Abstract
This Pituitary Society Consensus article presents an evidence-based consensus on the management of pituitary incidentaloma, defined as an unexpected sellar or parasellar finding incidentally discovered on an imaging study that was not performed for a clinically suspected pituitary lesion. Recommendations are offered for when endocrinology, neurosurgery and ophthalmology consultation, dedicated pituitary imaging, pituitary hormone testing and visual assessment are warranted for macroadenomas, microadenomas, cystic lesions and empty sella, as well as when surgical resection is indicated for incidental pituitary adenomas and cystic sellar lesions. Special considerations in patients with multiple endocrine neoplasia type 1, children and adolescents, older people, and pregnant women are addressed. The Consensus workshop concluded that diagnostic and management approaches should be individualized to the specific clinical context of an incidentally discovered pituitary lesion. Consultation with a multidisciplinary pituitary tumour centre of excellence should be considered in the presence of new or deteriorating lesion-specific signs or symptoms, particularly when surgical or other adjuvant interventions are being considered and when there is uncertainty about the most appropriate subsequent management.
Similar content being viewed by others
Introduction
A pituitary incidentaloma is a sellar or parasellar finding incidentally discovered during an imaging study. Although symptoms associated with hormone excess, hypopituitarism or mass effect might be covertly present in these patients, imaging is not undertaken because of a suspected pituitary lesion. Of all lesions discovered incidentally, pituitary adenomas are the most commonly diagnosed, followed by Rathke cleft cyst (RCC) and empty sella1,2,3; vascular, inflammatory, infiltrative and infectious aetiologies are also encountered1 (Box 1). Very rarely, a double pituitary incidentaloma might be detected, most commonly with growth hormone-secreting and adrenocorticotrophic hormone (ACTH)-secreting pituitary adenomas and more rarely with an RCC4,5.
Pituitary adenomas are predominantly benign and clinically indolent, and morbidity results from hormone hypersecretion and/or growth into or compression of local structures6. Although clinically non-functioning pituitary adenomas (NFPAs) comprise only one type of incidentaloma, the terms ‘NFPA’ and ‘pituitary incidentalomas’ are often used interchangeably in the literature. MicroNFPAs, defined as adenomas <10 mm in diameter, are nearly always detected with no overt signs or symptoms, show no hormone secretory activity, and have a low likelihood of inducing hormonal deficits. MicroNFPAs are generally managed with regular observation rather than through surgical resection7. MacroNFPAs, defined as ≥10 mm in diameter, are more likely than microNFPAs to be associated with visual field changes or cavernous sinus invasion at the point of NFPA identification8. Characteristics of NFPAs found incidentally or due to symptoms might differ markedly. MacroNFPAs are managed conservatively in many countries9, enabling longitudinal study of NFPA behaviour. Thus, this conservative practice has enabled a generalized understanding of how a pituitary adenoma with no or few overt clinical symptoms might progress over time.
Within the constraints of limited evidence-based reports, this Pituitary Society Consensus summarizes the epidemiology and natural history, diagnostic work-up, and treatment options for patients with pituitary incidentalomas. We distinguish between pituitary adenomas and other types of incidentalomas and particularly distinguish between microadenomas and macroadenomas given the lower likelihood of the latter to be a truly incidental finding.
We offer recommendations on a range of topics: when referral to specialists is warranted; when to pursue more advanced imaging, endocrine and other clinical evaluation and diagnostic studies; and when to consider surgery for each type of lesion detected. We present management approaches for incidentalomas discovered in unique populations, including those with multiple endocrine neoplasia type 1, children, adolescents, older individuals and pregnant women.
Overall, diagnostic and management approaches should be individualized to the specific clinical context. In the presence of new or deteriorating signs or symptoms, consultation with a multidisciplinary pituitary tumour centre of excellence should be considered10,11. These centres bring together endocrinologists, neurosurgeons, neuroradiologists, ophthalmologists, pathologists and radiation oncologists with expertise in the diagnosis and treatment of pituitary disease. Such a collaborative, experienced approach might be particularly important for patients with pituitary incidentalomas in whom surgical intervention is being considered and when there is uncertainty about the most appropriate next step in management10,11.
Methods
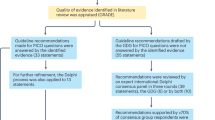
The Pituitary Society hosted four virtual meetings of the Incidentaloma Consensus Group between January and September 2024 to draft and discuss recommendations. Workshop Chairs (M.F. and S.M.) identified topics related to diagnosis and management to be addressed and assigned them during a virtual Steering Committee planning meeting comprising members selected for their expertise based on their respective publication records and recognized standing in the field (members: M.F., M.G., A.M., H.F., A. Glezer, F.L., T.H.S., Y.G., N.K. and S.M.). Evidence-based topic summaries were developed and entailed critically reviewing English-language, PubMed-indexed papers published before January 2024 using search terms including “pituitary incidentaloma” and “incidentaloma” with terms associated with topics for discussion, including “epidemiology”, “natural history”, “diagnosis”, “imaging”, “surveillance”, “hormones”, “hypopituitarism”, “symptoms”, “visual field testing” and “surgery”. Each of these terms was also considered within the context of the lesion type, including “pituitary adenoma”, “arachnoid cyst”, “Rathke cleft cyst”, “craniopharyngioma” and “empty sella”. The written summaries and draft recommendations were reviewed by all Steering Committee members in four rounds and discussed during subsequent virtual meetings.
In September 2024, recommendations were sent to the full Consensus Group, comprising 40 expert endocrinologists and neurosurgeons representing 21 countries with different health-care systems, then discussed during a virtual meeting in small breakout groups. Key discussion points and suggested changes were reported to the entire group, after which consensus recommendations were finalized based on majority opinion.
Previously published consensus statements from the Pituitary Society used GRADE principles for grading of evidence supporting recommendations12,13. Recommendations based on very-low-quality or low-quality evidence were graded as weak, and those based on medium-quality or high-quality evidence were graded as strong14,15. As there is no high-quality evidence and very limited medium-quality evidence for the management of pituitary incidentaloma, we considered factors that affect the strength of the GRADE expert recommendation, such as balance between desirable and undesirable effects13, and distinguish between stronger ‘recommended’ and weaker ‘suggested’ recommendations. Consensus was achieved by voting for all recommendations (labelled recommendation 1 (R1), R2 and so on).
After the meeting, the approved consensus recommendations and all discussion points were collated. Data published after the initial cut-off date of January 2024 were identified in subsequent literature reviews using the same keywords and were added as appropriate. The draft manuscript was then circulated to all authors for final approval.
Epidemiology and natural history
Epidemiology
The frequency of pituitary incidentalomas is derived from autopsy, imaging and population studies1,7,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46. A brief overview of available data is provided here. See Supplementary Box 1 for additional details.
Incidentally detected yet unsuspected sellar and/or parasellar lesions at autopsy are, by definition, not incidentalomas, as they are not identified on imaging for another indication. Nevertheless, these studies are often considered when surveying the incidentaloma epidemiology as they are indicators of the frequency of lesions that, had imaging been performed, might have been detected during the patient’s lifetime.
Not surprisingly, autopsy studies show the highest rates of incidental pituitary adenomas16,47 (Supplementary Table 1). A survey of 33 studies comprising >19,000 autopsies showed a total of 2,084 lesions discovered, yielding a mean prevalence of 10.7% (range, 1.5–31.1%)16. By contrast, when limited to pituitary incidentalomas detected on MRI, studies show a 3.4% pooled frequency; however, the populations included in the studies and the methods used were heterogeneous (Supplementary Table 2). A meta-analysis of 29 MRI studies comprising 38,406 patients reported a crude estimate for pituitary neoplasms of 2.7 (95% CI 1.7–4.0) per 1,000 scans30.
Population-based studies estimate the overall frequency of pituitary adenomas within a specific region; however, these studies do not all distinguish pituitary adenomas that are incidentally detected. The prevalence of clinically relevant pituitary adenomas based on cross-sectional regional reports is 78–94 cases per 100,000 persons37,38. Country-specific standardized incidence rates have increased over the years, rising from 0.59–0.73 cases per 100,000 to 1.6–2.0 cases per 100,000 persons per year over a span of 8–14 years39,40 (Supplementary Box 1).
Pooled estimates across 18 clinical studies comprising >2,000 patients suggest that approximately 43% of incidental pituitary adenomas detected on imaging are macroadenomas (Table 1); however, reported rates vary widely8,27,48.
Natural history
NFPAs found incidentally are often followed conservatively, which enables estimates of the overall natural history of incidentalomas7,8,23,26,44,49,50,51,52,53,54,55,56,57,58,59,60. A systematic review of 11 studies61 found that 3.3 (95% CI 2.1–4.5) per 100 person-years of microadenomas and 12.5 (95% CI 7.9–17.2) of macroadenomas grew. However, stability, growth and even adenoma shrinkage are variably reported, with heterogeneity attributed to differences in study design, cohort size, imaging techniques and duration of follow-up, as well as the inclusion of patients with other pituitary pathologies. Using these reports to inform intervals for follow-up in management algorithms is therefore challenging. See Supplementary Box 2 for details.
Diagnosis
Headache, trauma, cerebrovascular symptoms, internal ear dysfunction, cognitive deficits and visual disturbances are common clinical features that trigger an imaging procedure with subsequent detection of a pituitary incidentaloma1,2,3,7,8,16 (Box 2).
Primary care and internal medicine physicians, neurologists, otolaryngologists, ophthalmologists, emergency room physicians and oncologists are most likely to order imaging for these symptoms1,44. Although headache might be partially related to, or caused by, the incidentaloma, a sellar lesion might not be considered prior to its incidental detection on imaging.
When is an endocrinology consultation warranted?
-
R1. We recommend that an endocrinologist be consulted, when possible, for patients with newly discovered pituitary incidentalomas to establish an initial diagnosis, undertake hormonal evaluation and detailed imaging, and establish the frequency of subsequent evaluations.
-
R2. We recommend consultation with an endocrinologist and, if appropriate, also a neurosurgeon, especially for pituitary incidentalomas with one or more of the following features that might indicate a need for early intervention:
-
Adenoma maximal diameter ≥10 mm or cavernous sinus invasion
-
Evidence of 15–20% growth in maximum diameter compared with previous imaging studies
-
Visual field deficits or localization of the lesion abutting or near the optic chiasm or in the suprasellar space
-
Ophthalmoplegia or severe headaches
-
Clinical signs and symptoms suggestive of hypopituitarism or hormone hypersecretion
-
Desire for pregnancy or diagnosis during pregnancy
-
Following detection of a pituitary incidentaloma, imaging, endocrine and clinical evaluation should determine a need for subsequent surveillance and/or surgical intervention. Personalized assessment should consider the risks associated with an increase in lesion size, specifically as it relates to mass effect, hormonal dysfunction and vision loss. Patients with lesions showing a 15–20% increase in maximum diameter on repeat imaging should be referred to an endocrinologist or neurosurgeon. Although such growth might not signal a need for intervention, close observation is warranted for a clearly growing pituitary mass. For all lesions, a multidisciplinary team approach comprising endocrinologists, neurosurgeons and neuroradiologists should be considered, particularly when there is uncertainty about appropriate management.
The cost burden of regular surveillance imaging and subsequent hormonal testing is an important consideration in managing pituitary incidentalomas. An informal survey of Consensus Group members found that the cost of an MRI in countries with a national health system can range from a low of US$ 175 equivalent in a public hospital to a high of US$ 750 equivalent if performed at a private clinic or hospital. In the USA, the cost of an MRI can be more than US$ 5,000. The cost of a pituitary hormone panel can be as high as US$ 500 equivalent at a private clinic outside of the USA and at least twice that in the USA. Determining the clinical need for each assessment in each patient can help minimize the cost burden on both the patient and the health-care system.
When is dedicated pituitary imaging warranted?
At diagnosis
-
R3. We recommend performing an MRI with pituitary-dedicated sequences for most patients after incidental detection of a pituitary or sellar lesion on other brain imaging (for example, CT, brain MRI or 18F-fluorodeoxyglucose (FDG) PET-CT).
-
R4. We recommend that, unless contraindicated, gadolinium enhancement be used with the pituitary MRI to improve characterization of the incidental pituitary lesion.
-
R5. We recommend, where possible, use of a standardized imaging protocol to include T1-weighted spin echo MRI in coronal and sagittal planes pre-gadolinium and post-gadolinium, as well as T2-weighted fast (turbo) spin echo MRI in the coronal plane, with a slice thickness ≤3 mm.
-
R6. We suggest that dedicated pituitary imaging might not be required when existing images provide sufficient information for possible diagnosis.
Intervals for regular surveillance
-
R7. We recommend that timing of the first imaging procedure after a diagnosis be individualized. In the absence of new or worsening signs or symptoms, acceptable intervals are 2–3 years for microadenomas, 1 year for macroadenomas that are ≥5 mm away from the optic chiasm and 6–12 months for macroadenomas that are <5 mm from the optic chiasm, assuming that surgical resection is not undertaken.
-
R8. To further determine the subsequent imaging interval, we suggest considering age and the rate and direction of adenoma growth after the first repeat MRI, as well as the adenoma consistency (solid versus cystic), intensity on T2-weighted images and pituitary stalk deviation.
-
R9. We recommend repeat MRI every 1–2 years for slowly enlarging macroadenomas and for stable macroadenomas that are <5 mm away from the optic chiasm. Enlarging or invasive (including cavernous sinus extension) macroadenomas could require closer imaging intervals and could be considered for surgical intervention.
-
R10. We recommend repeat MRI every 2–3 years for stable macroadenomas that are ≥5 mm away from optic chiasm.
Discontinuing surveillance imaging
-
R11. In the absence of new or worsening signs or symptoms, we suggest reassessing the need for regular surveillance imaging of microadenomas that are <5 mm in size and that remain stable on repeat MRI performed 2–3 years after the initial MRI, especially in patients who have reduced life expectancy, poor functional status or contraindication to surgery. Consensus was not reached on when to stop imaging. Some members of the Consensus Group considered suggesting repeat imaging after another 5 years, depending on clinical circumstances, for example, in patients <40 years of age.
Indications for MRI with pituitary-dedicated sequences
An incidentally detected sellar or parasellar lesion might not be adequately characterized on initial brain imaging, and additional dedicated pituitary-focused imaging sequences might be required1,62.
We do not recommend routine CT, as soft tissue contrast is inferior compared with MRI for imaging adenomas, and invasion of adjacent structures, including the cavernous sinus, is less readily appreciated22,63. Sellar or parasellar lesions might be identified on a wide array of magnetic resonance sequences, including during imaging of the cervical spine, where the pituitary gland is often visualized on sagittal sections. However, magnetic resonance sequences and planes vary according to the primary indication for intracranial imaging. With use of thicker sections (such as 5 mm slices), the sellar region is often only captured on one or two images in each plane, and the pituitary might be visualized opportunistically for some indications.
Despite case reports of pituitary lesions detected incidentally on PET64,65, multiple retrospective series of patients undergoing whole-body 18F-FDG PET-CT for other clinical indications demonstrate focally increased pituitary uptake in <1% of patients34,35,36. Nevertheless, further evaluation of these findings can yield high rates of pituitary pathology. In a chart review of 40,967 patients, incidental pituitary FDG uptake was seen in only 30 patients (an incidence rate of 0.073%), after excluding those with a history of pituitary adenoma or other lesions. Yet, of these 30 patients, a pituitary adenoma was found in 18 of 19 patients who underwent follow-up MRI, and none was a metastasis34. Similarly, in two other studies of 13,145 and 24,007 patients, incidental pituitary FDG uptake was observed in 0.8% and 0.13% of patients, respectively. Pituitary adenomas, metastatic spread of a primary tumour, and non-adenomatous inflammatory or infiltrative lesions were all detected35,36. Focal uptake of other PET radiotracers (such as 68Ga-DOTATATE, 68Ga-DOTATOC and 18F-choline) by a pituitary adenoma has been described in a small number of reports, precluding accurate estimates of incidence rates66,67.
In patients exhibiting increased pituitary uptake on PET performed for another indication, dedicated MRI of the pituitary gland and surrounding structures is indicated due to the high probability of identifying primary sellar pathology63,68. Discussion with the patient is warranted when there are notable comorbidities or limited life expectancy that might justify a conservative approach63.
Adoption of a standardized MRI protocol increases the likelihood of correctly identifying the nature of an incidentally discovered pituitary lesion and facilitates comparison with subsequent follow-up scans. A fine matrix (for example, 256 × 256 or greater) and a small field of view (16–18 cm) are favoured to focus on the sella and immediate surrounding structures63,64,69,70.
Gadolinium enhancement should be used for dedicated pituitary MRI; however, concerns regarding the effects of repeated exposure to gadolinium-based contrast agents have led to reconsideration of which contrast agents to use as well as when contrast enhancement might not be required as a result of increased use of T2-weighted sequences71,72. Nevertheless, the acquisition of a complete set of baseline pre-contrast and post-contrast images is important to facilitate optimal diagnosis and potentially reduce or eliminate the need for follow-up imaging63. CT witout contrast enhancement might be considered to assess mass calcification and skull base bony extension64,69.
Factors influencing intervention versus regular surveillance
Although they are not all well defined, several factors predictive of incidentaloma enlargement might help identify patients who are likely to benefit from surgical intervention. Studies of NFPAs where the patients are undergoing observation versus surgery inform the likelihood of adenoma progression without intervention7,8,59. In general, macroadenomas grow more rapidly than microadenomas. Macroadenomas are also more likely to show symptomatic enlargement, with visual field deficits, new or deteriorating pituitary hormone deficiencies, and apoplexy6,61,73.
Adenoma size
Larger size at diagnosis has been widely studied as a potential risk factor for subsequent lesion growth. Accordingly, a new proposed comprehensive classification for pituitary adenoma prognosis74 includes adenoma size as a risk factor in a calculated score to assess pituitary disease severity.
In a meta-analysis of 19 studies including 1,057 patients with NFPAs, macroadenomas were more likely to undergo growth (34% versus 12%; P <0.01) or apoplexy (5% versus <1%; P = 0.01) compared with microadenomas75. The incidence of macroadenoma growth is 10–14 events per 100 person-years versus 2–5 per 100 person-years for microadenomas50,51,59,61. Approximately 40–45% of macroadenomas show increased diameter or volume within 3–4 years8,52 and an additional 5–10% grow when followed up for >4 years58. Compared with microadenomas, macroadenomas are associated with a fivefold higher risk of apoplexy, fourfold increased risk of visual field deficits and a threefold higher risk of pituitary hormone deficits61. Nevertheless, growth is not linear; most macroadenomas and microadenomas remain unchanged in size over time and some might shrink or disappear on follow-up imaging7,50,76. Furthermore, the probability of enlargement increases with longer follow-up times. For instance, in some studies, increases were only seen after 24–60 months of follow-up, regardless of initial adenoma size, with some patients showing growth up to 14 years after diagnosis24,48,50,51,55.
Two series found fairly low growth rates for microadenomas. Among 133 incidental NFPAs and non-pituitary sellar masses, overall, adenoma growth occurred in 2% at 1 year, 5% at 2 years and 9% at 3 years. Yet, when considering growth based on size, none of the microadenomas <5 mm and 14% of those ≥5 mm grew during follow-up, compared with 25% of macroadenomas27. Another study of 271 microNFPAs found a low growth rate of 8% over approximately 3 years (range, 3–154 months)50. Initial adenoma size, sex and age did not affect median time to growth. Among the 92% of adenomas that did not grow over time, 10% shrunk and 25.8% were not visible on last MRI; adenomas <5 mm in size had a higher likelihood of shrinkage than those ≥5 mm (ref. 50). In 177 patients with microadenomas, approximately two-thirds of the microadenomas remained unchanged or decreased in size during long-term follow-up77.
Adenoma size can drive referral for surgical resection, which was undertaken in 20–30% of patients in whom macroadenoma enlargement occurred52,55,59,78, versus <4% of patients who did not exhibit macroadenoma enlargement51,52. The effect of growth on clinical symptoms, and particularly on visual function, is also an important driver of surgical resection56. A growth rate of 5.9 mm per year51 as well as an increase >3.5 mm overall61 predicted deteriorating visual function, and a macroadenoma volume growth rate >10 mm3 per month assessed at 2 years was highly predictive of the requirement for surgery52. Untreated macroNFPAs can also shrink over time. In the UK NFPA Consortium of 949 macroNFPAs, the cumulative probability of adenoma shrinkage was 9.6% after 5 years of follow-up59, whereas 20% of 49 patients followed up for a mean of 4.9 years showed a decrease in size (mean, 3.5 ± 1.3 mm)79.
Mass effects
Clinically meaningful growth is more frequent in macroadenomas than in microadenomas. In a cohort of 42 patients (88% of whom had macroadenomas), 24% showed symptomatic enlargement with visual symptoms, panhypopituitarism and apoplexy 4 years after diagnosis24. Although size and likelihood of enlargement did not correlate, the risk of symptomatic enlargement increased in those with adenoma height ≥15 mm versus those with adenoma height <15 mm (P = 0.007)24. By contrast, in 271 microNFPAs, 8.1% showed a mean size increase of 3.1 mm (SD 1.0) over a median follow-up time of 29 months (range, 3–154 months)50.
Visual field deficits are uncommon and almost invariably occur with macroadenomas rather than with microadenomas. A meta-analysis reported a low rate of visual symptoms of 0.65 per 100 person-years (95% CI 47–0.82) in untreated patients with pituitary incidentalomas61. New hypopituitarism or worsening pituitary function was seen in 4% of 197 patients with untreated NFPAs followed up for a median of 37 months (95% CI 12–170)51. Higher rates of hypopituitarism have been reported in those with macroadenomas than in those with microadenomas, with approximately 8% of patients with macroadenoma and no patients with microadenoma developing hypopituitarism in a series of 50 patients followed up for 3 years52.
Pituitary apoplexy rates in patients with macroadenomas vary from 3% in patients followed up for a median of 35 months to 14% in those followed up for 85 months51,58 and might be associated with both adenoma size enlargement51,76 or reduction58. Adenoma size is one of the few independent predictors of apoplexy80. In 385 patients with NFPA treated surgically, 75% of whom had macroadenomas, clinically silent apoplexy was identified in 13% of patients81. Apoplexy is rarely encountered in microadenomas7,51,52. Apoplexy can be the first manifestation of a pituitary incidentaloma82 or might be identified only on imaging or sometimes during surgery24, and could be precipitated by head trauma82,83,84,85,86. Nevertheless, apoplexy was rarely encountered in a systematic review of 11 studies of pituitary incidentalomas, with an overall incidence of 0.6 per 100 person-years61.
Adenoma consistency
Determining adenoma consistency based on imaging alone can be challenging87. However, such information could be useful as incidental lesions appearing solid on imaging and presumed to be NFPAs are more likely to enlarge than cystic lesions. Of 265 incidental lesions not surgically resected, risk of enlargement was higher for NFPA than for non-pituitary sellar masses, 75% of which were RCCs or other cysts, with an odds ratio of 3.92 (95% CI 1.27–12.07; P = 0.017)27. Two other studies showed that 20% of solid lesions presumed to be NFPAs increased in size during follow-up of 51 months and 23 months compared with 5% of lesions presumed to be RCCs followed up over 23–24 months55,78. In the UK NFPA cohort, the presence of a cystic or haemorrhagic component predicted macroNFPA shrinkage (HR 0.30, 95% CI 0.19–0.48 for purely solid lesions)59.
Intervals for imaging during regular surveillance
Previous guidance recommended considering the size of an incidentally discovered pituitary adenoma together with other key clinical features to inform the need and timing for subsequent surveillance62,63,68. For example, a macroadenoma with aggressive biology in a patient <65 years old has a potentially higher risk of enlargement than in an older patient and requires closer surveillance88. Yet, it remains difficult to define independent predictors of outcomes based on presenting features; indeed, some studies did not identify any such factors8. Potentially predictive factors for increasing adenoma size include adenoma location <1 mm from the optic chiasm51 and adenomas >15 mm in size at diagnosis24. Age was not an independent predictor of the need for surgery in one series27 although, in another study, progression was found to be more likely in women aged >50 years than in women aged ≤50 years or in men60. Both male sex59 and female sex60 predicted growth. Pituitary stalk deviation78 as well as presence of hormone deficiency at diagnosis44 are associated with pituitary incidentaloma progression. In addition, contrast enhancement on T1-weighted images and high signal intensity on T2-weighted images were more common among incidentalomas increasing in size than in those that remained stable78.
Although methodologies to assess risk and predictors of progression vary between studies, any of these findings could potentially prove helpful when defining intervals for imaging during regular surveillance. The presence of new or worsening signs or symptoms should prompt repeat imaging regardless of the planned interval schedule, and a need for intervention rather than continued surveillance should be determined.
Macroadenomas
MRI of incidental macroadenomas has traditionally been recommended every 6 months for the first 2 years then annually for 3–5 years55,62; however, data from the UK NFPA showed less growth in the first year than in every subsequent year up to 5 years59. Generally acceptable imaging intervals after the initial finding are 1 year for macroadenomas ≥5 mm from the optic chiasm and 6 months for macroadenomas <5 mm from the optic chiasm, assuming surgery is not performed. If size remains stable, imaging can be performed less frequently and eventually discontinued according to the patient and adenoma characteristics.
Microadenomas
Recommendations for surveillance of microadenomas consider the lower likelihood of clinically significant lesion growth during follow-up compared with macroadenomas. Based on large observational studies published in the past 5 years, the first repeat MRI should be performed 2–3 years after diagnosis, unless the lesion is in close proximity to the optic chiasm7,50,51.
Microadenoma size could be used to guide intervals. For small microadenomas (those <5 mm), some published reviews suggest no imaging surveillance is needed68,89. A more prudent approach might be to discontinue surveillance of microadenomas shown to remain stable on repeat MRI performed 2–3 years after the initial MRI. However, some members of the Consensus Group suggested repeat imaging after another 5 years, depending on clinical circumstances, before discontinuing surveillance. For microadenomas ≥5 mm in size, a review suggested MRI at 6 months, then annually if growth is detected, and repeated at 2 years only if the microadenoma remains stable68. More frequent imaging can be considered in rare instances of microadenomas in close proximity to the optic chiasm.
Adenoma growth rate
Regular MRIs during the first 1–2 years after diagnosis can be used to assess incidentaloma growth rate58, which might inform subsequent imaging intervals. For example, a study of 197 NFPAs evaluated cut-off values for adenoma growth between the first and second annual MRIs and found that volumetric growth ≥0.88 cm3 per year predicted deteriorating visual function. Thus, for adenomas growing more slowly than this cut-off, delay of repeat MRI might be warranted51. Generally, however, annual repeat MRI is warranted for slowly enlarging macroadenomas and stable macroadenomas <5 mm from the optic chiasm versus MRI every 2–3 years for stable macroadenomas ≥5 mm from the optic chiasm (Fig. 1).
Type of assessment to be performed and timing of assessment depend on clinical and tumour features. If initial imaging was a brain MRI or CT, dedicated pituitary MRI with specific sequences is desired in most cases. Patients should undergo pituitary function testing at presentation to rule out hypopituitarism and/or hormone hypersecretion and should undergo visual assessment (including visual field testing). Further repeat evaluation depends on adenoma size and proximity to the optic chiasm. If a microadenoma <5 mm in size remains stable on repeat MRIs performed at intervals of 2–3 years, reassess the need for continued imaging. For macroadenomas and microadenomas <5 mm from the optic chiasm, visual field testing could be performed in the interval between MRIs done after 6–12 months. Evaluation and follow-up for cystic lesions, cystic adenomas, or other sellar or parasellar masses, including Rathke cleft cyst and craniopharyngiomas, should be individualized based on both patient and lesion characteristics. *We recommend that patients with documented hormone hypersecretion or hypopituitarism be followed up as per current guidelines. **CT of head and/or sella might help confirm a diagnosis of craniopharyngioma, chordoma or other non-pituitary lesions.
Factors influencing consultation with an expert neuroradiologist
Although many centres are equipped to deliver optimized pituitary imaging, interpretation is not always straightforward and might require special expertise. For example, normal pituitary physiology can vary during adolescence, menopause or pregnancy. Cystic lesions with haemorrhagic components might be difficult to assess, and less experienced radiologists might overestimate the importance of a subclinical sellar or parasellar haemorrhage or one that might resolve without intervention90.
Early consultation with a neuroradiologist with expertise in pituitary imaging and with the broader pituitary tumour centre of excellence team is advised when intervention is being considered or when there is uncertainty about the nature and consistency of a lesion or the most appropriate next step in management10,11 (Fig. 2).
Management decisions are based on whether initial work-up and/or additional dedicated sellar or parasellar imaging is sufficient for diagnosis or whether referral for expert evaluation at a pituitary tumour centre of excellence (PTCOE) is required. (1) Examples of incidental lesions in which initial imaging, together with clinical and laboratory assessment, enabled diagnosis and management without requiring additional baseline imaging. Top, 25-year-old woman with incidental Rathke cleft cyst (yellow arrows) detected during migraine evaluation. Bottom, 27-year-old man with incidental enlarged, partial empty sella due to an arachnoid cyst (yellow arrows) detected during evaluation for limb weakness and sensory loss. (2) Examples of incidental lesions in which additional dedicated sellar or parasellar imaging was required to ascertain the diagnosis and guide management. Top, 70-year-old woman with incidental cavernous sinus aneurysm (yellow arrows) detected during evaluation for hearing loss and tinnitus. Bottom, 62-year-old man with incidental pituitary macroadenoma (yellow arrows) detected during investigation of sudden onset dysphasia. (3) Examples of incidental lesions in which uncertainty remained following dedicated sellar or parasellar imaging, necessitating further assessment at a PTCOE. Top, 59-year-old man with incidental left parasellar mass (yellow arrows) detected during evaluation of retro-orbital headache with diplopia. The nature of the lesion remained unclear on dedicated sellar and parasellar imaging. A biopsy revealed a pituitary adenoma. Bottom, 45-year-old woman with incidental sellar mass (yellow arrows) detected during evaluation for frontal headaches. Initially, a pituitary adenoma was suspected. However, review by an expert pituitary neuroradiologist suggested a broader differential diagnosis, considering the atypical appearance and possible clival invasion. At surgery, a chordoma was successfully resected. *Lesion might be evident on careful review of historical imaging (for example, brain CT or MRI, or cervical spine MRI). FIESTA, Fast Imaging Employing Steady-state Acquisition; Gad, gadolinium; T1, T1-weighted; T2, T2-weighted.
Endocrine evaluation
When is pituitary function testing warranted?
At diagnosis
-
R12. We recommend that pituitary hormone testing be performed in all patients with incidental sellar lesions at initial presentation, irrespective of size, as well as in patients with incidentally discovered empty sella. Presence or absence of symptoms might not correlate with pituitary dysfunction at initial presentation.
-
R13. We recommend screening for prolactin and growth hormone hypersecretion by measuring levels of prolactin and insulin-like growth factor 1 (IGF1) in all patients with incidentally discovered pituitary adenomas; screening for hypercortisolism should be performed if clinically warranted.
-
R14. We recommend baseline screening for hypopituitarism in all patients with sellar lesions by measuring levels of free thyroxine (T4), thyroid-stimulating hormone (TSH), cortisol (in the morning), ACTH, luteinizing hormone, follicle-stimulating hormone (FSH) and IGF1 levels; testosterone in men and oestradiol in women should be measured as needed. Dynamic testing of the hypothalamus–pituitary–adrenal axis should be performed if morning cortisol levels suggest hypocortisolism. Dynamic testing for growth hormone deficiency might be required in select patients after correction of other pituitary hormone deficiencies. Premenopausal women with regular menses do not require gonadal hormonal testing.
During follow-up
-
R15. We recommend patients with documented hormone hypersecretion or hypopituitarism be followed as per current guidelines for these conditions.
-
R16. We suggest repeat pituitary hormone evaluation in all patients with microadenoma and macroadenomas at 1–2 years or earlier in the presence of new clinical symptoms, as new hypopituitarism, albeit not frequently, might develop without evidence of concurrent lesion growth.
-
R17. We recommend repeat pituitary hormonal evaluation in patients with enlarging microadenomas and macroadenomas.
Screening for a functioning pituitary adenoma
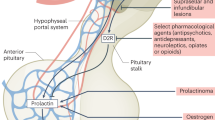
Prolactinomas comprised 6–18% of incidentalomas in pooled case series (Table 1). Of 159 surgically resected incidentalomas, 6.9% were prolactin-secreting and 5% growth hormone-secreting91. A smaller series of 65 patients with incidentalomas undergoing endocrine evaluation found rates of 6.2% for prolactin-secreting and 3.1% for growth hormone-secreting adenomas92. An evaluation of 35 incidentalomas found that 9% were growth hormone-secreting adenomas93, and in an autopsy study that identified 334 clinically silent pituitary adenomas, 39.5% stained for prolactin, 13.8% for ACTH and only 2% for growth hormone17.
Variation in rates of growth hormone-secreting adenomas might relate to unrecognized clinical evidence of growth hormone hypersecretion. Of 112 patients with surgically treated acromegaly, 17% were diagnosed following an incidental image finding94. Yet, 58% of these patients had ‘look back’ clinical features of acromegaly, and 47% presented with otolaryngology manifestations, including hearing loss, sinusitis and tinnitus. Similar symptoms were the most common presenting features prompting imaging in a study of 67 incidentally diagnosed pituitary adenomas, and evaluation revealed loss of libido in the three men with macroprolactinoma and oligomenorrhea in 1 of 5 women with macroprolactinoma26.
Screening for Cushing syndrome in the absence of clinical features is typically not recommended95. Screening tests for hypercortisolism have high false-positive rates15, which could prompt unnecessary patient anxiety. Nevertheless, in 68 consecutive patients with pituitary incidentaloma but without clinically overt hypercortisolism, 7.3% met biochemical criteria for Cushing disease and 4.4% had confirmed ACTH on histology of surgically resected tissue96. Similarly, in 105 patients without clinically overt hypercortisolism, 4.8% were diagnosed with Cushing disease after surgery97. It is not known whether ACTH-secreting adenomas without overt hypercortisolism confer an increased risk of cardiovascular, bone and other adverse effects associated with lengthy hypercortisolism15. Therefore, screening should be performed for clinical suspicion based on features and/or comorbidities suggestive of Cushing disease, even in the absence of classic hypercortisolism characteristics98.
As clinical evidence of a functioning pituitary adenoma might be subtle and/or might slowly progress over time16, initial evaluation of prolactin and growth hormone axes should be undertaken in all patients with incidentally discovered sellar lesions62,68. If initial endocrine assessment excludes a secretory adenoma, further assessment is not indicated if not clinically required or if no signs of an aggressive adenoma are found during surveillance. If screening is suggestive of hormone hypersecretion or if new clinical features appear, further testing and follow-up should be performed according to Cushing disease15, acromegaly99 and prolactinoma14 guidelines. Switch of a previously documented silent adenoma to a functioning, growth hormone-secreting or ACTH-secreting adenoma could signal a potentially aggressive pituitary tumour100 requiring intervention.
Screening for hypopituitarism
Hypopituitarism is reported in up to half of patients with incidentalomas of various aetiologies, including pituitary adenomas, RCCs and other sellar lesions. Deficiency of luteinizing hormone and/or FSH, TSH and growth hormone are the most common, and arginine vasopressin deficiency (AVP-D; formerly known as diabetes insipidus) is fairly uncommon91,92,101.
In general, hormone deficit is less commonly encountered in patients with microadenomas than in those with macroadenomas7,44,52,93,102. However, the risk of developing new endocrinopathies is not different (0.9 and 2.1, respectively, per 100 person-years; P = 0.15)9, and larger adenoma size does not always predict worsening hormone function27,29,56. Clinical symptoms also do not necessarily predict hormone dysfunction29,73. Pituitary function testing is typically recommended for macroadenomas regardless of symptomatology but recommendations for testing in cases of microadenoma are less consistent3,16,103.
Adenoma size
Retrospective analyses support the relevance of lesion size in contributing to the likelihood of hypopituitarism but careful literature interpretation is required. In 903 patients with sellar masses of various aetiologies (pituitary adenoma, RCC, craniopharyngioma and meningioma), 31% of 222 patients with pituitary incidentalomas had hormone deficiency in at least one axis, particularly hypogonadism and hypothyroidism101. Macroadenoma (P = 0.002) and male sex (P = 0.006) increased the risk of new or worsening hormone deficiency; as 71% of incidentalomas were macroadenomas, adenoma size might have contributed to the hypopituitarism.
In 371 patients with NFPA, at least one hormone deficiency was evident at diagnosis in 37% of those with macroadenoma and 11% of those with microadenoma (P <0.001). A macroadenoma was a significant predictor of hormone dysfunction (OR 3.38, 95% CI 1.81–6.29; P <0.001)56. Importantly, however, 3% of 187 patients with both imaging and hormone function and at least 3 years of follow-up showed worsening pituitary dysfunction even without evidence of adenoma growth56.
Results from the UK NFPA Consortium show that approximately 10% of 459 patients with microNFPAs had hypopituitarism, with 7.2% having hypogonadism and <2% having hypothyroidism and hypoadrenalism7. By contrast, in the macroNFPA cohort, re-evaluation of previously intact pituitary hormone axes showed biochemical evidence of new-onset hypoadrenalism, hypogonadism and hypothyroidism in 4.0%, 4.6% and 4.9% of patients, respectively, and pituitary failure in up to 2.2% of patients, despite imaging evidence of adenoma stability59.
Clinical symptoms
Comparison of incidentally discovered (n = 131) versus clinically symptomatic (n = 138) NFPAs that were >6 mm in 269 patients demonstrated more hypopituitarism in the symptomatic group than in the incidental group (58.7% versus 27.4%; P <0.0001). However, neither the negative predictive value of the lack of symptoms (71%) nor the area under the curve proved helpful in guiding a need for pituitary testing29.
Screening for pituitary dysfunction in patients with RCC
Hypopituitarism in patients with incidental RCCs is not well described, as studies vary with regard to the number of affected axes and diagnostic tests used to assess pituitary function104. Among 62 of 134 patients with RCCs not treated surgically, 48% of which were incidentally discovered, 16% had hypopituitarism at diagnosis, mostly hypoadrenalism (9.7%)105. After 37 months of follow-up, the rate of hypopituitarism decreased; hyperprolactinaemia resolved in 5 of 10 patients and no new deficits were noted, including in those with cyst enlargement. Nevertheless, maximum cyst diameter >10 mm correlated with hormone dysfunction (P = 0.04), suggesting a need for pituitary function monitoring in patients with cysts >10 mm (ref. 105). As 25% of patients with resected RCCs exhibit hypocortisolism at diagnosis106, hypothalamus–pituitary–adrenal axis testing is indicated at baseline.
Screening for pituitary dysfunction in patients with empty sella
Hypopituitarism rates are considerably higher in patients diagnosed with empty sella based on clinical suspicion than in those diagnosed incidentally107. Function and pituitary volume do not correlate, although patients with total empty sella are more likely to have hypopituitarism than those with partial empty sella108. Testing in 402 patients with primary empty sella revealed that 40.5% exhibited one or more axis deficiencies, with a rate of 29% in the 289 patients diagnosed incidentally107. Hypothyroidism (29.2% versus 2.8%), growth hormone deficiency (20.4% versus 12.5%), hypogonadism (50.4% versus 8.5%) and hyperprolactinaemia (15.7% versus 2.9%) were more frequent in patients with clinically suspicious pituitary disease than in those diagnosed incidentally, whereas rates of hypocortisolism (17.7% versus 13.5%) and AVP-D (1.7% versus 0.9%) were similar. Male sex (P = 0.02), clinical suspicion (P <0.001) and traumatic brain injury (P = 0.003) predicted hypopituitarism. Progression from partial to total empty sella could be associated with hormonal deterioration (P = 0.006)107.
Clinical evaluation
Cranial neuropathy, hypopituitarism and/or hormonal excess might develop over time in patients with pituitary incidentaloma due to central compressive effects, local inflammation or autonomous hormone secretion3,6,62. Headache, visual field disturbance, diminished visual acuity, diplopia and, extremely rarely, hypothalamic dysregulation and cerebrospinal fluid rhinorrhoea might occur. Ideally, clinical evaluation of patients with diagnosed incidentalomas should be undertaken by a collaborative multidisciplinary team.
Indications for repeat endocrine evaluation
New hormone deficiencies
The incidence of new-onset pituitary hormone deficiency in patients with pituitary incidentaloma ranges from 1.2 to 2.4 per 100 person-years51,52,56,61. Considerably higher rates, up to 11.9 per 100 patient-years, are encountered with some macroadenomas61.
The frequency of new-onset pituitary hormone deficiency associated with mass growth varies widely, with deficiency of ACTH seen in 0.2–39.0%, of growth hormone in 0.2–31.8%, of TSH in 0.5–21.4%, and of luteinizing hormone and/or FSH in 0.2–19.7%7,44,54,56,58. As gonadotropin axis impairment is frequently present from the time of diagnosis and growth hormone is not always assessed using dynamic tests, rates of new luteinizing hormone and/or FSH deficiencies might be overestimated and growth hormone deficiencies underestimated. ACTH stimulation testing is recommended to confirm adrenal insufficiency for most patients if morning levels of cortisol are not diagnostic109.
Although the rate of new hormone deficiencies in microNFPA is low, with only two found in the 459 patients of the UK NFPA Consortium cohort7, initial mass size might be unrelated to new-onset hormone dysfunction with pituitary adenoma growth. Among 111 patients with NFPA followed over a median of 3.75 years (interquartile range 4.33), approximately 5% developed new hormone deficiencies, but there was no association between new hormone deficits and initial adenoma diameter across size categories of <5 mm, 5–10 mm and >10 mm (ref. 27). Similarly, among 194 incidental NFPAs followed for a median of 3 years after diagnosis (interquartile range 2–5), new hormone deficiencies were unrelated to mass size and 10 of 194 patients (5.2%) with both repeat imaging and hormonal testing showed new-onset hormone deficiency despite stable or decreasing size56. In 35 patients with macroNFPAs managed conservatively and followed for a median of 50 months, those with hormone deficiencies showed a more rapid onset of adenoma growth in the first 2 years after diagnosis than patients without hormone deficiencies, and all patients with hypopituitarism at baseline had experienced growth by year 5 (ref. 44).
By contrast, in patients with non-adenomatous cysts, the risk of new hormone deficiencies is more closely related to lesion size than in all patients with macroNFPAs. The Swedish Pituitary Registry of patients with RCCs showed hypopituitarism in 3.3% at 1 year and 7% at 5 years among the 204 patients with cysts <10 mm in diameter versus 20% and 22%, respectively, among the 174 patients with cysts ≥10 mm in diameter110.
New hormone excess in NFPAs
Very rarely, clinically silent hormone-expressing pituitary adenomas might transform into secreting adenomas111. Of 20 patients identified in a systematic review, 4 silent corticotroph adenomas and 4 null cell adenomas converted to Cushing disease and 1 silent somatotroph adenoma converted to acromegaly, with a median time to transformation of 72 months (range 12–276)112. Silent adenomas that convert to hormone-secreting adenomas are potentially aggressive100.
Conversion of silent corticotroph adenoma to Cushing disease is well studied113, but frequency varies widely. Transformation to Cushing disease was reported in 5 of 176 (2.8%) patients with resected corticotroph adenomas over an average of 4.2 years114 whereas, in 75 silent corticotroph adenomas, transformation occurred in 3 (4%) patients over 7 years115. Among 16 patients with silent corticotroph adenoma that transformed to Cushing disease, median time from diagnosis to transformation was 30 months, with 50% transforming in 12–24 months116. These patients required a median of 2.5 surgeries (95% CI 2–4); 81% received radiation therapy and only one-third of those remitted.
There are few reports of silent somatotroph adenomas developing into acromegaly. These adenomas are usually more aggressive than both NFPAs and clinically overt acromegaly and are usually refractory to treatment. In 17 patients followed up for a mean of 3.9 years, 5 showed increased levels of IGF1 and required repeat surgery or radiotherapy117.
When is ophthalmological evaluation warranted?
At diagnosis
-
R18. We recommend ophthalmological consultation and visual field testing for patients with vision complaints or lesions abutting the optic nerves or chiasm regardless of lesion size.
-
R19. We suggest visual field testing for patients with incidentalomas ≥10 mm in diameter as well as those with sellar lesions <10 mm in diameter that are close to the optic chiasm (<3–5 mm).
At follow-up
-
R20. We suggest repeat visual field testing after 6 months in patients with suprasellar extension if surgery is not performed.
-
R21. We suggest repeat visual field testing at 1–2 years in all patients with macroadenomas, as adenoma growth and distance to the optic chiasm at baseline are not always predictive of deteriorating visual function.
-
R22. For patients with macroadenomas not compressing the optic chiasm who are followed up with annual MRIs, we suggest visual field testing be performed at 6–12 months to detect potential early adenoma growth during the extended MRI interval.
-
R23. We recommend that patients with abnormal visual field test results be evaluated by a neuro-ophthalmologist.
Recommendations for ophthalmological evaluation are based on data reviewed by the expert panel regarding the incidence of visual field abnormalities and predictors of visual loss in patients with macroNFPAs, microNFPAs and other incidental lesions7,8,24,26,44,51,54,56,58,61,62,118,119,120,121,122,123,124. See Supplementary Box 3 for details.
Indications for surgical management
As with other conditions that can manifest across all ages (for example, diabetes mellitus), a decision to pursue a more intensive or conservative treatment approach should be informed by the potential risks and benefits to the individual patient. Therefore, although age-specific thresholds have been included throughout these guidelines (derived from published studies where available), the Consensus Group advises taking account of other factors that might independently affect patient outcomes (for example, comorbidities and general frailty) when discussing management options.
When is surgical resection indicated for incidental NFPAs?
-
R24. We recommend surgery for incidentally detected hypersecreting pituitary adenomas as per current guidelines.
-
R25. We recommend surgery in patients with visual compromise, cranial neuropathy or another neurological deficit related to the adenoma.
-
R26. We recommend surgery in patients with an adenoma abutting the optic chiasm after evaluation in a pituitary tumour centre of excellence. However, in patients >65 years old or in patients of any age who have poor functional status but intact visual function, observation can be considered.
-
R27. If the adenoma is ≥5 mm from the optic chiasm, we suggest individualized care based on the size of the mass and the age of the patient. Surgical consultation could be considered for macroadenomas in young patients, in those with evidence of adenoma growth on serial MRIs or cavernous sinus invasion, and in women desiring pregnancy.
-
R28. We suggest surgery for most patients with severe and/or progressive vision loss or with a diminished level of consciousness due to apoplexy in a pituitary adenoma.
-
R29. We suggest that risks and benefits of surgery be considered for patients with severe headache refractory to anti-headache medication after evaluation in a multidisciplinary clinic, including by an expert neurologist.
-
R30. For asymptomatic macroadenomas, we suggest considering age and functional status when deciding between surgery and observation. In frail patients, close observation for evidence of growth might be suitable. In the absence of clinical factors driving a decision, patient preference is considered.
-
R31. We suggest considering surgery for patients with progressively enlarging macroadenomas, especially those close to the optic chiasm, and for patients with new-onset hypopituitarism; however, postoperative recovery of pituitary deficits is not assured.
The role of surgery in the management of a pituitary incidentaloma, especially for NFPAs, has not been well defined and no randomized studies have evaluated outcomes61,62,125,126. Indications for surgery as well as potential benefits and risks should be discussed with a collaborative multidisciplinary team3,6,62.
Macroadenomas, including those detected incidentally, generally have more adverse outcomes than microadenomas74. Nevertheless, the presence of a visual defect, cranial neuropathy, other neurological deficit or cavernous sinus invasion127 confirmed to be related to the incidentaloma should be referred for interdisciplinary discussion and surgery consideration regardless of mass size, except for prolactinomas, as per current guidelines14,15,99,128. Development of new hormone deficiencies is not always related to adenoma size, and recovery of postsurgical hypopituitarism has been observed59,109,129,130. In a patient who is a good surgical candidate with new-onset hormone deficiencies and progressive adenoma growth, we suggest that surgery be considered in a specialized centre to mitigate risks of new postoperative deficits.
Age, functional status, adenoma size and cavernous sinus invasion should be considered when weighing surgical resection versus observation of asymptomatic incidentally discovered pituitary adenomas. For example, in a female patient of childbearing age with a large microadenoma or a small macroadenoma, surgery or close observation until lesion growth is documented could be appropriate, especially if fertility is desired. However, in a frail patient with a macroadenoma who is asymptomatic and is not a good surgical candidate, observation might be considered even if the lesion abuts the optic chiasm. By contrast, if the risk of surgical complications is low and there are no clinical factors driving the decision-making process, patient preference could be considered. For example, prompt surgical resection might be preferred over observation given the cost burden of serial imaging and frequent clinical evaluations as well as the adverse effect on patient anxiety regarding the potential for continued adenoma growth.
Management of patients with pituitary apoplexy is complex and evolving. Initial recommendations mandated urgent pituitary surgery in all patients with apoplexy and visual or neurological abnormalities62. However, a multicentre, international prospective registry of 97 patients confirmed that 3 months after the apoplectic event, surgery did not confer benefit in improving vision compared with conservative management. Timing of surgery (≤3 days versus >3 days after the event) also did not affect outcomes, and underlying adenoma size decreased in both groups131. Nevertheless, as surgery was performed in more severely ill patients, it is difficult to generalize these findings to all patients with apoplexy. Notably, these results only apply to patients presenting with clinical apoplexy, and not to the 25% of patients with signs of prior subclinical pituitary haemorrhage when pituitary imaging is performed90.
Individualized approaches for patients with hypersecreting pituitary adenomas have been reviewed in detail14,73,132,133. Surgical resection is the mainstay of therapy for Cushing disease and acromegaly and is also sometimes used to manage certain prolactinomas14,15,128,134. TSH-secreting adenomas are exceedingly rare135, and rarer still are those incidentally discovered136. Surgical resection is a preferred approach135,137.
When is surgical resection indicated for cystic sellar incidentalomas?
-
R32. We recommend a detailed MRI and hormonal evaluation to establish a differential diagnosis for an incidentally discovered cystic sellar lesion.
-
R33. We suggest that the risks and benefits of surgery in patients with symptomatic cystic adenomas be discussed by a multidisciplinary team, especially for patients with severe headache refractory to medication and those with large cystic lesions and pituitary apoplexy.
-
R34. We suggest that asymptomatic small RCCs (<10 mm) be followed by serial pituitary MRI with frequency depending on clinical features, similar to pituitary adenomas.
-
R35. We suggest that risks and benefits of surgery be considered for patients with small RCCs (<10 mm) and severe headache refractory to anti-headache medication after evaluation in a multidisciplinary clinic, including by an expert neurologist.
-
R36. We recommend surgery for enlarging cystic lesions associated with visual and/or hormonal dysfunction.
-
R37. We suggest observation of small arachnoid cysts (<10 mm) and larger asymptomatic arachnoid cysts (≥10 mm).
-
R38. We suggest that a multidisciplinary team undertake an evidence-based discussion of observation versus surgery for small (<10 mm) stable craniopharyngiomas. In patients who are good surgical candidates, surgery by an expert neurosurgeon might be beneficial for complete safe resection and confirmed pathological diagnosis. For frail patients, observation might be reasonable in the absence of central signs and symptoms of mass effect.
-
R39. We recommend surgical resection of enlarging and large craniopharyngiomas (≥10 mm) followed by medication and/or radiation if gross total resection is not achieved. In patients ≥65 years old or frail patients, observation might be reasonable if vision is intact.
Overall, 20% of cystic sellar lesions, including RCCs, cystic pituitary adenomas and arachnoid cysts, are diagnosed as incidentalomas. Distinctive clinical, biochemical and radiological findings are important for diagnostic and treatment purposes138. The remainder usually present with mass compression leading to headache and vision compromise1,139,140,141.
Rathke cleft cysts
RCCs are the most common sellar lesion found at autopsy, accounting for up to one-third of incidental pituitary lesions104,142 and 19% of non-adenomatous masses found on MRI1. In 116 RCCs, the average maximal diameter was 13 mm (ref. 141) and ranged from 9 mm to 30 mm in 27 RCCs140.
On imaging, RCCs often contain a waxy nodule; approximately 40% are hypointense on T2-weighted images and hyperintense on T1-weighted images with no contrast enhancement, probably due to the increased protein content. In general, they show minimal enhancement, with a thin wall, and are located in the pars intermedia, with the stalk often displaced anteriorly139,143. During long-term follow-up, only a small percentage of lesions progress, and many regress over time144, with no morphological features predicting change in size78. In 229 RCCs followed up with serial MRIs for a median of 36.6 months, 32% spontaneously regressed123; in another series of 110 RCCs followed up for a median of 23 months, 13.6% regressed on MRI, and 6.4% became undetectable78. Similarly, of 94 RCCs followed up for a mean of 27 months, 76.5% showed no change in size on serial imaging, while 5% increased and 16% decreased in size55. Based on serial MRIs performed in 75 patients with incidentally discovered RCCs, 21 of which increased in size, the mean cyst growth rate was calculated as 0.0010 cm3 per month (95% CI 0.0015–0.0035) and was not statistically distinguishable from zero. Of note, only nine of these patients underwent surgical resection, which suggests that RCC growth might not be clinically significant145.
Given the slow rate of RCC growth, asymptomatic lesions are typically followed up with serial imaging. Small RCCs (<10 mm) with intractable headache146 and large (≥10 mm) or progressively growing lesions associated with visual and/or hormone dysfunction might be considered for surgical resection147. Cyst drainage leads to new AVP-D in 0–9% of patients, and complete cyst removal leads to new AVP-D in 19–69% of patients147,148. Postoperative cerebrospinal fluid leak should be avoided and cyst closure might be required147.
Cystic pituitary adenomas
Haemorrhage or ischaemic pituitary adenoma infarction can result in a cystic component evident on MRI139, with heterogeneity on T1-weighted images and T2-weighted images that are isointense, wall enhancing and sometimes nodular. In 82 patients with cystic pituitary adenomas or RCCs, MRI showed internal septations, eccentric location, invasion and distinct layers of fluid in the lesion from prior haemorrhage143.
Among 47 patients treated surgically, including 27 (57%) with RCCs and 17 (36%) with cystic pituitary adenomas, hormone dysfunction at presentation varied, with amenorrhoea and/or oligomenorrhoea, decreased libido, and galactorrhoea reported in 24–47% of those with cystic pituitary adenomas versus 12.8–34.0% of those with RCC140. Postoperatively, 70% of patients with cystic pituitary adenomas report improved headache, 80% improved visual function and 33% improved hormone function140. Accordingly, in patients with symptomatic cystic pituitary adenomas, surgery is often considered for those with severe refractory headache or hormonal excess.
Craniopharyngiomas
Craniopharyngiomas represent 1% of all intracranial tumours and 5–10% of sellar tumours in children149. Adamantinomatous tumours are invariably cystic and limited to the sella and often calcify. Papillary tumours often appear solid or as mixed cystic–solid. Wall enhancement and hyperintensity on T1-weighted MRI images are seen. Calcification favours a diagnosis of adamantinomatous craniopharyngioma over other cystic lesions, as does a normal-sized sella and a suprasellar cyst location. Post-contrast 3D T2-FLAIR images are sensitive for differentiating craniopharyngiomas from RCCs as the RCC cyst wall is less likely to enhance150. Craniopharyngiomas might have a more aggressive course than other cystic lesions. Treatment at diagnosis or when growth is detected might lead to more favourable outcomes, especially in patients <65 years old and in patients with papillary tumours where medical treatment could be efficacious151. Surgical decompression can improve symptoms related to mass effects; remission can be achieved with a gross total resection, and subtotal resection followed by radiation might be equally effective for long-term control. Nevertheless, given treatment-associated morbidity from both surgery and radiation therapy, observation could be reasonable until cyst growth is documented152.
Arachnoid cysts
Herniation of arachnoid tissue through a defect in the diaphragma sellae might lead to arachnoid cysts, representing 1.4% of sellar lesions153. These are isointense to cerebrospinal fluid, with no MRI contrast enhancement, as the cyst has a higher protein content than cerebrospinal fluid154. In 485 patients with arachnoid cysts identified over a 10-year period, 92.5% of adults and 72.4% of children were asymptomatic155. After an average of 31 months of follow-up, cyst volume was unchanged in 95% and no previously asymptomatic patients became symptomatic155. Similarly, in 213 arachnoid cysts followed up for 3.8 years, 5 (2.3%) patients showed an increase in size and 2 (0.9%) developed new or worsening symptoms153. Given the low rate of symptoms and small risk of enlargement, small arachnoid cysts (<10 mm) and larger cysts (≥10 mm) that remain asymptomatic can be observed conservatively156.
Special considerations
The expert panel reviewed the data on the management of pituitary incidentaloma in patients with multiple endocrine neoplasia type 1 (refs. 157,158,159,160,161,162,163,164,165,166,167,168), in children and adolescents25,167,169,170,171,172,173,174,175, in older patients (age >65 years)176,177,178,179,180,181,182,183,184,185,186, and in pregnant women6,14,15,128,134,187,188,189,190,191. See Supplementary Box 4 for details.
Managing patient expectations
The recommendations presented here are based on published peer-reviewed evidence as well as the collective expertise of Consensus Group members. We also assessed how patients with incidentalomas interact with the health-care system to better understand the effect of our recommendations on clinical practice.
Steering Committee members developed a patient survey to collect information on diagnosis and follow-up monitoring of pituitary incidentalomas192. The results provide important insight into managing the expectations of patients with pituitary incidentalomas.
A total of 275 patients responded to the survey, 84% of whom were from the UK, Australia, Japan and Brazil; 46% of respondents were aged <50 years and <10% were aged ≥70 years. Sex was not recorded. Although this limited sample might not be representative of patients with pituitary incidentalomas in all countries, the results show that accurate patient education about the nature of pituitary incidentalomas and their treatment is paramount. Communications with patients should clearly outline management options and recommendations for the assessment of pituitary incidentalomas. The importance of accurate specialist assessment and follow-up should be highlighted, particularly emphasizing the value of expert multidisciplinary subspecialty care.
Conclusion
Finding a pituitary incidentaloma on imaging requires careful evaluation from an endocrinologist followed by relevant referrals to neurosurgery and/or ophthalmology specialists, as determined by lesion and patient characteristics. This Consensus Statement from the Pituitary Society offers recommendations on imaging, as well as endocrine and clinical assessments for diagnosis, and guides pituitary incidentaloma management and follow-up. Decisions about MRI surveillance or surgical intervention for NFPAs should be based on patient characteristics and adenoma size and location as well as guided by visual, neurological and associated central signs and symptoms of mass effects. Cystic lesions, those associated with genetic syndromes and those found in children, older people and pregnant women require an individualized approach by an expert multidisciplinary team to ensure optimal management. Prospective studies are needed to define criteria for surgical intervention in patients with small lesions, to optimize management in challenging clinical settings, and to understand cost–benefit and mental health effects of long-term imaging and endocrine surveillance in patients with pituitary incidentalomas (Box 3).
References
Famini, P., Maya, M. M. & Melmed, S. Pituitary magnetic resonance imaging for sellar and parasellar masses: ten-year experience in 2598 patients. J. Clin. Endocrinol. Metab. 96, 1633–1641 (2011).
Giraldi, E., Allen, J. W. & Ioachimescu, A. G. Pituitary incidentalomas: best practices and looking ahead. Endocr. Pract. 29, 60–68 (2023).
Langlois, F. & Fleseriu, M. What to do with incidentally discovered pituitary abnormalities? Med. Clin. North Am. 105, 1081–1098 (2021).
Budan, R. M. & Georgescu, C. E. Multiple pituitary adenomas: a systematic review. Front. Endocrinol. 7, 1 (2016).
Ogando-Rivas, E., Alalade, A. F., Boatey, J. & Schwartz, T. H. Double pituitary adenomas are most commonly associated with GH- and ACTH-secreting tumors: systematic review of the literature. Pituitary 20, 702–708 (2017).
Melmed, S. et al. Clinical biology of the pituitary adenoma. Endocr. Rev. 43, 1003–1037 (2022).
Hamblin, R. et al. Natural history of non-functioning pituitary microadenomas: results from the UK non-functioning pituitary adenoma consortium. Eur. J. Endocrinol. 189, 87–95 (2023).
Karavitaki, N. et al. What is the natural history of nonoperated nonfunctioning pituitary adenomas? Clin. Endocrinol. 67, 938–943 (2007).
Rikvold, S. D., Pedersen, M. B., Andreassen, M. & Krogh, J. Natural history of non-functioning pituitary adenomas: a systematic review and meta-analysis. Horm. Metab. Res. 55, 443–451 (2023).
Casanueva, F. F. et al. Criteria for the definition of pituitary tumor centers of excellence (PTCOE): a pituitary society statement. Pituitary 20, 489–498 (2017).
Giustina, A. et al. Pilot study to define criteria for pituitary tumors centers of excellence (PTCOE): results of an audit of leading international centers. Pituitary 26, 583–596 (2023).
Swiglo, B. A. et al. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J. Clin. Endocrinol. Metab. 93, 666–673 (2008).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926 (2008).
Petersenn, S. et al. Diagnosis and management of prolactin-secreting pituitary adenomas: a pituitary society international consensus statement. Nat. Rev. Endocrinol. 19, 722–740 (2023).
Fleseriu, M. et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 9, 847–875 (2021).
Molitch, M. E. Pituitary tumours: pituitary incidentalomas. Best Pract. Res. Clin. Endocrinol. Metab. 23, 667–675 (2009).
Buurman, H. & Saeger, W. Subclinical adenomas in postmortem pituitaries: classification and correlations to clinical data. Eur. J. Endocrinol. 154, 753–758 (2006).
Schoning, J. V. et al. Multiple tumorous lesions of the pituitary gland. Hormones 21, 653–663 (2022).
Gobara, A. et al. T2 hypointense signal discovered incidentally at the posterior edge of the adenohypophysis on MRI: its prevalence and morphology and their relationship to age. Neuroradiology 64, 1755–1761 (2022).
Chong, B. W., Kucharczyk, W., Singer, W. & George, S. Pituitary gland MR: a comparative study of healthy volunteers and patients with microadenomas. AJNR Am. J. Neuroradiol. 15, 675–679 (1994).
Pineyro, M. M. et al. Strikingly low prevalence of pituitary incidentalomas in a teaching hospital in Uruguay. Front. Endocrinol. 14, 1254180 (2023).
Kuo, M., Maya, M. M., Bonert, V. & Melmed, S. Prospective evaluation of incidental pituitary imaging findings in the Sella turcica. J. Endocr. Soc. 5, bvaa186 (2021).
Anagnostis, P. et al. Pituitary incidentalomas: a single-centre experience. Int. J. Clin. Pract. 65, 172–177 (2011).
Arita, K. et al. Natural course of incidentally found nonfunctioning pituitary adenoma, with special reference to pituitary apoplexy during follow-up examination. J. Neurosurg. 104, 884–891 (2006).
Esteves, C. et al. Pituitary incidentalomas: analysis of a neuroradiological cohort. Pituitary 18, 777–781 (2015).
Feldkamp, J. et al. Incidentally discovered pituitary lesions: high frequency of macroadenomas and hormone-secreting adenomas — results of a prospective study. Clin. Endocrinol. 51, 109–113 (1999).
Imran, S. A. et al. Analysis and natural history of pituitary incidentalomas. Eur. J. Endocrinol. 175, 1–9 (2016).
Day, P. F. et al. Retrospective multicentric study of pituitary incidentalomas. Pituitary 7, 145–148 (2004).
Freda, P. U. et al. Presenting features in 269 patients with clinically nonfunctioning pituitary adenomas enrolled in a prospective study. J. Endocr. Soc. 4, bvaa021 (2020).
Sunny, D. E. et al. Prevalence of incidental intracranial findings on magnetic resonance imaging: a systematic review and meta-analysis. Acta Neurochir. 164, 2751–2765 (2022).
Hall, W. A., Luciano, M. G., Doppman, J. L., Patronas, N. J. & Oldfield, E. H. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann. Intern. Med. 120, 817–820 (1994).
Yue, N. C. et al. Clinically serious abnormalities found incidentally at MR imaging of the brain: data from the cardiovascular health study. Radiology 202, 41–46 (1997).
Vernooij, M. W. et al. Incidental findings on brain MRI in the general population. N. Engl. J. Med. 357, 1821–1828 (2007).
Jeong, S. Y. et al. Incidental pituitary uptake on whole-body 18F-FDG PET/CT: a multicentre study. Eur. J. Nucl. Med. Mol. Imaging 37, 2334–2343 (2010).
Hyun, S. H., Choi, J. Y., Lee, K. H., Choe, Y. S. & Kim, B. T. Incidental focal 18F-FDG uptake in the pituitary gland: clinical significance and differential diagnostic criteria. J. Nucl. Med. 52, 547–550 (2011).
Ju, H., Zhou, J., Pan, Y., Lv, J. & Zhang, Y. Evaluation of pituitary uptake incidentally identified on 18F-FDG PET/CT scan. Oncotarget 8, 55544–55549 (2017).
Fernandez, A., Karavitaki, N. & Wass, J. A. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 72, 377–382 (2010).
Daly, A. F. et al. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J. Clin. Endocrinol. Metab. 91, 4769–4775 (2006).
Raappana, A., Koivukangas, J., Ebeling, T. & Pirila, T. Incidence of pituitary adenomas in Northern Finland in 1992-2007. J. Clin. Endocrinol. Metab. 95, 4268–4275 (2010).
Watanabe, G., Choi, S. Y. & Adamson, D. C. Pituitary incidentalomas in the United States: a national database estimate. World Neurosurg. 158, e843–e855 (2022).
Agustsson, T. T. et al. The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur. J. Endocrinol. 173, 655–664 (2015).
Graffeo, C. S. et al. Pituitary adenoma incidence, management trends, and long-term outcomes: a 30-year population-based analysis. Mayo Clin. Proc. 97, 1861–1871 (2022).
Iglesias, P. et al. Prevalence, clinical features, and natural history of incidental clinically non-functioning pituitary adenomas. Horm. Metab. Res. 49, 654–659 (2017).
Constantinescu, S. M. et al. Natural history and surgical outcome of incidentally discovered clinically nonfunctioning pituitary macroadenomas. Endocr. Connect. 12, e230224 (2023).
McComb, D. J., Ryan, N., Horvath, E. & Kovacs, K. Subclinical adenomas of the human pituitary. New light on old problems. Arch. Pathol. Lab. Med. 107, 488–491 (1983).
Suzuki, M. et al. Expression of proliferation markers in human pituitary incidentalomas. Endocr. Pathol. 17, 263–275 (2006).
Tahara, S. et al. An overview of pituitary incidentalomas: diagnosis, clinical features, and management. Cancers 14, 4324 (2022).
Oyama, K., Sanno, N., Tahara, S. & Teramoto, A. Management of pituitary incidentalomas: according to a survey of pituitary incidentalomas in Japan. Semin. Ultrasound CT MR 26, 47–50 (2005).
Donovan, L. E. & Corenblum, B. The natural history of the pituitary incidentaloma. Arch. Intern. Med. 155, 181–183 (1995).
Han, A. J., Varlamov, E. V. & Fleseriu, M. Nonfunctioning pituitary microadenomas: should imaging interval be extended? A large single-center cohort study. J. Clin. Endocrinol. Metab. 107, e1231–e1241 (2022).
Kim, J. H. et al. Developing an optimal follow-up strategy based on the natural history of nonfunctioning pituitary adenomas. J. Neurosurg. 131, 500–506 (2018).
Lenders, N. et al. Longitudinal evaluation of the natural history of conservatively managed nonfunctioning pituitary adenomas. Clin. Endocrinol. 84, 222–228 (2016).
Reincke, M., Allolio, B., Saeger, W., Menzel, J. & Winkelmann, W. The ‘incidentaloma’ of the pituitary gland. Is neurosurgery required? JAMA 263, 2772–2776 (1990).
Sam, A. H. et al. Clinical outcomes in patients with nonfunctioning pituitary adenomas managed conservatively. Clin. Endocrinol. 83, 861–865 (2015).
Sanno, N., Oyama, K., Tahara, S., Teramoto, A. & Kato, Y. A survey of pituitary incidentaloma in Japan. Eur. J. Endocrinol. 149, 123–127 (2003).
Tresoldi, A. S. et al. Clinically nonfunctioning pituitary incidentalomas: characteristics and natural history. Neuroendocrinology 110, 595–603 (2020).
Yuen, K. C. et al. Prevalence of GH and other anterior pituitary hormone deficiencies in adults with nonsecreting pituitary microadenomas and normal serum IGF-1 levels. Clin. Endocrinol. 69, 292–298 (2008).
Dekkers, O. M. et al. The natural course of non-functioning pituitary macroadenomas. Eur. J. Endocrinol. 156, 217–224 (2007).
Fountas, A. et al. Conservatively managed non-functioning pituitary macroadenomas — cohort study from the UK Non-functioning Pituitary Adenoma Consortium. Eur. J. Endocrinol. 192, 680–690 (2025).
Park, S. S., Kang, H., Kim, Y. H. & Kim, J. H. Different tumor growth pattern of clinically nonfunctioning pituitary neuroendocrine tumor according to sex and age: a longitudinal study. J. Endocrinol. Invest. 47, 1911–1921 (2024).
Fernandez-Balsells, M. M. et al. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J. Clin. Endocrinol. Metab. 96, 905–912 (2011).
Freda, P. U. et al. Pituitary incidentaloma: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 894–904 (2011).
Hoang, J. K. et al. Management of incidental pituitary findings on CT, MRI, and 18F-fluorodeoxyglucose PET: a white paper of the ACR incidental findings committee. J. Am. Coll. Radiol. 15, 966–972 (2018).
Bashari, W. A. et al. Modern imaging of pituitary adenomas. Best Pract. Res. Clin. Endocrinol. Metab. 33, 101278 (2019).
MacFarlane, J. et al. Advances in the imaging of pituitary tumors. Endocrinol. Metab. Clin. North Am. 49, 357–373 (2020).
Bashari, W. A. et al. Using molecular imaging to enhance decision making in the management of pituitary adenomas. J. Nucl. Med. 62, 57S–62S (2021).
Bentestuen, M., Gossili, F., Almasi, C. E. & Zacho, H. D. Prevalence and significance of incidental findings on 68 Ga-DOTA-conjugated somatostatin receptor-targeting peptide PET/CT: a systematic review of the literature. Cancer Imaging 22, 44 (2022).
Galland, F. et al. Management of nonfunctioning pituitary incidentaloma. Ann. Endocrinol. 76, 191–200 (2015).
Bashari, W. A., Gillett, D., MacFarlane, J., Scoffings, D. & Gurnell, M. in The Pituitary (ed. Melmed, S.) 677–721 (Elsevier, 2022).
Raverot, G. et al. Biological and radiological exploration and management of non-functioning pituitary adenoma. Ann. Endocrinol. 76, 201–209 (2015).
Bonneville, J. F. A plea for the T2W MR sequence for pituitary imaging. Pituitary 22, 195–197 (2019).
Nachtigall, L. B. et al. Physicians’ awareness of gadolinium retention and MRI timing practices in the longitudinal management of pituitary tumors: a “Pituitary Society” survey. Pituitary 22, 37–45 (2019).
Tritos, N. A. & Miller, K. K. Diagnosis and management of pituitary adenomas: a review. JAMA 329, 1386–1398 (2023).
Ho, K. K. Y. et al. A proposed clinical classification for pituitary neoplasms to guide therapy and prognosis. Lancet Diabetes Endocrinol. 12, 209–214 (2024).
Pernik, M. N. et al. The natural history of non-functioning pituitary adenomas: a meta-analysis of conservatively managed tumors. J. Clin. Neurosci. 95, 134–141 (2022).
Molitch, M. E. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol. Metab. Clin. North Am. 37, 151–171 (2008).
Hordejuk, D. et al. Long-term changes in the size of pituitary microadenomas. Ann. Intern. Med. 176, 298–302 (2023).
Jung, H., Yang, S. Y. & Cho, K. T. Suggestion of follow-up period in nonfunctioning pituitary incidentaloma based on MRI characteristics. Brain Tumor Res. Treat. 12, 40–49 (2024).
Ayalon-Dangur, I. et al. Natural history of nonfunctioning pituitary macroadenomas followed without intervention: a retrospective cohort study. Clin. Endocrinol. 98, 559–566 (2023).
Kajal, S., Ahmad, Y. E. S., Halawi, A., Gol, M. A. K. & Ashley, W. Pituitary apoplexy: a systematic review of non-gestational risk factors. Pituitary 27, 320–334 (2024).
Chen, L., White, W. L., Spetzler, R. F. & Xu, B. A prospective study of nonfunctioning pituitary adenomas: presentation, management, and clinical outcome. J. Neurooncol. 102, 129–138 (2011).
Donegan, D. & Erickson, D. Revisiting pituitary apoplexy. J. Endocr. Soc. 6, bvac113 (2022).
Bao, Y. J. et al. Pituitary apoplexy complicated with subarachnoid hemorrhage caused by incidentaloma following a head injury: case report. Chin. Med. J. 120, 2341–2343 (2007).
Holness, R. O., Ogundimu, F. A. & Langille, R. A. Pituitary apoplexy following closed head trauma. Case report. J. Neurosurg. 59, 677–679 (1983).
Nishizawa, S., Ohta, S., Yokoyama, T. & Uemura, K. Therapeutic strategy for incidentally found pituitary tumors (“pituitary incidentalomas”). Neurosurgery 43, 1344–1348 (1998).
Saeed, O. & Braga, M. A 68-year-old man with an incidentally discovered pituitary lesion. CMAJ 189, E605–E607 (2017).
Kamimura, K. et al. Consistency of pituitary adenoma: prediction by pharmacokinetic dynamic contrast-enhanced MRI and comparison with histologic collagen content. Cancers 13, 3914 (2021).
Kim, Y. S., Ahn, S., Lee, Y. S., Jeun, S. S. & Park, J. S. Clinicopathological analysis of non-functioning pituitary adenomas (PAs) according to the 2022 WHO classification. Pituitary 27, 665–672 (2024).
Boguszewski, C. L., de Castro Musolino, N. R. & Kasuki, L. Management of pituitary incidentaloma. Best Pract. Res. Clin. Endocrinol. Metab. 33, 101268 (2019).
Briet, C., Salenave, S., Bonneville, J. F., Laws, E. R. & Chanson, P. Pituitary apoplexy. Endocr. Rev. 36, 622–645 (2015).
Ono, M. et al. A survey of surgically resected pituitary incidentalomas and a comparison of the clinical features and surgical outcomes of non-functioning pituitary adenomas discovered incidentally versus symptomatically. Endocr. J. 68, 561–571 (2021).
Ishii, K. et al. Clinical investigation of pituitary incidentalomas: a two-center study. Intractable Rare Dis. Res. 8, 239–244 (2019).
Morinaga, Y. et al. Characteristics and clinical outcomes in pituitary incidentalomas and non-incidental pituitary tumors treated with endoscopic transsphenoidal surgery. Medicine 99, e22713 (2020).
Giraldi, E. A., Veledar, E., Oyesiku, N. M. & Ioachimescu, A. G. Incidentally detected acromegaly: single-center study of surgically treated patients over 22 years. J. Investig. Med. 69, 351–357 (2021).
Torpy, D. J. Screening for ACTH-dependent hypercortisolism in patients with pituitary incidentaloma. Eur. J. Endocrinol. 172, C1–C4 (2015).
Toini, A. et al. Screening for ACTH-dependent hypercortisolism in patients affected with pituitary incidentaloma. Eur. J. Endocrinol. 172, 363–369 (2015).
Tamada, D. et al. Clinical significance of screening for subclinical Cushing’s disease in patients with pituitary tumors. Endocr. J. 63, 47–52 (2016).
Coscia, K., Verrienti, M., Di Dalmazi, G. & Zatelli, M. C. Who and how to screen for endogenous hypercortisolism in adrenal and pituitary incidentaloma. J. Endocrinol. Invest. 48, 63–71 (2025).
Giustina, A. et al. Consensus on criteria for acromegaly diagnosis and remission. Pituitary 27, 7–22 (2024).
Burman, P. et al. Aggressive pituitary tumours and carcinomas, characteristics and management of 171 patients. Eur. J. Endocrinol. 187, 593–605 (2022).
Vaninetti, N. M. et al. A comparative, population-based analysis of pituitary incidentalomas vs clinically manifesting sellar masses. Endocr. Connect. 7, 768–776 (2018).
Carosi, G. et al. Hypothalamic-pituitary axis in non-functioning pituitary adenomas: focus on the prevalence of isolated central hypoadrenalism. Neuroendocrinology 102, 267–273 (2015).
Tritos, N. A. et al. Pituitary Society Delphi survey: an international perspective on endocrine management of patients undergoing transsphenoidal surgery for pituitary adenomas. Pituitary 25, 64–73 (2022).
Trifanescu, R., Ansorge, O., Wass, J. A., Grossman, A. B. & Karavitaki, N. Rathke’s cleft cysts. Clin. Endocrinol. 76, 151–160 (2012).
Sala, E. et al. Natural history of Rathke’s cleft cysts: a retrospective analysis of a two centres experience. Clin. Endocrinol. 89, 178–186 (2018).
Langlois, F. et al. High prevalence of adrenal insufficiency at diagnosis and headache recovery in surgically resected Rathke’s cleft cysts — a large retrospective single center study. Endocrine 63, 463–469 (2019).
Carosi, G. et al. A multicenter cohort study in patients with primary empty sella: hormonal and neuroradiological features over a long follow-up. Front. Endocrinol. 13, 925378 (2022).
Akkus, G. et al. Pituitary volume in patients with primary empty sella and clinical relevance to pituitary hormone secretion: a retrospective single center study. Curr. Med. Imaging 17, 1018–1024 (2021).
Fleseriu, M., Christ-Crain, M., Langlois, F., Gadelha, M. & Melmed, S. Hypopituitarism. Lancet 403, 2632–2648 (2024).
Petersson, M. et al. Natural history and surgical outcome of Rathke’s cleft cysts — a study from the swedish pituitary registry. Clin. Endocrinol. 96, 54–61 (2022).
Melmed, S. Pituitary-tumor endocrinopathies. N. Engl. J. Med. 382, 937–950 (2020).
Guerrero-Perez, F., Marengo, A. P., Vidal, N. & Villabona, C. Pituitary adenomas with changing phenotype: a systematic review. Exp. Clin. Endocrinol. Diabetes 128, 835–844 (2020).
Ben-Shlomo, A. & Cooper, O. Silent corticotroph adenomas. Pituitary 21, 183–193 (2018).
Righi, A. et al. The changing faces of corticotroph cell adenomas: the role of prohormone convertase 1/3. Endocrine 56, 286–297 (2017).
Jahangiri, A. et al. A comprehensive long-term retrospective analysis of silent corticotrophic adenomas vs hormone-negative adenomas. Neurosurgery 73, 8–17 (2013).
Zheng, G. et al. Clinical, laboratory, and treatment profiles of silent corticotroph adenomas that have transformed to the functional type: a case series with a literature review. Front. Endocrinol. 11, 558593 (2020).
Langlois, F. et al. Clinical profile of silent growth hormone pituitary adenomas; higher recurrence rate compared to silent gonadotroph pituitary tumors, a large single center experience. Endocrine 58, 528–534 (2017).
Ryu, W. H. et al. Conservative management of pituitary macroadenoma contacting the optic apparatus. Can. J. Neurol. Sci. 37, 837–842 (2010).
Lithgow, K., Batra, R., Matthews, T. & Karavitaki, N. Management of endocrine disease: visual morbidity in patients with pituitary adenoma. Eur. J. Endocrinol. 181, R185–R197 (2019).
Gan, L. et al. The predictive value of suprasellar extension for visual function evaluation in Chinese patients with nonfunctioning pituitary adenoma with optic chiasm compression. World Neurosurg. 116, e960–e967 (2018).
Bonomo, G. et al. The suprasellar volume of nonfunctioning pituitary adenomas: a useful tool for predicting visual field deficits. Pituitary 23, 552–557 (2020).
Castle-Kirszbaum, M. et al. Surgical outcomes and quality of life in Rathke’s cleft cysts undergoing endoscopic transsphenoidal resection: a multicentre study and systematic review of the literature. Pituitary 25, 285–295 (2022).
Kinoshita, Y. et al. Natural course of Rathke’s cleft cysts and risk factors for progression. J. Neurosurg. 138, 1426–1432 (2023).
Vosoughi, A. & Micieli, J. A. Optical coherence tomography abnormalities as the presenting sign of an involuted sellar/suprasellar mass. Case Rep. Ophthalmol. 15, 757–761 (2024).
Aghi, M. K. et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the management of patients with nonfunctioning pituitary adenomas: executive summary. Neurosurgery 79, 521–523 (2016).
Seltzer, J. et al. Outcomes following transsphenoidal surgical management of incidental pituitary adenomas: a series of 52 patients over a 17-year period. J. Neurosurg. 130, 1584–1592 (2019).
Lefevre, E. et al. Clinical and therapeutic implications of cavernous sinus invasion in pituitary adenomas. Endocrine 85, 1058–1065 (2024).
Giustina, A. et al. Multidisciplinary management of acromegaly: a consensus. Rev. Endocr. Metab. Disord. 21, 667–678 (2020).
Mavromati, M. et al. The impact of transsphenoidal surgery on pituitary function in patients with non-functioning macroadenomas. Endocrine 81, 340–348 (2023).
Molitch, M. E. Nonfunctioning pituitary tumors. Handb. Clin. Neurol. 124, 167–184 (2014).
Mamelak, A. N. et al. A prospective, multicenter, observational study of surgical vs nonsurgical management for pituitary apoplexy. J. Clin. Endocrinol. Metab. 109, e711–e725 (2024).
Fleseriu, M., Langlois, F., Lim, D. S. T., Varlamov, E. V. & Melmed, S. Acromegaly: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 10, 804–826 (2022).
Fleseriu, M., Varlamov, E. V., Hinojosa-Amaya, J. M., Langlois, F. & Melmed, S. An individualized approach to the management of Cushing disease. Nat. Rev. Endocrinol. 19, 581–599 (2023).
Fleseriu, M. et al. A Pituitary Society update to acromegaly management guidelines. Pituitary 24, 1–13 (2021).
De Herdt, C., Philipse, E. & De Block, C. Endocrine tumors: thyrotropin-secreting pituitary adenoma: a structured review of 535 adult cases. Eur. J. Endocrinol. 185, R65–R74 (2021).
Rabbiosi, S. et al. Asymptomatic thyrotropin-secreting pituitary macroadenoma in a 13-year-old girl: successful first-line treatment with somatostatin analogs. Thyroid 22, 1076–1079 (2012).
Dutta, A. et al. The outcome of TSHoma from a tertiary care institute in India. Surg. Neurol. Int. 12, 161 (2021).
Tavakol, S. et al. Cyst type differentiates Rathke cleft cysts from cystic pituitary adenomas. Front. Oncol. 11, 778824 (2021).
Gadelha, M. R. et al. Approach to the patient: differential diagnosis of cystic sellar lesions. J. Clin. Endocrinol. Metab. 107, 1751–1758 (2022).
Tafreshi, A. R. et al. Differential clinical presentation, intraoperative management strategies, and surgical outcomes after endoscopic endonasal treatment of cystic sellar masses. World Neurosurg. 133, e241–e251 (2020).
Montaser, A. S., Catalino, M. P. & Laws, E. R. Professor Rathke’s gift to neurosurgery: the cyst, its diagnosis, surgical management, and outcomes. Pituitary 24, 787–796 (2021).
Teramoto, A., Hirakawa, K., Sanno, N. & Osamura, Y. Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiology 193, 161–164 (1994).
Park, M. et al. Differentiation between cystic pituitary adenomas and Rathke cleft cysts: a diagnostic model using MRI. AJNR Am. J. Neuroradiol. 36, 1866–1873 (2015).
Menendez-Torre, E. L. et al. Natural history and surgical outcomes of Rathke’s cleft cysts: a Spanish multicenter study. Front. Endocrinol. 15, 1413810 (2024).
Culver, S. A. et al. A case for conservative management: characterizing the natural history of radiographically diagnosed Rathke cleft cysts. J. Clin. Endocrinol. Metab. 100, 3943–3948 (2015).
Fleseriu, M., Yedinak, C., Campbell, C. & Delashaw, J. B. Significant headache improvement after transsphenoidal surgery in patients with small sellar lesions. J. Neurosurg. 110, 354–358 (2009).
Arko, L. et al. Endonasal endoscopic fenestration of Rathke’s cleft cysts: whether to leave the fenestration open or closed? J. Neurol. Surg. B Skull Base 82, e101–e104 (2021).
Aho, C. J., Liu, C., Zelman, V., Couldwell, W. T. & Weiss, M. H. Surgical outcomes in 118 patients with Rathke cleft cysts. J. Neurosurg. 102, 189–193 (2005).
Momin, A. A. et al. Descriptive epidemiology of craniopharyngiomas in the United States. Pituitary 24, 517–522 (2021).
Azuma, M. et al. Usefulness of contrast-enhanced 3D-FLAIR MR imaging for differentiating Rathke cleft cyst from cystic craniopharyngioma. AJNR Am. J. Neuroradiol. 41, 106–110 (2020).
Prieto, R. et al. Papillary craniopharyngioma: an integrative and comprehensive review. Endocr. Rev. 46, 151–213 (2025).
Kulkarni, A., Konar, S., Shukla, D., Sadashiva, N. & Devi, B. I. Transventricular endoscopic approach for cystic craniopharyngioma: case series. J. Neurol. Surg. B Skull Base 84, 591–597 (2023).
Al-Holou, W. N. et al. Prevalence and natural history of arachnoid cysts in adults. J. Neurosurg. 118, 222–231 (2013).
Nomura, M. et al. Contrast-enhanced MRI of intrasellar arachnoid cysts: relationship between the pituitary gland and cyst. Neuroradiology 38, 566–568 (1996).
Hall, S. et al. Natural history of intracranial arachnoid cysts. World Neurosurg. 126, e1315–e1320 (2019).
Carbone, J. & Sadasivan, A. P. Intracranial arachnoid cysts: review of natural history and proposed treatment algorithm. Surg. Neurol. Int. 12, 621 (2021).
Thakker, R. V. et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J. Clin. Endocrinol. Metab. 97, 2990–3011 (2012).
de Laat, J. M. et al. Long-term natural course of pituitary tumors in patients with MEN1: results from the DutchMEN1 study group (DMSG). J. Clin. Endocrinol. Metab. 100, 3288–3296 (2015).
Verges, B. et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J. Clin. Endocrinol. Metab. 87, 457–465 (2002).
Newey, P. J. & Newell-Price, J. MEN1 surveillance guidelines: time to (re)think? J. Endocr. Soc. 6, bvac001 (2022).
Pieterman, C. R. C. & Valk, G. D. Update on the clinical management of multiple endocrine neoplasia type 1. Clin. Endocrinol. 97, 409–423 (2022).
Damianse, S. S. P. et al. The importance of periodical screening for primary hyperparathyroidism in a pituitary tumor cohort in searching patients with MEN1 and its genetic profile. Endocr. Pract. 28, 509–514 (2022).
Nunes, V. S., Souza, G. L., Perone, D., Conde, S. J. & Nogueira, C. R. Frequency of multiple endocrine neoplasia type 1 in a group of patients with pituitary adenoma: genetic study and familial screening. Pituitary 17, 30–37 (2014).
Brandi, M. L. et al. Multiple endocrine neoplasia type 1: latest insights. Endocr. Rev. 42, 133–170 (2021).
Cuny, T. et al. Genetic analysis in young patients with sporadic pituitary macroadenomas: besides AIP don’t forget MEN1 genetic analysis. Eur. J. Endocrinol. 168, 533–541 (2013).
Kamilaris, C. D. C. & Stratakis, C. A. Multiple endocrine neoplasia type 1 (MEN1): an update and the significance of early genetic and clinical diagnosis. Front. Endocrinol. 10, 339 (2019).
Korbonits, M. et al. Consensus guideline for the diagnosis and management of pituitary adenomas in childhood and adolescence: part 1, general recommendations. Nat. Rev. Endocrinol. 20, 278–289 (2024).
Trouillas, J. et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am. J. Surg. Pathol. 32, 534–543 (2008).
Thaker, V. V., Lage, A. E., Kumari, G., Silvera, V. M. & Cohen, L. E. Clinical course of nonfunctional pituitary microadenoma in children: a single-center experience. J. Clin. Endocrinol. Metab. 104, 5906–5912 (2019).
Shareef, M. et al. Pituitary incidentalomas in paediatric population: incidence and characteristics. Clin. Endocrinol. 94, 269–276 (2021).
Souteiro, P. et al. Pituitary incidentalomas in paediatric age are different from those described in adulthood. Pituitary 22, 124–128 (2019).
Boekhoff, S., Bison, B., Eveslage, M., Sowithayasakul, P. & Muller, H. L. Craniopharyngiomas presenting as incidentalomas: results of KRANIOPHARYNGEOM 2007. Pituitary 22, 532–541 (2019).
Derrick, K. M., Gomes, W. A. & Gensure, R. C. Incidence and outcomes of pituitary microadenomas in children with short stature/growth hormone deficiency. Horm. Res. Paediatr. 90, 151–160 (2018).
Hirsch, W. et al. Microadenomas of the pituitary gland in children with and without hypophyseal dysfunction in magnetic resonance imaging. J. Pediatr. Endocrinol. Metab. 15, 157–162 (2002).
Korbonits, M. et al. Consensus guideline for the diagnosis and management of pituitary adenomas in childhood and adolescence: part 2, specific diseases. Nat. Rev. Endocrinol. 20, 290–309 (2024).
Minniti, G. et al. Diagnosis and management of pituitary tumours in the elderly: a review based on personal experience and evidence of literature. Eur. J. Endocrinol. 153, 723–735 (2005).
Chalif, E. J. et al. Pituitary adenoma in the elderly: surgical outcomes and treatment trends in the United States. J. Neurosurg. 137, 1687–1698 (2022).
Spina, A., Losa, M. & Mortini, P. Pituitary adenomas in elderly patients: clinical and surgical outcome analysis in a large series. Endocrine 65, 637–645 (2019).
Stalldecker, G., Ballarino, C., Diez, S. & Mallea-Gil, M. S. Pituitary adenomas in elderly patients [Spanish]. Medicina 79, 191–196 (2019).
Araujo-Castro, M., Berrocal, V. R. & Pascual-Corrales, E. Pituitary tumors: epidemiology and clinical presentation spectrum. Hormones 19, 145–155 (2020).
Thakur, J. D. et al. Pituitary adenomas in older adults (>/= 65 years): 90-day outcomes and readmissions: a 10-year endoscopic endonasal surgical experience. Pituitary 24, 14–26 (2021).
Liu, J. et al. Comparison of pituitary adenomas in elderly and younger adults: clinical characteristics, surgical outcomes, and prognosis. J. Am. Geriatr. Soc. 63, 1924–1930 (2015).
Tardivo, V. et al. Surgical management of pituitary adenomas: does age matter? Pituitary 23, 92–102 (2020).
Pereira, M. P. et al. Clinical characteristics and outcomes in elderly patients undergoing transsphenoidal surgery for nonfunctioning pituitary adenoma. Neurosurg. Focus. 49, E19 (2020).
Wilson, P. J., Omay, S. B., Kacker, A., Anand, V. K. & Schwartz, T. H. Endonasal endoscopic pituitary surgery in the elderly. J. Neurosurg. 128, 429–436 (2018).
Memel, Z. et al. Outcomes following transsphenoidal pituitary surgery in the elderly: a retrospective single-center review. Oper. Neurosurg. 16, 302–309 (2019).
Nair, A., Sagili, H., Dorairaj, J. & Parvathi, T. Acromegaly incidentally diagnosed at term in a pregnant woman presenting with ventricular premature complexes. Int. J. Reprod. Contracept. Obstet. Gynecol. 10, 2907 (2021).
Rosmino, J. et al. Non-functioning pituitary adenomas and pregnancy: one-center experience and review of the literature. Arch. Endocrinol. Metab. 64, 614–622 (2021).
Luger, A. et al. ESE clinical practice guideline on functioning and nonfunctioning pituitary adenomas in pregnancy. Eur. J. Endocrinol. 185, G1–G33 (2021).
Karaca, Z. et al. How does pregnancy affect the patients with pituitary adenomas: a study on 113 pregnancies from Turkey. J. Endocrinol. Invest. 41, 129–141 (2018).
Lambert, K. et al. Macroprolactinomas and nonfunctioning pituitary adenomas and pregnancy outcomes. Obstet. Gynecol. 129, 185–194 (2017).
Fukuoka, H. et al. Initial interactions of patients harboring pituitary incidentalomas with the healthcare system: a Pituitary Society international patient survey. OSF https://osf.io/5e74g (2025).
Acknowledgements
M.G. is supported by the National Institute for Health and Care Research Cambridge Biomedical Research Centre (NIHR 203312). H.J.M. is supported by the University College London Hospitals/University College London Biomedical Research Centre. E.N. is supported by the Clinician Scientist Program RISE (Rare Important Syndromes in Endocrinology), supported by the Else-Kröner-Fresenius Stiftung and Eva Luise und Horst Köhler Stiftung. M.M.U. received a scholarship from The Society of Endocrinology and Metabolism of Türkiye. The authors thank Shira Berman for editorial support.
Author information
Authors and Affiliations
Consortia
Contributions
M.F., M.G., A.M., H.F., A. Glezer, F.L., T.H.S., Y.G., N.K. and S.M. comprise the Steering Committee and wrote the initial draft manuscript. All authors contributed to the literature review, consensus recommendation discussions and manuscript editing. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Carla Scaroni, who co-reviewed with Alessandro Mondin; Pinar Kadioglu; and Pedro Marques for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fleseriu, M., Gurnell, M., McCormack, A. et al. Pituitary incidentaloma: a Pituitary Society international consensus guideline statement. Nat Rev Endocrinol 21, 638–655 (2025). https://doi.org/10.1038/s41574-025-01134-8
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41574-025-01134-8