303 (2019). This paper demonstrated that TGFβ2 is secreted from adipose tissue in response to exercise and improves glucose tolerance.
Subbotina, E. et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc. Natl Acad. Sci. USA 112, 16042–16047 (2015).
McPherron, A. C., Lawler, A. M. & Lee, S. J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 387, 83–90 (1997). This is a key paper describing myostatin (GDF8), which inhibits muscle growth.
Jensen, L., Bangsbo, J. & Hellsten, Y. Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J. Physiol. 557, 571–582 (2004).
Richardson, R. S. et al. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 279, H772–H778 (2000).
Kim, I. et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ. Res. 86, 24–29 (2000).
Frydelund-Larsen, L. et al. Exercise induces interleukin-8 receptor (CXCR2) expression in human skeletal muscle. Exp. Physiol. 92, 233–240 (2007).
Ding, Y. H. et al. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr. Neurovasc Res. 3, 15–23 (2006).
Pedersen, B. K. Muscles and their myokines. J. Exp. Biol. 214, 337–346 (2011).
McConell, G. K., Rattigan, S., Lee-Young, R. S., Wadley, G. D. & Merry, T. L. Skeletal muscle nitric oxide signaling and exercise: a focus on glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 303, E301–E307 (2012).
Tejero, J., Shiva, S. & Gladwin, M. T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 99, 311–379 (2019).
Koliatsos, V. E., Clatterbuck, R. E., Winslow, J. W., Cayouette, M. H. & Price, D. L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 10, 359–367 (1993).
Knudsen, J. G. et al. Skeletal muscle IL-6 regulates muscle substrate utilization and adipose tissue metabolism during recovery from an acute bout of exercise. PLoS ONE 12, e0189301 (2017).
Roberts, L. D. et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 19, 96–108 (2014). Using a mouse model, this paper demonstrated that a myokine, BAIBA, can ‘brown’ WAT to improve glucose homeostasis.
Fisher, F. M. et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Nielsen, A. R. et al. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J. Clin. Endocrinol. Metab. 93, 4486–4493 (2008).
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 (2002).
Kitase, Y. et al. β-aminoisobutyric Acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 22, 1531–1544 (2018).
Weitzmann, M. N., Roggia, C., Toraldo, G., Weitzmann, L. & Pacifici, R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J. Clin. Invest. 110, 1643–1650 (2002).
Dankbar, B. et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat. Med. 21, 1085–1090 (2015).
Watanabe-Takano, H. et al. Mechanical load regulates bone growth via periosteal osteocrin. Cell Rep. 36, 109380 (2021).
Melouane, A., Yoshioka, M., Kanzaki, M. & St-Amand, J. Sparc, an EPS-induced gene, modulates the extracellular matrix and mitochondrial function via ILK/AMPK pathways in C2C12 cells. Life Sci. 229, 277–287 (2019).
Heinemeier, K. M., Bjerrum, S. S., Schjerling, P. & Kjaer, M. Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand. J. Med. Sci. Sports 23, e150–e161 (2013).
Kim, J. & Lee, J. Role of transforming growth factor-β in muscle damage and regeneration: focused on eccentric muscle contraction. J. Exerc. Rehabil. 13, 621–626 (2017).
Imai, T. et al. Identification and molecular characterization of fractalkine receptor CX(3)CR1, which mediates both leukocyte migration and adhesion. Cell 91, 521–530 (1997).
Gao, Y. & Galis, Z. S. Exploring the role of endothelial cell resilience in cardiovascular health and disease. Arterioscler. Thromb. Vasc. Biol. 41, 179–185 (2020).
Cullberg, K. B. et al. Effect of weight loss and exercise on angiogenic factors in the circulation and in adipose tissue in obese subjects. Obesity 21, 454–460 (2013).
Gavin, T. P., Drew, J. L., Kubik, C. J., Pofahl, W. E. & Hickner, R. C. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol. 191, 139–146 (2007).
Catoire, M., Mensink, M., Kalkhoven, E., Schrauwen, P. & Kersten, S. Identification of human exercise-induced myokines using secretome analysis. Physiol. Genomics 46, 256–267 (2014).
Wong, B. W. C., Wong, D. & McManus, B. M. Characterization of fractalkine (CX3CL1) and CX3CR1 in human coronary arteries with native atherosclerosis, diabetes mellitus, and transplant vascular disease. Cardiovasc. Pathol. 11, 332–338 (2002).
Keihanian, A., Arazi, H. & Kargarfard, M. Effects of aerobic versus resistance training on serum fetuin-A, fetuin-B, and fibroblast growth factor-21 levels in male diabetic patients. Physiol. Int. 106, 70–80 (2019).
He, Z. et al. Myokine response to high-intensity interval vs. resistance exercise: an individual approach. Front. Physiol. 9, 1735 (2018).
Nieman, D. C. et al. Cytokine changes after a marathon race. J. Appl. Physiol. 91, 109–114 (2001).
Otaka, N. et al. Myonectin is an exercise-induced myokine that protects the heart from ischemia-reperfusion injury. Circ. Res. 123, 1326–1338 (2018).
Fabel, K. et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18, 2803–2812 (2003).
Kim, K. H. et al. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS ONE 8, e63517 (2013).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Catoire, M. et al. Fatty acid-inducible ANGPTL4 governs lipid metabolic response to exercise. Proc. Natl Acad. Sci. USA 111, E1043–E1052 (2014).
Laurens, C. et al. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight 5, e131870 (2020).
Wedell-Neergaard, A.-S. et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 29, 844–855.e3 (2019).
Little, H. C. et al. Myonectin deletion promotes adipose fat storage and reduces liver steatosis. FASEB J. 33, 8666–8687 (2019).
Becher, T. et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 27, 58–65 (2021).
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012). The first paper to describe irisin, a PGC1α-dependent myokine, that drives ‘browning’ of WAT in an animal model; the extent to which these results might apply to humans remains under investigation.
Terada, S. et al. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem. Biophys. Res. Commun. 296, 350–354 (2002).
Pilegaard, H., Saltin, B. & Neufer, P. D. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J. Physiol. 546, 851–858 (2003).
Fox, J. et al. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: a meta-analysis. Scand. J. Med. Sci. Sports 28, 16–28 (2018).
Qiu, S. et al. Chronic exercise training and circulating irisin in adults: a meta-analysis. Sports Med. 45, 1577–1588 (2015).
Vosselman, M. J. et al. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int. J. Obes. 39, 1696–1702 (2015).
Tsiloulis, T. et al. No evidence of white adipocyte browning after endurance exercise training in obese men. Int. J. Obes. 42, 721–727 (2017).
Stanford, K. I. et al. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 27, 1357–1357 (2018). This paper demonstrated that levels of a lipokine produced from brown adipose tissue increase with acute and chronic training to affect white adipose tissue lipolysis.
Vasan, S. K. et al. The proposed systemic thermogenic metabolites succinate and 12,13-diHOME are inversely associated with adiposity and related metabolic traits: evidence from a large human cross-sectional study. Diabetologia 62, 2079–2087 (2019).
Pinckard, K. M. et al. A novel endocrine role for the BAT-released lipokine 12,13-diHOME to mediate cardiac function. Circulation 143, 145–159 (2021).
Besse-Patin, A. et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int. J. Obes. 38, 707–713 (2014).
Kwak, S. E. et al. Effects of exercise-induced apelin on muscle function and cognitive function in aged mice. Exp. Gerontol. 127, 110710 (2019).
Vinel, C. et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 24, 1360–1371 (2018).
Sargeant, J. A. et al. The influence of adiposity and acute exercise on circulating hepatokines in normal-weight and overweight/obese men. Appl. Physiol. Nutr. Metab. 43, 482–490 (2018).
Seo, M. W. et al. Effects of 16 weeks of resistance training on muscle quality and muscle growth factors in older adult women with sarcopenia: a randomized controlled trial. Int. J. Env. Res. Public Health 18, 6762 (2021).
Domin, R. et al. Effect of various exercise regimens on selected exercise-induced cytokines in healthy people. Int. J. Env. Res. Public Health 18, 1261 (2021).
Peppler, W. T. et al. Regulation of hepatic follistatin expression at rest and during exercise in mice. Med. Sci. Sports Exerc. 51, 1116–1125 (2019).
Malin, S. K., del Rincon, J. P., Huang, H. & Kirwan, J. P. Exercise-induced lowering of fetuin-A may increase hepatic insulin sensitivity. Med. Sci. Sports Exerc. 46, 2085–2090 (2014).
Haugen, F. et al. IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol. Cell Physiol. 298, C807–C816 (2010).
Broholm, C. et al. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J. Appl. Physiol. 111, 251–259 (2011).
Rønning, S. B. et al. Syndecan-4 regulates muscle differentiation and is internalized from the plasma membrane during myogenesis. PLoS One 10, e0129288 (2015).
Takahashi, H., Kotani, K., Tanaka, K., Egucih, Y. & Anzai, K. Therapeutic approaches to nonalcoholic fatty liver disease: exercise intervention and related mechanisms. Front. Endocrinol. 9, 588 (2018).
Keipert, S. et al. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am. J. Physiol. Endocrinol. Metab. 306, E469–E482 (2014).
Febbraio, M. A. et al. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J. Physiol. 544, 957–962 (2002). Using sophisticated arterial-venous sampling techniques, this paper demonstrated that the splanchnic tissue bed, rather than the muscle, releases HSP72 during acute exercise in humans.
Mailing, L. J., Allen, J. M., Buford, T. W., Fields, C. J. & Woods, J. A. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc. Sport. Sci. Rev. 47, 75–85 (2019).
Allen, J. M. et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 50, 747–757 (2018).
Packer, N. & Hoffman-Goetz, L. Exercise training reduces inflammatory mediators in the intestinal tract of healthy older adult mice. Can. J. Aging 31, 161–171 (2012).
van Wijck, K. et al. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS ONE 6, e22366 (2011).
Meissner, M. et al. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 218, 323–329 (2011).
Jung, T. W. et al. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK–PPARδ-dependent pathway in mice. Diabetologia 58, 2096–2105 (2015).
Lee, Y. S. et al. The fractalkine/CX3CR1 system regulates β cell function and insulin secretion. Cell 153, 413–425 (2013).
Gerst, F. et al. Metabolic crosstalk between fatty pancreas and fatty liver: effects on local inflammation and insulin secretion. Diabetologia 60, 2240–2251 (2017).
Tao, R. et al. Inactivating hepatic follistatin alleviates hyperglycemia. Nat. Med. 24, 1058–1069 (2018).
Zhang, D., Xie, T. & Leung, P. S. Irisin ameliorates glucolipotoxicity-associated β-cell dysfunction and apoptosis via AMPK signaling and anti-inflammatory actions. Cell Physiol. Biochem. 51, 924–937 (2018).
Lang Lehrskov, L. et al. Interleukin-6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab. 27, 1201–1211.e3 (2018).
Ellingsgaard, H. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 17, 1481–1489 (2011).
Carey, A. L. et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55, 2688–2697 (2006).
van Hall, G. et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J. Clin. Endocrinol. Metab. 88, 3005–3010 (2003).
Benatti, F. B. & Pedersen, B. K. Exercise as an anti-inflammatory therapy for rheumatic diseases–myokine regulation. Nat. Rev. Rheumatol. 11, 86–97 (2015).
Pedersen, L. et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 23, 554–562 (2016).
Pedersen, B. K. & Toft, A. D. Effects of exercise on lymphocytes and cytokines. Br. J. Sports Med. 34, 246–251 (2000).
Steensberg, A., Fischer, C. P., Keller, C., Møller, K. & Pedersen, B. K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 285, E433–E437 (2003).
Dinarello, C. A. The interleukin-1 family: 10 years of discovery. FASEB J. 8, 1314–1325 (1994).
de Waal Malefyt, R., Abrams, J., Bennett, B., Figdor, C. G. & de Vries, J. E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174, 1209–1220 (1991).
Starkie, R., Ostrowski, S. R., Jauffred, S., Febbraio, M. & Pedersen, B. K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J. 17, 884–886 (2003). An important paper demonstrating that increasing IL-6 either by exercise or by infusion blunts the inflammatory effect of TNF.
Moore, S. C. et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med. 176, 816–825 (2016).
Rundqvist, H. et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. eLife 9, e59996 (2020).
Matsuo, K. et al. A mechanism underlying preventive effect of high-intensity training on colon cancer. Med. Sci. Sports Exerc. 49, 1805–1816 (2017).
Aoi, W. et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 62, 882–889 (2013).
Strömberg, A. et al. CX3CL1–a macrophage chemoattractant induced by a single bout of exercise in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R297–R304 (2016).
Rao, R. R. et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157, 1279–1291 (2014).
Kang, K. et al. Adipocyte-derived Th2 cytokines and myeloid PPARδ regulate macrophage polarization and insulin sensitivity. Cell Metab. 7, 485–495 (2008).
Knudsen, N. H. et al. Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science 368, eaat3987 (2020). This paper showed that type 2 innate lymphoid cells (ILC2s) in skeletal muscle secrete IL-13 to affect the metabolic adaptation to exercise training.
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Valenzuela, P. L. et al. Exercise benefits on Alzheimer’s disease: state-of-the-science. Ageing Res. Rev. 62, 101108 (2020).
Etnier, J. L., Shih, C.-H. & Piepmeier, A. T. in Exercise-Cognition Interaction (ed. McMorris, T.) 29–42 (Academic Press, 2016).
Voss, M. W. et al. Exercise and hippocampal memory systems. Trends Cogn. Sci. 23, 318–333 (2019).
Etnier, J. L. et al. The influence of physical fitness and exercise upon cognitive functioning: a meta-analysis. J. Sport. Exerc. Psychol. 19, 249–277 (1997).
Colcombe, S. & Kramer, A. F. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 14, 125–130 (2003).
Neylan, T. C. Memory and the medial temporal lobe. J. Neuropsychiatry Clin. Neurosci. 12, 103 (2000).
Erickson, K. I. et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl Acad. Sci. USA 108, 3017–3022 (2011). This was a randomized controlled trial in older adults that showed that chronic exercise training increases hippocampal size and improves memory function.
Gaitán, J. M. et al. Effects of aerobic exercise training on systemic biomarkers and cognition in late middle-aged adults at risk for Alzheimer’s disease. Front. Endocrinol. 12, 660181 (2021).
Liu, P. Z. & Nusslock, R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front. Neurosci. 12, 52 (2018).
Vivar, C. & van Praag, H. Running changes the brain: the long and the short of it. Physiology 32, 410–424 (2017).
Horowitz, A. M. et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 369, 167–173 (2020). This paper demonstrated that transfer of plasma from exercised aged mice to sedentary aged mice can confer benefits on the ageing brain, including improvements in cognitive function.
De Miguel, Z. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600, 494–499 (2021). This paper demonstrated that plasma transfer from exercised young mice to sedentary young mice reduces brain inflammation and improves cognitive function, supporting the potential of transferable anti-inflammatory exerkines in improving brain function.
Agudelo, L. Z. et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45 (2014).
Yau, S. Y. et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl Acad. Sci. USA 111, 15810–15815 (2014).
Wrann, C. D. et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659 (2013).
Fu, Y. C., Luo, N. L., Klein, R. L. & Garvey, W. T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 46, 1369–1379 (2005).
Schön, M. et al. Effects of running on adiponectin, insulin and cytokines in cerebrospinal fluid in healthy young individuals. Sci. Rep. 9, 1959 (2019).
Lourenco, M. V. et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 25, 165–175 (2019).
Islam, M. R. et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 3, 1058–1070 (2021).
Moon, H. Y. et al. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 24, 332–340 (2016).
Naito, A. T. et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 149, 1298–1313 (2012).
Baker, S. K. et al. Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 115, E9687–E9696 (2018).
Heinonen, A. et al. Bone mineral density of female athletes in different sports. Bone Min. 23, 1–14 (1993).
Bergmann, P. et al. Loading and skeletal development and maintenance. J. Osteoporos. 2011, 786752 (2010).
Janssens, K., ten Dijke, P., Janssens, S. & Van Hul, W. Transforming growth factor-β1 to the bone. Endocr. Rev. 26, 743–774 (2005).
Amrein, K. et al. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J. Clin. Endocrinol. Metab. 97, 148–154 (2012).
Silverman, S. L. Sclerostin. J. Osteoporos. 2010, 941419 (2010).
Li, G. et al. Muscle-bone crosstalk and potential therapies for sarco-osteoporosis. J. Cell Biochem. 120, 14262–14273 (2019).
Luo, J., Liu, W., Feng, F. & Chen, L. Apelin/APJ system: a novel therapeutic target for locomotor system diseases. Eur. J. Pharmacol. 906, 174286 (2021).
Kim, H. et al. Irisin mediates effects on bone and fat via αV integrin receptors. Cell 175, 1756–1768.e17 (2018).
Harmer, D., Falank, C. & Reagan, M. R. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front. Endocrinol. 9, 788 (2019).
Lynes, M. D. et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 23, 631–637 (2017).
Neeper, S. A., Gómez-Pinilla, F., Choi, J. & Cotman, C. Exercise and brain neurotrophins. Nature 373, 109 (1995).
Matthews, V. B. et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 52, 1409–1418 (2009).
Lin, Z. et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17, 779–789 (2013).
Chung, J. et al. HSP72 protects against obesity-induced insulin resistance. Proc. Natl Acad. Sci. USA 105, 1739–1744 (2008).
Fujimoto, T. et al. Overexpression of interleukin-15 exhibits improved glucose tolerance and promotes GLUT4 translocation via AMP-activated protein kinase pathway in skeletal muscle. Biochem. Biophys. Res. Commun. 509, 994–1000 (2019).
Willkomm, L. et al. Lactate regulates myogenesis in C2C12 myoblasts in vitro. Stem Cell Res. 12, 742–753 (2014).
Shimomura, M. et al. Decreased muscle-derived musclin by chronic resistance exercise is associated with improved insulin resistance in rats with type 2 diabetes. Physiol. Rep. 9, e14823 (2021).
Thomas, G. et al. Osteocrin, a novel bone-specific secreted protein that modulates the osteoblast phenotype. J. Biol. Chem. 278, 50563–50571 (2003).
Ataman, B. et al. Evolution of osteocrin as an activity-regulated factor in the primate brain. Nature 539, 242–247 (2016).
Palmer, R. M. J., Ferrige, A. G. & Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526 (1987). This paper described the vasodilatory role of nitric oxide.
Davies, K. J. A., Quintanilha, A. T., Brooks, G. A. & Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 107, 1198–1205 (1982).
Aoi, W. et al. Secreted protein acidic and rich in cysteine (SPARC) improves glucose tolerance via AMP-activated protein kinase activation. FASEB J. 33, 10551–10562 (2019).
Li, Y. et al. Transforming growth factor-β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am. J. Pathol. 164, 1007–1019 (2004).
Hunt, L. C., Upadhyay, A., Jazayeri, J. A., Tudor, E. M. & White, J. D. An anti-inflammatory role for leukemia inhibitory factor receptor signaling in regenerating skeletal muscle. Histochem. Cell Biol. 139, 13–34 (2013).
Ito, Y. et al. Differential temporal expression of mRNAs for ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), interleukin-6 (IL-6), and their receptors (CNTFRα, LIFRβ, IL-6Rα and gp130) in injured peripheral nerves. Brain Res. 793, 321–327 (1998).
Lee, S., Kolset, S. O., Birkeland, K. I., Drevon, C. A. & Reine, T. M. Acute exercise increases syndecan-1 and -4 serum concentrations. Glycoconj. J. 36, 113–125 (2019).
Bouassida, A. et al. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br. J. Sports Med. 44, 620–630 (2010).
Barlow, J. P. et al. Beta-aminoisobutyric acid is released by contracting human skeletal muscle and lowers insulin release from INS-1 832/3 cells by mediating mitochondrial energy metabolism. Metab. Open 7, 100053 (2020).
Lin, W., Zhang, T., Zhou, Y., Zheng, J. & Lin, Z. Advances in biological functions and clinical studies of FGF21. Diabetes Metab. Syndr. Obes. 14, 3281–3290 (2021).
Lee, S. J. & McPherron, A. C. Regulation of myostatin activity and muscle growth. Proc. Natl Acad. Sci. USA 98, 9306–9311 (2001).
Kleinert, M. et al. Exercise increases circulating GDF15 in humans. Mol. Metab. 9, 187–191 (2018).
Andersson, H. et al. Differences in the inflammatory plasma cytokine response following two elite female soccer games separated by a 72-h recovery. Scand. J. Med. Sci. Sports 20, 740–747 (2010).
Irving, B. A. et al. Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. J. Clin. Endocrinol. Metab. 100, 1654–1663 (2015).
Bae, J. Y. Aerobic exercise increases meteorin-like protein in muscle and adipose tissue of chronic high-fat diet-induced obese mice. Biomed. Res. Int. 2018, 6283932 (2018).
Pourranjbar, M., Arabnejad, N., Naderipour, K. & Rafie, F. Effects of aerobic exercises on serum levels of myonectin and insulin resistance in obese and overweight women. J. Med. Life 11, 381–386 (2018).
Nishizawa, H. et al. Musclin, a novel skeletal muscle-derived secretory factor. J. Biol. Chem. 279, 19391–19395 (2004).
Hjorth, M. et al. The effect of acute and long-term physical activity on extracellular matrix and serglycin in human skeletal muscle. Physiol. Rep. 3, e12473 (2015).
Gopal, S. Syndecans in Inflammation at a glance. Front. Immunol. 11, 227 (2020).
Breen, E. C. et al. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J. Appl. Physiol. 81, 355–361 (1996). This paper demonstrated that a single bout of exercise can increase VEGF1 and TGFβ1 expression in muscle to promote the angiogenic response to exercise.
Smith, J. K., Dykes, R., Douglas, J. E., Krishnaswamy, G. & Berk, S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 281, 1722–1727 (1999).
Klein, A. B. et al. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat. Commun. 12, 1041 (2021).
Boulé, N. G., Haddad, E., Kenny, G. P., Wells, G. A. & Sigal, R. J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 286, 1218–1227 (2001).
Pandey, A. et al. Metabolic effects of exercise training among fitness-nonresponsive patients with type 2 diabetes: the HART-D study. Diabetes Care 38, 1494–1501 (2015).
Acknowledgements
The authors thank A. Honkala (Stanford University) and N. C. Oldenburg (University of Minnesota) for their assistance with the manuscript. The workshop that set the foundation for this Review article was supported by the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH). The listed authors acknowledge the support of the following grants. L.S.C.: NIH grant R01DK098203; B.H.G.: NIH grant U01AR071133; A.L.: Spanish Ministry of Economy and Competitiveness and Fondos FEDER (PI18/00139); C.M.: European Federation of the Study of Diabetes/MSD, Occitanie Region/FEDER funds (DIABKINES, MP0021755 and Enterosys); B.K.P.: TrygFonden for the Centre for PA Research (CFAS); A.P.: Texas Health Resources Clinical Scholarship, Gilead Sciences Research Scholar Program, NIH National Institute of Aging GEMSSTAR grant (1R03AG067960-01), and Applied Therapeutics; J.M.R.: NIH grant HL150327-01A1; H.v.P.: NIH National Institute on Aging (NIA)/NIH IRP and the FDOH Ed and Ethel Moore Alzheimer’s Disease Research program; K.I.S.: NIH grants R01-HL138738 and R01-AG060542; A.E.T.: National Institute for Health Research (NIHR) Leicester Biomedical Research Centre; J.M.T.: NIH grant 1R01HL142879; S.T.: NIH grants U01AR071133; J.R.Z.: Swedish Research Council for Sport Science (P2018-0097), Swedish Research Council (Vetenskapsrådet) (2015-00165), and Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen (NNF18CC0034900); M.P.S.: NIH grants U24DK112348 and U54DK102556. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
L.S.C. has received a Dexcom investigator initiated grant (product only). C.J.L. is a consultant for PAIhealth on their Personalized Activity Intelligence applications. A.P. is on the advisory board of Roche Diagnostics. M.P.S. is a cofounder and scientific advisory board member of Personalis, SensOmics, Qbio, January, Filtricine, Protos, NiMo, Mirvie, and an advisor for Genapsys. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks P. Atherton who co-reviewed the manuscript with D. Wilkinson, M. Fukui and E. Ziemann for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Molecular Transducers of Physical Activity Consortium: https://www.motrpac.org/
Resilience: https://ods.od.nih.gov/Research/resilience.aspx#defining
Supplementary information
Glossary
- Resilience
-
Resilience is the ability of the body to resist, adapt to, recover or grow in response to stressors.
- High-intensity interval training
-
High-intensity interval training is a form of exercise training characterized by bursts of high-intensity activity followed by less intense recovery periods.
- Exerkines
-
Exerkines encompass a broad variety of signalling moieties that are released in response to acute and/or chronic exercise that exert their effects through endocrine, paracrine and/or autocrine pathways.
- Acute exercise
-
Acute exercise is typically considered a single episode of exercise (often resistant or aerobic exercise) that is completed during one visit.
- Chronic exercise
-
Chronic exercise is typically described as multiple exercise episodes (often resistant or aerobic exercise) performed over the course of weeks to months.
- MicroRNA
-
MicroRNAs are non-protein-coding RNA molecules that are regulated in a transcriptional or post-transcriptional fashion to affect mRNA transcription and/or degradation.
- Exosomes
-
Exosomes are a type of extracellular vesicle released by parent cells, which contain RNAs, proteins and lipids, to facilitate crosstalk between tissues.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chow, L.S., Gerszten, R.E., Taylor, J.M. et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol 18, 273–289 (2022). https://doi.org/10.1038/s41574-022-00641-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41574-022-00641-2
This article is cited by
-
Effects of irisin on inflammatory and apoptotic markers in the Caco-2 colon cancer cell line
BMC Molecular and Cell Biology (2026)
-
The relationship between secondary school exam performance and lifestyle behaviors at the onset of university education
Scientific Reports (2026)
-
Serum extracellular vesicle RNA profiles in long COVID: insights from exercise-induced gene modulation
Scientific Reports (2026)
-
Physical exercise protects cardiovascular fitness in long-term spaceflight: what have we learned?
Science China Life Sciences (2026)
-
Comparative study: trimetazidine versus olanzapine on cognitive dysfunction and behavioral changes in ketamine-induced psychosis model in mice
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2025)