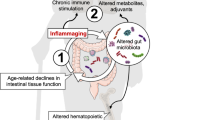
Abstract
The gut microbiome is a contributory factor in ageing-related health loss and in several non-communicable diseases in all age groups. Some age-linked and disease-linked compositional and functional changes overlap, while others are distinct. In this Review, we explore targeted studies of the gut microbiome of older individuals and general cohort studies across geographically distinct populations. We also address the promise of the targeted restoration of microorganisms associated with healthier ageing.
Key points
-
The gut microbiome is a transducer of environmental signals, modifies the risk of disease across all age groups and changes with host age.
-
Age-related alterations in the gut microbiome are influenced by personal factors, including progressive physiological deterioration, as well as by lifestyle-linked factors such as diet, medication and reduced social contact.
-
Age-related and disease-related deterioration in the gut microbiome of older people reflect overlapping interactive but distinct processes.
-
Resetting gut microbiome-derived signals of ‘unhealthy’ ageing through personalized or subpopulation-level microbiome-associated interventions is a new area of research informed by large shotgun metagenomics-based studies and data analytics.
-
Gut microbiome-based therapeutics for older people will need combined approaches, including dietary intervention with microbial restoration of lost strains.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
UN. World Population Ageing 2019: Highlights (ST/ESA/SER.A/430) (United Nations, 2019).
Shanahan, F., Ghosh, T. S. & O’Toole, P. W. The healthy microbiome — what is the definition of a healthy gut microbiome? Gastroenterology 160, 483–494 (2021). This review investigates the concept of a ‘healthy’ gut microbiome and the factors that complicate the precise definition of a universal healthy gut microbiome.
Ley, R. E. et al. Evolution of mammals and their gut microbes. Science 320, 1647–1651 (2008).
Almeida, A. et al. A new genomic blueprint of the human gut microbiota. Nature 568, 499–504 (2019).
Sender, R., Fuchs, S. & Milo, R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cell humans. Cell 164, 337–340 (2016).
Lynch, S. V. & Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379 (2016). A comprehensive review that describes the role of the human intestinal microbiota in modulating various aspects of our physiology.
Brown, J. M. & Hazen, S. L. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 66, 343–359 (2015).
Valles-Colomer, M. et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632 (2019). This study identified specific gut bacterial lineages and metabolites predicted to act upon the brain–gut axis.
Borrel, G. et al. Genomics and metagenomics of trimethylamine-utilizing Archaea in the human gut microbiome. ISME J. 11, 2059–2074 (2017).
Janney, A., Powrie, F. & Mann, E. H. Host-microbiota maladaptation in colorectal cancer. Nature 585, 509–517 (2020).
Flint, H. J., Scott, K. P., Louis, P. & Duncan, S. H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 9, 577–589 (2012).
Krautkramer, K. A., Fan, J. & Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 19, 77–94 (2021). This review provides an overview of the major classes of gut microbial metabolites with clinical and translational implications, an overview of available methodologies for profiling the gut microbial metabolome, and a framework for integrating this data with other multi-omics layers.
Fischbach, M. A. & Segre, J. A. Signaling in host-associated microbial communities. Cell 164, 1288–1300 (2016).
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016).
Scheithauer, T. P. M. et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 11, 571731 (2020).
Couto, M. R., Gonçalves, P., Magro, F. & Martel, F. Microbiota-derived butyrate regulates intestinal inflammation: focus on inflammatory bowel disease. Pharmacol. Res. 159, 104947 (2020).
Lebeer, S. et al. Identification of probiotic effector molecules: present state and future perspectives. Curr. Opin. Biotechnol. 49, 217–223 (2018).
Schiavi, E. et al. The surface-associated exopolysaccharide of bifidobacterium longum 35624 plays an essential role in dampening host proinflammatory responses and repressing local TH17 responses. Appl. Environ. Microbiol. 82, 7185–7196 (2016).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Quévrain, E. et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 65, 415–425 (2016).
Miquel, S. et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio https://doi.org/10.1128/mBio.00300-15 (2015).
Landman, C. et al. Inter-kingdom effect on epithelial cells of the N-acyl homoserine lactone 3-oxo-C12:2, a major quorum-sensing molecule from gut microbiota. PLoS ONE 13, e0202587 (2018).
Burokas, A., Moloney, R. D., Dinan, T. G. & Cryan, J. F. Microbiota regulation of the Mammalian gut-brain axis. Adv. Appl. Microbiol. 91, 1–62 (2015).
Wang, H., Braun, C., Murphy, E. F. & Enck, P. Bifidobacterium longum 1714™ strain modulates brain activity of healthy volunteers during social stress. Am. J. Gastroenterol. 114, 1152–1162 (2019).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013). A pioneering review that describes nine physiological hallmarks of biological ageing.
Pellanda, P., Ghosh, T. S. & O’Toole, P. W. Understanding the impact of age-related changes in the gut microbiome on chronic diseases and the prospect of elderly-specific dietary interventions. Curr. Opin. Biotechnol. 70, 48–55 (2020).
Cryan, J. F. et al. The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012). One of the earliest large-scale studies investigating the gut microbiome composition in older people.
Stein, M. M. et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N. Engl. J. Med. 375, 411–421 (2016).
Sarkar, A. et al. Microbial transmission in animal social networks and the social microbiome. Nat. Ecol. Evol. 4, 1020–1035 (2020).
Lax, S. et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345, 1048–1052 (2014).
Brito, I. L. et al. Transmission of human-associated microbiota along family and social networks. Nat. Microbiol. 4, 964–971 (2019).
Song, S. J. et al. Cohabiting family members share microbiota with one another and with their dogs. eLife 2, e00458 (2013).
Johnson, K. V. A. Gut microbiome composition and diversity are related to human personality traits. Hum. Microbiome J. 15, 100069 (2020).
Keohane, D. M. et al. Microbiome and health implications for ethnic minorities after enforced lifestyle changes. Nat. Med. https://doi.org/10.1038/s41591-020-0963-8 (2020).
Xu, C., Zhu, H. & Qiu, P. Aging progression of human gut microbiota. BMC Microbiol. 19, 236 (2019).
Odamaki, T. et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 16, 90 (2016).
Biagi, E. et al. Gut microbiota and extreme longevity. Curr. Biol. 26, 1480–1485 (2016). The first study to investigate the gut microbiome of semi-centenarians and identify an enrichment of sub-dominant species and specific health-associated taxa in centenarians.
Rampelli, S. et al. Shotgun metagenomics of gut microbiota in humans with up to extreme longevity and the increasing role of xenobiotic degradation. mSystems https://doi.org/10.1128/mSystems.00124-20 (2020).
Wu, L. et al. A cross-sectional study of compositional and functional profiles of gut microbiota in Sardinian centenarians. mSystems https://doi.org/10.1128/mSystems.00325-19 (2019).
Kong, F. et al. Gut microbiota signatures of longevity. Curr. Biol. 26, R832–R833 (2016).
Wang, N. et al. Enriched taxa were found among the gut microbiota of centenarians in East China. PLoS ONE 14, e0222763 (2019).
Tuikhar, N. et al. Comparative analysis of the gut microbiota in centenarians and young adults shows a common signature across genotypically non-related populations. Mech. Ageing Dev. 179, 23–35 (2019).
Kashtanova, D. A. et al. A cross-sectional study of the gut microbiota composition in Moscow long-livers. Microorganisms https://doi.org/10.3390/microorganisms8081162 (2020).
Jeffery, I. B., Lynch, D. B. & O’Toole, P. W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 10, 170–182 (2016). This study identified distinct microbiome configurations associated with community-dwelling older people and older individuals in residential care homes, with individuals in residential care homes demonstrating a gradual shift from their initial microbiome.
Ghosh, T. S., Das, M., Jeffery, I. B. & O’Toole, P. W. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. eLife 9, e50240 (2020). A meta-analysis of gut microbiome compositions covering patients with five major diseases, the study observed distinct variations in gut microbiome alterations (for the same disease) in older people and younger individuals, in addition to distinct disease-shared microbiome alterations.
Jackson, M. A. et al. Signatures of early frailty in the gut microbiota. Genome Med. 8, 8 (2016).
Lim, M. Y., Hong, S., Kim, J. H. & Nam, Y. D. Association between gut microbiome and frailty in the older adult population in Korea. J. Gerontol. Ser. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glaa319 (2021).
Maffei, V. J. et al. Biological aging and the human gut microbiota. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 72, 1474–1482 (2017).
Zhang, X. et al. Viral and host factors related to the clinical outcome of COVID-19. Nature https://doi.org/10.1038/s41586-020-2355-0 (2020).
Picca, A. et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the BIOSPHERE Study. Nutrients https://doi.org/10.3390/nu12010065 (2019).
Langsetmo, L. et al. The association between objectively measured physical activity and the gut microbiome among older community dwelling men. J. Nutr. Health Aging 23, 538–546 (2019).
Fart, F. et al. Differences in gut microbiome composition between senior orienteering athletes and community-dwelling older adults. Nutrients https://doi.org/10.3390/nu12092610 (2020).
Taniguchi, H. et al. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol. Rep. 6, e13935 (2018).
Anderson, J. R. et al. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 38, 104–107 (2017).
Manderino, L. et al. Preliminary evidence for an association between the composition of the gut microbiome and cognitive function in neurologically healthy older adults. J. Int. Neuropsychol. Soc. 23, 700–705 (2017).
Haran, J. P. et al. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio https://doi.org/10.1128/mBio.00632-19 (2019).
Chen, J., Wang, Q., Wang, A. & Lin, Z. Structural and functional characterization of the gut microbiota in elderly women with migraine. Front. Cell. Infect. Microbiol. 9, 470 (2019).
Das, M. et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 58, 2295–2304 (2019).
Li, C. et al. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos. Int. 30, 1003–1013 (2019).
Le Roy, C. I. et al. Dissecting the role of the gut microbiota and diet on visceral fat mass accumulation. Sci. Rep. 9, 9758 (2019).
Zhong, X. et al. Gut microbiota associations with metabolic health and obesity status in older adults. Nutrients https://doi.org/10.3390/nu12082364 (2020).
Margiotta, E. et al. Gut microbiota composition and frailty in elderly patients with chronic kidney disease. PLoS ONE 15, e0228530 (2020).
Luan, Z. et al. Metagenomics study reveals changes in gut microbiota in centenarians: a cohort study of Hainan centenarians. Front. Microbiol. 11, 1474 (2020).
Duvallet, C., Gibbons, S. M., Gurry, T., Irizarry, R. A. & Alm, E. J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 8, 1784 (2017).
Pasolli, E., Truong, D. T., Malik, F., Waldron, L. & Segata, N. Machine learning meta-analysis of large metagenomic datasets: tools and biological insights. PLoS Comput. Biol. 12, e1004977 (2016).
DeJong, E. N., Surette, M. G. & Bowdish, D. M. E. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe 28, 180–189 (2020).
Tomás-Barberán, F. A., García-Villalba, R., González-Sarrías, A., Selma, M. V. & Espín, J. C. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 62, 6535–6538 (2014).
Cortés-Martín, A. et al. The gut microbiota urolithin metabotypes revisited: the human metabolism of ellagic acid is mainly determined by aging. Food Funct. 9, 4100–4106 (2018).
Selma, M. V. et al. Isolation of human intestinal bacteria capable of producing the bioactive metabolite isourolithin a from ellagic acid. Front. Microbiol. 8, 1521 (2017).
He, Z. et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 68, 289–300 (2019).
Pleguezuelos-Manzano, C. et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature 580, 269–273 (2020).
Wu, J., Li, Q. & Fu, X. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl. Oncol. 12, 846–851 (2019).
Sung, J. et al. Global metabolic interaction network of the human gut microbiota for context-specific community-scale analysis. Nat. Commun. 8, 15393 (2017).
Cani, P. D. et al. Microbial regulation of organismal energy homeostasis. Nat. Metab. 1, 34–46 (2019).
Boehme, M. et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat. Aging 1, 666–676 (2021).
Marizzoni, M. et al. Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J. Alzheimers Dis. 78, 683–697 (2020).
Getachew, B., Csoka, A. B., Bhatti, A., Copeland, R. L. & Tizabi, Y. Butyrate protects against salsolinol-induced toxicity in SH-SY5Y cells: implication for Parkinson’s disease. Neurotox. Res. 38, 596–602 (2020).
Bodogai, M. et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aat4271 (2018).
Shao, Y., Gao, Z., Marks, P. A. & Jiang, X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc. Natl Acad. Sci. USA 101, 18030–18035 (2004).
Sovran, B. et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci. Rep. 9, 1437 (2019).
van Beek, A. A. et al. Frontline science: tryptophan restriction arrests B cell development and enhances microbial diversity in WT and prematurely aging Ercc1(-/Δ7) mice. J. Leukoc. Biol. 101, 811–821 (2017).
Fransen, F. et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front. Immunol. 8, 1385 (2017).
Bárcena, C. et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 25, 1234–1242 (2019). This study showed the efficacy of FMT in modulating progeria in a mouse model.
Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019). This study linked supplementation with the health-associated Akkermansia muciniphila and improved insulin sensitivity and cardiometabolic health.
Cekanaviciute, E. et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl Acad. Sci. USA 114, 10713–10718 (2017).
Haikal, C., Chen, Q. Q. & Li, J. Y. Microbiome changes: an indicator of Parkinson’s disease? Transl. Neurodegener. 8, 38 (2019).
Ansaldo, E. et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 364, 1179–1184 (2019).
Siddharth, J. et al. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging 9, 1698–1720 (2017).
Fielding, R. A. et al. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 127, 110722 (2019).
Conley, M. N. et al. Aging and serum MCP-1 are associated with gut microbiome composition in a murine model. PeerJ 4, e1854 (2016).
Hirose, M. et al. Mitochondrial gene polymorphism is associated with gut microbial communities in mice. Sci. Rep. 7, 15293 (2017).
Sato, Y. et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 599, 458–464 (2021).
Han, B. et al. Microbial genetic composition tunes host longevity. Cell 169, 1249–1262.e13 (2017).
La-Ongkham, O., Nakphaichit, M., Nakayama, J., Keawsompong, S. & Nitisinprasert, S. Age-related changes in the gut microbiota and the core gut microbiome of healthy Thai humans. 3 Biotech 10, 276 (2020).
Park, S. H. et al. Comparative analysis of gut microbiota in elderly people of urbanized towns and longevity villages. BMC Microbiol. 15, 49 (2015).
Zhang, X. et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat. Aging 1, 87–100 (2021).
Rahayu, E. S. et al. Gut microbiota profile in healthy Indonesians. World J. Gastroenterol. 25, 1478–1491 (2019).
Wang, W. et al. Cholecystectomy damages aging-associated intestinal microbiota construction. Front. Microbiol. 9, 1402 (2018).
Bian, G. et al. The gut microbiota of healthy aged Chinese is similar to that of the healthy young. mSphere https://doi.org/10.1128/mSphere.00327-17 (2017).
de la Cuesta-Zuluaga, J. et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems https://doi.org/10.1128/mSystems.00261-19 (2019).
Zhang, S. et al. Gut microbiota in healthy and unhealthy long-living people. Gene 779, 145510 (2021).
Wilmanski, T. et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 3, 274–286 (2021).
Ghosh, T. S. et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 69, 1218–1228 (2020). One of the largest dietary intervention studies for older people that demonstrated the role of the gut microbiota in transducing the signals of a beneficial diet.
Ostan, R. et al. Impact of diet and nutraceutical supplementation on inflammation in elderly people. Results from the RISTOMED study, an open-label randomized control trial. Clin. Nutr. 35, 812–818 (2016).
Sanborn, V., Azcarate-Peril, M. A., Updegraff, J., Manderino, L. & Gunstad, J. Randomized clinical trial examining the impact of lactobacillus rhamnosus GG probiotic supplementation on cognitive functioning in middle-aged and older adults. Neuropsychiatr. Dis. Treat. 16, 2765–2777 (2020).
Shinkai, S. et al. Immunoprotective effects of oral intake of heat-killed Lactobacillus pentosus strain b240 in elderly adults: a randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 109, 1856–1865 (2013).
Agrawal, M. et al. The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated Clostridium difficile infection in 146 elderly individuals. J. Clin. Gastroenterol. 50, 403–407 (2016).
Qu, H., Zhang, Y., Chai, H., Gao, Z. Y. & Shi, D. Z. Effects of microbiota-driven therapy on inflammatory responses in elderly individuals: a systematic review and meta-analysis. PLoS ONE 14, e0211233 (2019).
Macfarlane, S., Cleary, S., Bahrami, B., Reynolds, N. & Macfarlane, G. T. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 38, 804–816 (2013).
Nyangale, E. P., Farmer, S., Keller, D., Chernoff, D. & Gibson, G. R. Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of Bacillus coagulans GBI-30, 6086. Anaerobe 30, 75–81 (2014).
Björklund, M. et al. Gut microbiota of healthy elderly NSAID users is selectively modified with the administration of Lactobacillus acidophilus NCFM and lactitol. Age 34, 987–999 (2012).
Spaiser, S. J. et al. Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2 ingestion induces a less inflammatory cytokine profile and a potentially beneficial shift in gut microbiota in older adults: a randomized, double-blind, placebo-controlled, crossover study. J. Am. Coll. Nutr. 34, 459–469 (2015).
Leblhuber, F., Steiner, K., Schuetz, B., Fuchs, D. & Gostner, J. M. Probiotic supplementation in patients with Alzheimer’s dementia — an explorative intervention study. Curr. Alzheimer Res. 15, 1106–1113 (2018).
Nilsson, A. G., Sundh, D., Bäckhed, F. & Lorentzon, M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 284, 307–317 (2018).
An, R. et al. Age-dependent changes in GI physiology and microbiota: time to reconsider? Gut 67, 2213–2222 (2018). This review describes age-related alterations in gastrointestinal physiology and function and gut microbiome changes during frailty.
Buigues, C. et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int. J. Mol. Sci. https://doi.org/10.3390/ijms17060932 (2016).
Igwe, E. O. et al. Anthocyanin-rich plum juice reduces ambulatory blood pressure but not acute cognitive function in younger and older adults: a pilot crossover dose-timing study. Nutr. Res. 47, 28–43 (2017).
Przemska-Kosicka, A. et al. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Immun. Ageing 13, 6 (2016).
Enani, S. et al. Impact of ageing and a synbiotic on the immune response to seasonal influenza vaccination; a randomised controlled trial. Clin. Nutr. 37, 443–451 (2018).
Akatsu, H. et al. Lactobacillus in jelly enhances the effect of influenza vaccination in elderly individuals. J. Am. Geriatr. Soc. 61, 1828–1830 (2013).
Maruyama, M. et al. The effects of non-viable Lactobacillus on immune function in the elderly: a randomised, double-blind, placebo-controlled study. Int. J. Food Sci. Nutr. 67, 67–73 (2016).
Assisi, R. F. Combined butyric acid/mesalazine treatment in ulcerative colitis with mild-moderate activity. Results of a multicentre pilot study. Minerva Gastroenterol. Dietol. 54, 231–238 (2008).
Govindarajan, N., Agis-Balboa, R. C., Walter, J., Sananbenesi, F. & Fischer, A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J. Alzheimers Dis. 26, 187–197 (2011).
Bamba, S. et al. Successful treatment by fecal microbiota transplantation for Japanese patients with refractory Clostridium difficile infection: a prospective case series. J. Microbiol. Immunol. 52, 663–666 (2019).
Friedman-Korn, T. et al. Fecal transplantation for treatment of clostridium difficile infection in elderly and debilitated patients. Dig. Dis. Sci. 63, 198–203 (2018).
Cheng, Y. W. & Fischer, M. The present status of fecal microbiota transplantation and its value in the elderly. Curr. Treat. Options Gastroenterol. 15, 349–362 (2017).
Xie, W. R., Yang, X. Y., Xia, H. H., Wu, L. H. & He, X. X. Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: a case report and review of the literature. World J. Clin. Cases 7, 3074–3081 (2019).
Ntemiri, A. et al. Whole blueberry and isolated polyphenol-rich fractions modulate specific gut microbes in an in vitro colon model and in a pilot study in human consumers. Nutrients https://doi.org/10.3390/nu12092800 (2020).
Del Bo, C. et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: The MaPLE randomised controlled trial. Clin. Nutr. https://doi.org/10.1016/j.clnu.2020.12.014 (2020).
Tran, T. T. T. et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome 7, 39 (2019).
Alfa, M. J. et al. A randomized placebo controlled clinical trial to determine the impact of digestion resistant starch MSPrebiotic(®) on glucose, insulin, and insulin resistance in elderly and mid-age adults. Front. Med. 4, 260 (2017).
Cancello, R. et al. Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients https://doi.org/10.3390/nu11123011 (2019).
Theou, O. et al. Can a prebiotic formulation reduce frailty levels in older people? J. Frailty Aging 8, 48–52 (2019).
Hidalgo-Liberona, N. et al. Increased intestinal permeability in older subjects impacts the beneficial effects of dietary polyphenols by modulating their bioavailability. J. Agric. Food Chem. 68, 12476–12484 (2020).
Piggott, D. A. & Tuddenham, S. The gut microbiome and frailty. Transl. Res. 221, 23–43 (2020).
Sanders, M. Lactic Acid Bacteria as Promoters of Human Health 1st edn (Chapman & Hall, 1994).
Picard, C. et al. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 22, 495–512 (2005).
Fukuda, S., Toh, H., Taylor, T. D., Ohno, H. & Hattori, M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 3, 449–454 (2012).
Kumar, A., Alrefai, W. A., Borthakur, A. & Dudeja, P. K. Lactobacillus acidophilus counteracts enteropathogenic E. coli-induced inhibition of butyrate uptake in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G602–G607 (2015).
Alfa, M. J. et al. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 37, 797–807 (2018).
Kim, C. S. et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 76, 32–40 (2021).
Nyangale, E. P. et al. Bacillus coagulans GBI-30, 6086 modulates Faecalibacterium prausnitzii in older men and women. J. Nutr. 145, 1446–1452 (2015).
Costabile, A. et al. Effects of soluble corn fiber alone or in synbiotic combination with lactobacillus rhamnosus gg and the pilus-deficient derivative GG-PB12 on fecal microbiota, metabolism, and markers of immune function: a randomized, double-blind, placebo-controlled, crossover study in healthy elderly (Saimes Study). Front. Immunol. 8, 1443 (2017).
Nagpal, R., Neth, B. J., Wang, S., Craft, S. & Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47, 529–542 (2019).
Kojima, G., Avgerinou, C., Iliffe, S. & Walters, K. Adherence to Mediterranean diet reduces incident frailty risk: systematic review and meta-analysis. J. Am. Geriatr. Soc. 66, 783–788 (2018).
Shanahan, F., van Sinderen, D., O’Toole, P. W. & Stanton, C. Feeding the microbiota: transducer of nutrient signals for the host. Gut 66, 1709–1717 (2017). This review describes the tripartite interaction between diet, gut microbiota, and human health and how it can be investigated to formulate interventions for addressing non-communicable inflammatory diseases.
Wang, D. D. et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 27, 333–343 (2021). A key study showing how the baseline gut microbiome of the host influences the beneficial response to a Mediterranean diet.
Meslier, V. et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 69, 1258–1268 (2020).
Vitale, M. et al. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: can SCFAs play a role? Clin. Nutr. 40, 428–437 (2021).
Asnicar, F. et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 27, 321–332 (2021).
Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26, 964–973 (2020).
Menni, C. et al. High intake of vegetables is linked to lower white blood cell profile and the effect is mediated by the gut microbiome. BMC Med. 19, 37 (2021).
O’Toole, P. W. & Jeffery, I. B. Gut microbiota and aging. Science 350, 1214–1215 (2021).
Marmot, M. Health Equity in England: The Marmot Review 10 Years On (Institute of Health Equity, Health Foundation, UK, 2020).
The Food Foundation. The Broken Plate. The Food Foundation https://foodfoundation.org.uk/publication/broken-plate-2019 (2019).
Rastelli, M., Knauf, C. & Cani, P. D. Gut microbes and health: a focus on the mechanisms linking microbes, obesity, and related disorders. Obesity 26, 792–800 (2018).
Régnier, M., Van Hul, M., Knauf, C. & Cani, P. D. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J. Endocrinol. https://doi.org/10.1530/joe-20-0473 (2020).
Pittayanon, R. et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterology 158, 930–946.e1 (2020).
Ni, J., Wu, G. D., Albenberg, L. & Tomov, V. T. Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584 (2017).
Jeffery, I. B. et al. Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Gastroenterology 158, 1016–1028.e1018 (2020).
Bhattarai, Y., Muniz Pedrogo, D. A. & Kashyap, P. C. Irritable bowel syndrome: a gut microbiota-related disorder? Am. J. Physiol. Gastrointest. Liver Physiol. https://doi.org/10.1152/ajpgi.00338.2016 (2017).
Pathak, P. et al. Small molecule inhibition of gut microbial choline trimethylamine lyase activity alters host cholesterol and bile acid metabolism. Am. J. Physiol. Heart Circ. Physiol. https://doi.org/10.1152/ajpheart.00584.2019 (2020).
Lach, G., Schellekens, H., Dinan, T. G. & Cryan, J. F. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15, 36–59 (2018).
Ruiz-Ruiz, S. et al. Functional microbiome deficits associated with ageing: chronological age threshold. Aging Cell 19, e13063 (2020).
Iwauchi, M. et al. Relationship between oral and gut microbiota in elderly people. Immun. Inflamm. Dis. 7, 229–236 (2019).
Collino, S. et al. Metabolic signatures of extreme longevity in Northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS ONE 8, e56564 (2013).
Ke, Y. et al. Gut flora-dependent metabolite trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic. Biol. Med. 116, 88–100 (2018).
Kim, B. S. et al. Comparison of the gut microbiota of centenarians in longevity villages of South Korea with those of other age groups. J. Microbiol. Biotechnol. 29, 429–440 (2019).
Huang, S. et al. Human skin, oral, and gut microbiomes predict chronological age. mSystems https://doi.org/10.1128/mSystems.00630-19 (2020).
Galkin, F. et al. Human gut microbiome aging clock based on taxonomic profiling and deep learning. iScience 23, 101199 (2020).
Verdi, S. et al. An investigation into physical frailty as a link between the gut microbiome and cognitive health. Front. Aging Neurosci. 10, 398 (2018).
Heinzel, S. et al. Gut microbiome signatures of risk and prodromal markers of Parkinson disease. Ann. Neurol. 88, 320–331 (2020).
Singh, H. et al. Gastro-intestinal and oral microbiome signatures associated with healthy aging. GeroScience 41, 907–921 (2019).
Brunt, V. E. et al. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 76, 101–112 (2020).
He, W. et al. Trimethylamine N-oxide, a gut microbiota-dependent metabolite, is associated with frailty in older adults with cardiovascular disease. Clin. Interv. Aging 15, 1809–1820 (2020).
Brunt, V. E. et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J. Physiol. 597, 2361–2378 (2019).
Brunt, V. E. et al. The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience https://doi.org/10.1007/s11357-020-00257-2 (2020).
Li, D. et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 17, e12768 (2018).
Lin, H. et al. The role of gut microbiota metabolite trimethylamine N-oxide in functional impairment of bone marrow mesenchymal stem cells in osteoporosis disease. Ann. Transl. Med. 8, 1009 (2020).
Thomas, A. M. et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. https://doi.org/10.1038/s41591-019-0405-7 (2019).
Pignanelli, M. et al. Moderate renal impairment and toxic metabolites produced by the intestinal microbiome: dietary implications. J. Ren. Nutr. 29, 55–64 (2019).
Gutiérrez-Díaz, I. et al. Could fecal phenylacetic and phenylpropionic acids be used as indicators of health status? J. Agric. Food Chem. 66, 10438–10446 (2018).
Pascucci, T. et al. P-cresol alters brain dopamine metabolism and exacerbates autism-like behaviors in the BTBR mouse. Brain Sci. https://doi.org/10.3390/brainsci10040233 (2020).
MahmoudianDehkordi, S. et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease — an emerging role for gut microbiome. Alzheimers Dement. 15, 76–92 (2019).
Wirbel, J. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. https://doi.org/10.1038/s41591-019-0406-6 (2019).
Sears, C. L. & Garrett, W. S. Microbes, microbiota, and colon cancer. Cell Host Microbe 15, 317–328 (2014).
Awoyemi, A., Trøseid, M., Arnesen, H., Solheim, S. & Seljeflot, I. Markers of metabolic endotoxemia as related to metabolic syndrome in an elderly male population at high cardiovascular risk: a cross-sectional study. Diabetol. Metab. Syndr. 10, 59 (2018).
Lin, H. V. et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 7, e35240 (2012).
Jia, Y. et al. Butyrate stimulates adipose lipolysis and mitochondrial oxidative phosphorylation through histone hyperacetylation-associated β(3) -adrenergic receptor activation in high-fat diet-induced obese mice. Exp. Physiol. 102, 273–281 (2017).
Litvak, Y., Byndloss, M. X. & Bäumler, A. J. Colonocyte metabolism shapes the gut microbiota. Science https://doi.org/10.1126/science.aat9076 (2018).
Sahuri-Arisoylu, M. et al. Reprogramming of hepatic fat accumulation and ‘browning’ of adipose tissue by the short-chain fatty acid acetate. Int. J. Obes. 40, 955–963 (2016).
Mitchell, S. M. et al. A period of 10 weeks of increased protein consumption does not alter faecal microbiota or volatile metabolites in healthy older men: a randomised controlled trial. J. Nutr. Sci. 9, e25 (2020).
Igwe, E. O. et al. Low anthocyanin plum nectar does not impact cognition, blood pressure and gut microbiota in healthy older adults: A randomized crossover trial. Nutr. Res. 82, 74–87 (2020).
Ruiz-Saavedra, S. et al. Comparison of different dietary indices as predictors of inflammation, oxidative stress and intestinal microbiota in middle-aged and elderly subjects. Nutrients https://doi.org/10.3390/nu12123828 (2020).
Zengul, A. G. et al. Associations between dietary fiber, the fecal microbiota and estrogen metabolism in postmenopausal women with breast cancer. Nutr. Cancer https://doi.org/10.1080/01635581.2020.1784444 (2020).
An, R. et al. Sugar beet pectin supplementation did not alter profiles of fecal microbiota and exhaled breath in healthy young adults and healthy elderly. Nutrients https://doi.org/10.3390/nu11092193 (2019).
Chung, W. S. F. et al. Relative abundance of the Prevotella genus within the human gut microbiota of elderly volunteers determines the inter-individual responses to dietary supplementation with wheat bran arabinoxylan-oligosaccharides. BMC Microbiol. 20, 283 (2020).
Watson, A. W. et al. Changes in stool frequency following chicory inulin consumption, and effects on stool consistency, quality of life and composition of gut microbiota. Food Hydrocoll. 96, 688–698 (2019).
Birkeland, E. et al. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: a randomised controlled trial. Eur. J. Nutr. 59, 3325–3338 (2020).
Gao, R., Zhang, X., Huang, L., Shen, R. & Qin, H. Gut microbiota alteration after long-term consumption of probiotics in the elderly. Probiotics Antimicrob. Proteins 11, 655–666 (2019).
Eloe-Fadrosh, E. A. et al. Functional dynamics of the gut microbiome in elderly people during probiotic consumption. mBio https://doi.org/10.1128/mBio.00231-15 (2015).
Kotani, Y. et al. Oral intake of Lactobacillus pentosus strain b240 accelerates salivary immunoglobulin A secretion in the elderly: A randomized, placebo-controlled, double-blind trial. Immun. Ageing 7, 11 (2010).
Andreux, P. A. et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 1, 595–603 (2019).
Cai, T., Shi, X., Yuan, L.-Z., Tang, D. & Wang, F. Fecal microbiota transplantation in an elderly patient with mental depression. Int. Psychogeriatr. 31, 1525–1526 (2019).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
F.S. is a cofounder of three campus companies: Alimentary Health Ltd, Tucana Health Ltd (now named 4D Pharma Cork) and Atlantia Food Clinical Trials. P.W.O.T. is a cofounder of 4D Pharma Cork. T.S.G. declares no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology and Hepatology thanks Silvia Turroni, Liping Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Probiotics
-
Microbial species (or lineages) that have a beneficial effect on the host.
- Psychobiotics
-
Subgroup of probiotic bacteria that have a beneficial impact on the gut–brain axis resulting in improved behaviour or cognitive function.
- Internal transcribed spacer
-
A method of microbial community profiling similar to 16S profiling that relies on specific PCR-based amplification, sequencing and characterization of the internal spacer DNA regions between the small subunit rRNA components (16S for bacteria/archaea and 18S for eukaryotes) and the large subunit rRNA components, and is typically used for the characterization of the mycobiome of a given environment due to the superior phylogenetic granularity afforded by the sequence.
- Inter-study variations
-
Variations in the patterns of gut microbiome alterations detected across different studies investigating the same (or similar) host trait or phenotype (for example, age, BMI, a disease or disorder, or specific dietary patterns).
- Shotgun metagenomics
-
A method of community genomic profiling that involves the extraction and sequencing of the entire genomic content of all cells belonging to a given microbial community.
- Prebiotic
-
Nutritional supplements designed to increase the abundance of specific target groups of beneficial microorganisms based on restricted catabolic ability in certain taxa.
- Synbiotics
-
Single or consortia of beneficial microorganisms that are administered in combination with prebiotics.
- Postbiotics
-
Deliberately inactivated (heat-killed) microbial cells, cell components or microbiota-derived metabolites that confer health benefits.
- Quantitative polymerase chain reaction
-
(qPCR). A method of real-time microbial quantification in a given sample that relies on measuring the change in PCR amplicon copy number (in this context, amplified by primers designed against a phylogenetic marker gene corresponding to a given species or a microbial group) during the cycles of the PCR; copy numbers of phylogenetic marker gene regions belonging to microbial lineages in higher abundance are expected to be amplified faster, resulting in a faster increase in the strength of fluorescent oligonucleotide probes targeted at these lineages.
- Fluorescence in situ hybridization
-
(FISH). A method of physically locating or enumerating the population of a specific microorganism or cell type by using fluorescent oligonucleotide probes that bind to specific genomic sequences in the target cells with high sequence complementarity; it can be used to measure either specific bacterial species or overall bacterial cell abundance (by using probes towards the universally conserved regions of the 16S rRNA gene).
- 16S profiling
-
(Also known as 16S rRNA sequencing). A method of microbial community profiling that relies on specific polymerase chain reaction (PCR)-based amplification, sequencing and phylogenetic profiling of either the full length or specific sub-regions of the 16S rRNA marker gene across all bacterial and archaeal cells present in a given sample.
Rights and permissions
About this article
Cite this article
Ghosh, T.S., Shanahan, F. & O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat Rev Gastroenterol Hepatol 19, 565–584 (2022). https://doi.org/10.1038/s41575-022-00605-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-022-00605-x
This article is cited by
-
Exploring the gut microbiota alterations with obesity in older adults: a case-control study in the context of Bushehr Elderly Health (BEH) program
Journal of Health, Population and Nutrition (2026)
-
Exploring gut microbiota mechanisms in frailty induced by 5-hydroxymethylfurfural: evidence from mouse models and Mendelian randomization
Nutrition & Metabolism (2026)
-
Lifetime existence of a core of mutualistic symbionts and functionally uncoupled taxa in the gut of a Mediterranean cohort
Scientific Reports (2026)
-
Gut dysbiosis in oncology: a risk factor for immunoresistance
Cell Research (2026)
-
Early-onset Palmijihwang-hwan treatment modulates phospholipid metabolism and gut microbiota for healthy aging: reducing adipose inflammation and oxidative stress
npj Aging (2026)