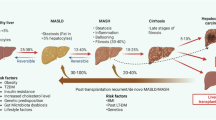
Abstract
Since 1980, the cumulative effort of scientists and health-care stakeholders has advanced the prerequisites to address metabolic dysfunction-associated steatotic liver disease (MASLD), a prevalent chronic non-communicable liver disease. This effort has led to, among others, the approval of the first drug specific for metabolic dysfunction-associated steatohepatitis (MASH; formerly known as nonalcoholic steatohepatitis). Despite substantial progress, MASLD is still a leading cause of advanced chronic liver disease, including primary liver cancer. This Perspective contextualizes the nomenclature change from nonalcoholic fatty liver disease to MASLD and proposes important considerations to accelerate further progress in the field, optimize patient-centric multidisciplinary care pathways, advance pharmacological, behavioural and diagnostic research, and address health disparities. Key regulatory and other steps necessary to optimize the approval and access to upcoming additional pharmacological therapeutic agents for MASH are also outlined. We conclude by calling for increased education and awareness, enhanced health system preparedness, and concerted action by policy-makers to further the public health and policy agenda to achieve at least parity with other non-communicable diseases and to aid in growing the community of practice to reduce the human and economic burden and end the public health threat of MASLD and MASH by 2030.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zelman, S. The liver in obesity. Arch. Intern. Med. 90, 141–156 (1952).
Adler, M. & Schaffner, F. Fatty liver hepatitis and cirrhosis in obese patients. Am. J. Med. 67, 811–816 (1979).
Ludwig, J., Viggiano, T. R., McGill, D. B. & Oh, B. J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 55, 434–438 (1980).
Matteoni, C. A. et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116, 1413–1419 (1999).
Loomba, R., Friedman, S. L. & Shulman, G. I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184, 2537–2564 (2021).
Targher, G., Corey, K. E., Byrne, C. D. & Roden, M. The complex link between NAFLD and type 2 diabetes mellitus — mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 18, 599–612 (2021).
Smith, G. I. et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest. 130, 1453–1460 (2020).
Yang, S. Q., Lin, H. Z., Lane, M. D., Clemens, M. & Diehl, A. M. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc. Natl Acad. Sci. USA 94, 2557–2562 (1997).
Lin, H. Z. et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat. Med. 6, 998–1003 (2000).
Li, Z. et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 37, 343–350 (2003).
Sawada, K., Chung, H., Softic, S., Moreno-Fernandez, M. E. & Divanovic, S. The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease. Cell Metab. 35, 1852–1871 (2023).
Cope, K., Risby, T. & Diehl, A. M. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology 119, 1340–1347 (2000).
Rangwala, F. et al. Increased production of sonic hedgehog by ballooned hepatocytes. J. Pathol. 224, 401–410 (2011).
Guy, C. D. et al. Treatment response in the PIVENS trial is associated with decreased Hedgehog pathway activity. Hepatology 61, 98–107 (2015).
Rui, L. & Lin, J. D. Reprogramming of hepatic metabolism and microenvironment in nonalcoholic steatohepatitis. Annu. Rev. Nutr. 42, 91–113 (2022).
Sanyal, A. J. et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med. 385, 1559–1569 (2021).
Fromenty, B. & Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 78, 415–429 (2023).
Cheng, Y.-W., Liu, J. & Finkel, T. Mitohormesis. Cell Metab. 35, 1872–1886 (2023).
Andréasson, C., Ott, M. & Büttner, S. Mitochondria orchestrate proteostatic and metabolic stress responses. EMBO Rep. 20, e47865 (2019).
Tzouanas, C. N. et al. Chronic metabolic stress drives developmental programs and loss of tissue functions in non-transformed liver that mirror tumor states and stratify survival. Preprint at bioRxiv https://doi.org/10.1101/2023.11.30.569407 (2023).
Chen, T. et al. Hepatocyte smoothened activity controls susceptibility to insulin resistance and nonalcoholic fatty liver disease. Cell Mol. Gastroenterol. Hepatol. 15, 949–970 (2023).
Chen, Y. et al. The oxidative aging model integrated various risk factors in type 2 diabetes mellitus at system level. Front. Endocrinol. 14, 1196293 (2023).
Ronayne, C. T. & Latorre-Muro, P. Navigating the landscape of mitochondrial-ER communication in health and disease. Front. Mol. Biosci. 11, 1356500 (2024).
Fyhrquist, F., Saijonmaa, O. & Strandberg, T. The roles of senescence and telomere shortening in cardiovascular disease. Nat. Rev. Cardiol. 10, 274–283 (2013).
Eckl, E.-M., Ziegemann, O., Krumwiede, L., Fessler, E. & Jae, L. T. Sensing, signaling and surviving mitochondrial stress. Cell Mol. Life Sci. 78, 5925–5951 (2021).
Avitan, I. et al. Towards a consensus on Alzheimer’s disease comorbidity? J. Clin. Med. 10, 4360 (2021).
Maeso-Díaz, R. et al. Targeting senescent hepatocytes using the thrombomodulin-PAR1 inhibitor vorapaxar ameliorates NAFLD progression. Hepatology 78, 1209–1222 (2023).
Loomba, R. et al. DNA methylation signatures reflect aging in patients with nonalcoholic steatohepatitis. JCI Insight 3, e96685 (2018).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Flegal, K. M., Carroll, M. D., Kuczmarski, R. J. & Johnson, C. L. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int. J. Obes. Relat. Metab. Disord. 22, 39–47 (1998).
Stierman, B. et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files — Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports Number vol. 158 https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm (2021).
Stepanova, M. et al. Nonalcoholic steatohepatitis is the most common indication for liver transplantation among the elderly: data from the United States scientific registry of transplant recipients. Hepatol. Commun. 6, 1506–1515 (2022).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335–1347 (2023).
Sweeny, K. F. & Lee, C. K. Nonalcoholic fatty liver disease in children. Gastroenterol. Hepatol. 17, 579–587 (2021).
Younossi, Z. M. et al. Global survey of stigma among physicians and patients with nonalcoholic fatty liver disease. J. Hepatol. 80, 419–430 (2024).
American Association for the Study of Liver Diseases. NAFLD Nomenclature Consensus Meeting High-Level Output https://www.aasld.org/news/nafld-nomenclature-consensus-meeting-high-level-output (2022).
Rinella, M. E. et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. https://doi.org/10.1016/j.jhep.2023.06.003 (2023).
Hagström, H. et al. The future of International Classification of Diseases coding in steatotic liver disease: an expert panel Delphi consensus statement. Hepatol. Commun. 8, e0386 (2024).
Ratziu, V., Boursier, J. & AFEF Group for the Study of Liver Fibrosis. Confirmatory biomarker diagnostic studies are not needed when transitioning from NAFLD to MASLD. J. Hepatol. 80, e51–e52 (2024).
Song, S. J., Lai, J. C.-T., Wong, G. L.-H., Wong, V. W.-S. & Yip, T. C.-F. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 80, e54–e56 (2024).
Cusi, K., Younossi, Z. & Roden, M. From NAFLD to MASLD: promise and pitfalls of a new definition. Hepatology 79, E13–E15 (2024).
Eslam, M., Sanyal, A. J., George, J., & International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158, 1999–2014.e1 (2020).
Fouad, Y., Barakat, S., Hashim, A. & Ghazinyan, H. Towards unifying fatty liver nomenclature: a voice from the Middle East and North Africa. Nat. Rev. Gastroenterol. Hepatol. 21, 297–298 (2024).
Peeraphatdit, T. B. et al. A cohort study examining the interaction of alcohol consumption and obesity in hepatic steatosis and mortality. Mayo Clin. Proc. 95, 2612–2620 (2020).
Åberg, F., Byrne, C. D., Pirola, C. J., Männistö, V. & Sookoian, S. Alcohol consumption and metabolic syndrome: clinical and epidemiological impact on liver disease. J. Hepatol. 78, 191–206 (2023).
Israelsen, M., Torp, N., Johansen, S., Thiele, M. & Krag, A. MetALD: new opportunities to understand the role of alcohol in steatotic liver disease. Lancet Gastroenterol. Hepatol. 8, 866–868 (2023).
Krag, A. & Rinella, M. E. Steatotic liver disease: a new name to reflect the combined role of alcohol and metabolic dysfunction. Nat. Med. https://doi.org/10.1038/s41591-024-02849-3 (2024).
Israelsen, M. et al. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: an analysis of data from a prospective cohort study. Lancet Gastroenterol. Hepatol. https://doi.org/10.1016/S2468-1253(23)00443-0 (2024).
De, A., Bhagat, N., Mehta, M., Taneja, S. & Duseja, A. Metabolic dysfunction-associated steatotic liver disease (MASLD) definition is better than MAFLD criteria for lean patients with NAFLD. J. Hepatol. https://doi.org/10.1016/j.jhep.2023.07.031 (2023).
Charlton, M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin. Gastroenterol. Hepatol. 2, 1048–1058 (2004).
Mofrad, P. et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37, 1286–1292 (2003).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Harrison, S. A., Allen, A. M., Dubourg, J., Noureddin, M. & Alkhouri, N. Challenges and opportunities in NASH drug development. Nat. Med. 29, 562–573 (2023).
Sandrin, L. et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 29, 1705–1713 (2003).
Rouvière, O. et al. MR elastography of the liver: preliminary results. Radiology 240, 440–448 (2006).
Yin, M. et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin. Gastroenterol. Hepatol. 5, 1207–1213.e2 (2007).
Younossi, Z. M. et al. Role of noninvasive tests in clinical gastroenterology practices to identify patients with nonalcoholic steatohepatitis at high risk of adverse outcomes: expert panel recommendations. Am. J. Gastroenterol. 116, 254–262 (2021).
Rosenberg, W. M. C. et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 127, 1704–1713 (2004).
Shah, A. G. et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 1104–1112 (2009).
Angulo, P. et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45, 846–854 (2007).
Söderberg, C. et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 51, 595–602 (2010).
Rinella, M. E. et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 77, 1797–1835 (2023).
Kanwal, F. et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 161, 1657–1669 (2021).
Cusi, K. et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 28, 528–562 (2022).
Duseja, A. et al. Indian National Association for Study of the Liver (INASL) guidance paper on nomenclature, diagnosis and treatment of nonalcoholic fatty liver disease (NAFLD). J. Clin. Exp. Hepatol. 13, 273–302 (2023).
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
McPherson, S. et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. J. Gastroenterol. 112, 740–751 (2017).
Petta, S. et al. Monitoring occurrence of liver-related events and survival by transient elastography in patients with nonalcoholic fatty liver disease and compensated advanced chronic liver disease. Clin. Gastroenterol. Hepatol. 19, 806–815.e5 (2021).
Gidener, T. et al. Change in serial liver stiffness measurement by magnetic resonance elastography and outcomes in NAFLD. Hepatology 77, 268–274 (2023).
Gidener, T. et al. Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation, and death in NAFLD. Clin. Gastroenterol. Hepatol. 19, 1915–1924.e6 (2021).
Mózes, F. E. et al. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: an individual participant data meta-analysis. Lancet Gastroenterol. Hepatol. 8, 704–713 (2023).
Allen, A. M. et al. The role of three-dimensional magnetic resonance elastography in the diagnosis of nonalcoholic steatohepatitis in obese patients undergoing bariatric surgery. Hepatology 71, 510–521 (2020).
Allen, A. M. et al. Multiparametric magnetic resonance elastography improves the detection of NASH regression following bariatric. Surg. Hepatol. Commun. 4, 185–192 (2020).
Loomba, R. et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. Am. J. Gastroenterol. 111, 986–994 (2016).
Lee, H. W. et al. Discrimination of nonalcoholic steatohepatitis using transient elastography in patients with nonalcoholic fatty liver disease. PLoS ONE 11, e0157358 (2016).
Newsome, P. N. et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 5, 362–373 (2020).
Ajmera, V. et al. Liver stiffness on magnetic resonance elastography and the MEFIB index and liver-related outcomes in nonalcoholic fatty liver disease: a systematic review and meta-analysis of individual participants. Gastroenterology 163, 1079–1089.e5 (2022).
Noureddin, M. et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J. Hepatol. 76, 781–787 (2022).
Taylor-Weiner, A. et al. A machine learning approach enables quantitative measurement of liver histology and disease monitoring in NASH. Hepatology 74, 133–147 (2021).
Naoumov, N. V. et al. Digital pathology with artificial intelligence analyses provides greater insights into treatment-induced fibrosis regression in NASH. J. Hepatol. 77, 1399–1409 (2022).
Brunt, E. M. et al. Complexity of ballooned hepatocyte feature recognition: defining a training atlas for artificial intelligence-based imaging in NAFLD. J. Hepatol. 76, 1030–1041 (2022).
Soon, G. S. T. et al. Artificial intelligence improves pathologist agreement for fibrosis scores in nonalcoholic steatohepatitis patients. Clin. Gastroenterol. Hepatol. 21, 1940–1942.e3 (2023).
Iyer, J. S. et al. AI-based histologic scoring enables automated and reproducible assessment of enrollment criteria and endpoints in NASH clinical trials. Preprint at medRxiv https://doi.org/10.1101/2023.04.20.23288534 (2023).
Ratziu, V. et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128, 1898–1906 (2005).
Larson, S. P. et al. Histopathologic variability between the right and left lobes of the liver in morbidly obese patients undergoing Roux-en-Y bypass. Clin. Gastroenterol. Hepatol. 5, 1329–1332 (2007).
Bedossa, P., Dargère, D. & Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38, 1449–1457 (2003).
Younossi, Z. M. et al. A genomic and proteomic study of the spectrum of nonalcoholic fatty liver disease. Hepatology 42, 665–674 (2005).
Masoodi, M. et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol. 18, 835–856 (2021).
Sharpton, S. R., Schnabl, B., Knight, R. & Loomba, R. Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metab. 33, 21–32 (2021).
Sharpton, S. R., Ajmera, V. & Loomba, R. Emerging role of the gut microbiome in nonalcoholic fatty liver disease: from composition to function. Clin. Gastroenterol. Hepatol. 17, 296–306 (2019).
Winder, G. S. et al. ‘But I didn’t drink!’: What to do with discordant phosphatidylethanol results. Liver Transpl. 30, 213–222 (2024).
Scholten, K. et al. You can’t handle the truth! Comparing serum phosphatidylethanol to self-reported alcohol intake in chronic liver disease patients. Dig. Liver Dis. https://doi.org/10.1016/j.dld.2024.01.195 (2024).
Choi, C. & Simonetto, D. A. Digital interventions in the management of advanced liver disease: Prescription and monitoring of healthy living in homes. Clin. Liver Dis. 20, 162–165 (2022).
Sordi Chara, B. et al. Artificial intelligence-enabled stool analysis for lactulose titration assistance in hepatic encephalopathy through a smartphone application. Am. J. Gastroenterol. https://doi.org/10.14309/ajg.0000000000002656 (2024).
Allen, A. M., Charlton, M. & Hoovler, A. A cross-sectional online survey to understand existing NAFLD/NASH curriculum gaps in US primary care provider and specialty training programs. AASLD The Liver Meeting 2131A https://journals.lww.com/hep/fulltext/2023/10001/the_liver_meeting__boston,_massachusetts_nov.1.aspx (2023).
Younossi, Z. M. et al. A global survey of physicians knowledge about nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 20, e1456–e1468 (2022).
Ajmera, V. et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J. Hepatol. 78, 471–478 (2023).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 71, 793–801 (2019).
Stefan, N. & Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 10, 284–296 (2022).
Abeysekera, K. W. M. et al. Implementation of a liver health check in patients with type 2 diabetes. Lancet Gastroenterol. Hepatol. 9, 83–91 (2024).
Allen, A. M., Lazarus, J. V. & Younossi, Z. M. Healthcare and socioeconomic costs of NAFLD: a global framework to navigate the uncertainties. J. Hepatol. 79, 209–217 (2023).
Brennan, P. N., Zelber-Sagi, S., Allen, A. M., Dillon, J .F. & Lazarus, J. V. Beyond a liver-gut focus: the evolution of gastroenterology and hepatology in challenging the obesity and steatotic liver disease paradigm. Gut 73, 560–563 (2024).
Srivastava, A. et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 71, 371–378 (2019).
Dillon, J. F. et al. Intelligent liver function testing (iLFT): a trial of automated diagnosis and staging of liver disease in primary care. J. Hepatol. 71, 699–706 (2019).
Lazarus, J. V. et al. Defining comprehensive models of care for NAFLD. Nat. Rev. Gastroenterol. Hepatol. 18, 717–729 (2021).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Allen, A. M. et al. Measuring NAFLD models of care. Nat. Rev. Gastroenterol. Hepatol. 20, 626–627 (2023).
US Food and Drug Administration. FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease (2024).
Lazarus, J. V. et al. A global research priority agenda to advance public health responses to fatty liver disease. J. Hepatol. 79, 618–634 (2023).
Rowe, I. A. Understanding the risks and benefits of policy action in NAFLD. J. Hepatol. 79, 22–24 (2023).
Allen, A. M. et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology 67, 1726–1736 (2018).
Ma, J. et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J. Hepatol. 66, 390–397 (2017).
Chen, B. et al. NAFLD in cardiovascular diseases: a contributor or comorbidity? Semin. Liver Dis. 42, 465–474 (2022).
Brunner, K. T. et al. Increasing liver fat is associated with incident cardiovascular risk factors. Clin. Gastroenterol. Hepatol. 18, 1884–1886 (2020).
Ahmed, H. S. et al. The association between hepatic steatosis and incident cardiovascular disease, cancer, and all-cause mortality in a US multicohort study. Hepatology 77, 2063–2072 (2023).
Tapper, E. B. & Loomba, R. Nonalcoholic fatty liver disease, metabolic syndrome, and the fight that will define clinical practice for a generation of hepatologists. Hepatology 67, 1657–1659 (2018).
Ivancovsky-Wajcman, D. et al. Integrating social nutrition principles into the treatment of steatotic liver disease. Commun. Med. 3, 165 (2023).
Gilmore, A. B. et al. Defining and conceptualising the commercial determinants of health. Lancet 401, 1194–1213 (2023).
Lee, W. W. M., Choi, K. C., Yum, R. W. Y., Yu, D. S. F. & Chair, S. Y. Effectiveness of motivational interviewing on lifestyle modification and health outcomes of clients at risk or diagnosed with cardiovascular diseases: a systematic review. Int. J. Nurs. Stud. 53, 331–341 (2016).
Hallsworth, K. & Adams, L. A. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 1, 468–479 (2019).
Hagström, H. et al. Administrative coding in electronic health care record‐based research of NAFLD: an expert panel consensus statement. Hepatology 74, 474–482 (2021).
US Food and Drug Administration. Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment https://www.fda.gov/regulatory-information/search-fda-guidance-documents/noncirrhotic-nonalcoholic-steatohepatitis-liver-fibrosis-developing-drugs-treatment (2018).
European Medicines Agency. Reflection Papers on Regulatory Requirements for the Development of Medicinal Products for Chronic Non-infectious Liver Diseases (PBC, PSC, NASH) — Scientific Guideline https://www.ema.europa.eu/en/reflection-papers-regulatory-requirements-development-medicinal-products-chronic-non-infectious-liver-diseases-pbc-psc-nash-scientific-guideline (2024).
Schlander, M., Hernandez-Villafuerte, K., Cheng, C.-Y., Mestre-Ferrandiz, J. & Baumann, M. How much does it cost to research and develop a new drug? A systematic review and assessment. Pharmacoeconomics 39, 1243–1269 (2021).
DiMasi, J. A., Grabowski, H. G. & Hansen, R. W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 47, 20–33 (2016).
Harrison, S. A. et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 390, 497–509 (2024).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
Saiyed, M. M., Ong, P. S. & Chew, L. Off-label drug use in oncology: a systematic review of literature. J. Clin. Pharm. Ther. 42, 251–258 (2017).
Van Norman, G. A. Off-label use vs off-label marketing of drugs: part 1: off-label use-patient harms and prescriber responsibilities. JACC Basic Transl. Sci. 8, 224–233 (2023).
Edwards, K., Li, X. & Lingvay, I. Clinical and safety outcomes with GLP-1 receptor agonists and SGLT2 inhibitors in type 1 diabetes: a real-world study. J. Clin. Endocrinol. Metab. 108, 920–930 (2023).
Rinella, M. E. et al. Practice patterns in NAFLD and NASH: real life differs from published guidelines. Ther. Adv. Gastroenterol. 9, 4–12 (2016).
LeBlanc, E. S. et al. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 320, 1172–1191 (2018).
Gribble, F. M. & Reimann, F. Metabolic messengers: glucagon-like peptide 1. Nat. Metab. 3, 142–148 (2021).
van Bloemendaal, L. et al. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes. Metab. 17, 878–886 (2015).
Chuong, V. et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight 8, e170671 (2023).
Aldawsari, M. et al. The efficacy of GLP-1 analogues on appetite parameters, gastric emptying, food preference and taste among adults with obesity: systematic review of randomized controlled trials. Diabetes Metab. Syndr. Obes. 16, 575–595 (2023).
Younossi, Z. M., Zelber-Sagi, S., Henry, L. & Gerber, L. H. Lifestyle interventions in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 20, 708–722 (2023).
Lewis, K. H., Moore, J. B. & Ard, J. D. Game changers: do new medications make lifestyle‐based treatment of obesity obsolete? Obesity 32, 237–239 (2024).
Younossi, Z. et al. The burden of non-alcoholic steatohepatitis: a systematic review of health-related quality of life and patient-reported outcomes. JHEP Rep. 4, 100525 (2022).
Stepanova, M., Henry, L. & Younossi, Z. M. Economic burden and patient-reported outcomes of nonalcoholic fatty liver disease. Clin. Liver Dis. 27, 483–513 (2023).
Younossi, Z. M. et al. The impact of fatigue on mortality of patients with non-alcoholic fatty liver disease: Data from National Health and Nutrition Examination Survey 2005-2010 and 2017-2018. Liver Int. 42, 2646–2661 (2022).
Younossi, Z. M., Stepanova, M., Myers, R. P., Younossi, I. & Henry, L. The potential role of fatigue in identifying patients with NASH and advanced fibrosis who experience disease progression. Clin. Gastroenterol. Hepatol. 21, 970–977.e1 (2023).
Younossi, Z. M. et al. Improvements of fibrosis and disease activity are associated with improvement of patient-reported outcomes in patients with advanced fibrosis due to nonalcoholic steatohepatitis. Hepatol. Commun. 5, 1201–1211 (2021).
Kardashian, A., Serper, M., Terrault, N. & Nephew, L. D. Health disparities in chronic liver disease. Hepatology 77, 1382–1403 (2023).
Talens, M., Tumas, N., Lazarus, J. V., Benach, J. & Pericàs, J. M. What do we know about inequalities in NAFLD distribution and outcomes? A scoping review. J. Clin. Med. 10, 5019 (2021).
Cooksey-Stowers, K., Schwartz, M. B. & Brownell, K. D. Food swamps predict obesity rates better than food deserts in the United States. Int. J. Env. Res. Public Health 14, 1366 (2017).
Mehta, P. D. In International Encyclopedia of the Social & Behavioral Sciences 2727–2730 https://doi.org/10.1016/B0-08-043076-7/00734-8 (Elsevier, 2001).
Richardson, A. S. et al. Neighborhood socioeconomic status and food environment: a 20-year longitudinal latent class analysis among CARDIA participants. Health Place. 30, 145–531 (2014).
Odoms-Young, A. & Bruce, M. A. Examining the impact of structural racism on food insecurity: implications for addressing racial/ethnic disparities. Fam. Commun. Health 41, S3–S6 (2018).
Food and Agriculture Organization of the United Nations. Putting a Number on Hunger. Different Measures for Different Purposes https://www.fao.org/interactive/state-of-food-security-nutrition/en/ (2023).
Lazarus, J. V. et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J. Hepatol. 76, 771–780 (2022).
Lazarus, J. V. et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 19, 60–78 (2022).
Lazarus, J. V. et al. A global action agenda for turning the tide on fatty liver disease. Hepatology 79, 502–523 (2024).
Lazarus, J. V. et al. Real-world evidence on non-invasive tests and associated cut-offs used to assess fibrosis in routine clinical practice. JHEP Rep. 5, 100596 (2023).
Krag, A. et al. Uniting to defeat steatotic liver disease: a global mission to promote healthy livers and healthy lives. J. Hepatol. 79, 1076–1078 (2023).
Anstee, Q. M. et al. Real-world management of non-alcoholic steatohepatitis differs from clinical practice guideline recommendations and across regions. JHEP Rep. 4, 100411 (2022).
Targher, G., Tilg, H. & Byrne, C. D. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 6, 578–588 (2021).
Chen, V. L., Song, M. W., Suresh, D., Wadhwani, S. I. & Perumalswami, P. Effects of social determinants of health on mortality and incident liver‐related events and cardiovascular disease in steatotic liver disease. Aliment. Pharmacol. Ther. 58, 537–545 (2023).
Hagström, H. et al. Maternal obesity increases the risk and severity of NAFLD in offspring. J. Hepatol. 75, 1042–1048 (2021).
Gutierrez Sanchez, L. H. et al. Perinatal nutritional reprogramming of the epigenome promotes subsequent development of nonalcoholic steatohepatitis. Hepatol. Commun. 2, 1493–1512 (2018).
Ayonrinde, O. T. et al. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. J. Hepatol. 67, 568–576 (2017).
Ayonrinde, O. T. et al. Sex differences between parental pregnancy characteristics and nonalcoholic fatty liver disease in adolescents. Hepatology 67, 108–122 (2018).
Karlsen, T. H. et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 399, 61–116 (2022).
Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol. Hepatol. 7, 396–415 (2022).
Joint United Nations Programme on HIV/AIDS. Global HIV & AIDS Statistics — Fact Sheet https://www.unaids.org/en/resources/fact-sheet (2023).
The International AIDS Society. Sessions https://programme.ias2023.org/ (2023).
Lazarus, J. V., Kopka, C. J., Younossi, Z. M. & Allen, A. M. It is time to expand the fatty liver disease community of practice. Hepatology 78, 1325–1328 (2023).
Vilar-Gomez, E. et al. Prevalence of high-risk nonalcoholic steatohepatitis (NASH) in the United States: results from NHANES 2017–2018. Clin. Gastroenterol. Hepatol. 21, 115–124.e7 (2023).
Simon, T. G., Roelstraete, B., Khalili, H., Hagström, H. & Ludvigsson, J. F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 70, 1375–1382 (2021).
Ilyas, F. et al. Increasing nonalcoholic fatty liver disease-related mortality rates in the United States from 1999 to 2022. Hepatol. Commun. 7, e00207 (2023).
Centers for Disease Control and Prevention. Hepatitis C Surveillance 2021 https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-c.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnchhstp%2Fnewsroom%2Ffact-sheets%2Fhepatitis%2Fhepatitis-c-by-the-numbers.html (2023).
US Preventive Services Task Force et al. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA 323, 970–975 (2020).
Goff, D. C. et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation 113, 647–656 (2006).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008).
Speliotes, E. K. et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 7, e1001324 (2011).
Trépo, E. & Valenti, L. Update on NAFLD genetics: from new variants to the clinic. J. Hepatol. 72, 1196–1209 (2020).
Cherubini, A. et al. Interaction between estrogen receptor-α and PNPLA3 p.I148M variant drives fatty liver disease susceptibility in women. Nat. Med. 29, 2643–2655 (2023).
Eng, P. C. et al. Non-alcoholic fatty liver disease in women — current knowledge and emerging concepts. JHEP Rep. 5, 100835 (2023).
Acknowledgements
J.V.L. acknowledges support to ISGlobal from grant CEX2018-000806-S, funded by MCIN/AEI/10.13039/501100011033, and the ‘Generalitat de Catalunya’, through the CERCA Programme, outside of the submitted work. The authors would like to thank Paul N. Brennan, Dana Ivancovsky Wajcman, Christopher J. Kopka and Marcela Villota-Rivas for their feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. J.V.L. and A.M.A. contributed substantially to discussion of the content and developed the outline. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.M.A. acknowledges grant support to her institution from the National Institutes of Health (DK128127), Novo Nordisk, Pfizer and Target Pharma and advisory board participation for Boehringer Ingelheim and Novo Nordisk outside of the submitted work. Z.M.Y. acknowledges consulting fees from Abbott, AstraZeneca, BMS, Boehringer Ingelheim, Cymabay, Gilead, GSK, Intercept, Madrigal, Merck, Novo Nordisk and Siemens outside of the submitted work. A.M.D. acknowledges grant support to her institution from Boehringer Ingelheim, GSK, Hanmi, Intercept, Inventiva, Madrigal, Novo Nordisk, Tune Therapeutics, and Viking and consulting for Boehringer Ingelheim outside of the submitted work. M.R.C. acknowledges consulting for Bristol-Myers-Squibb, Celgene, Histoindex, Intercept, Madrigal, NGM, Novo Nordisk, Pfizer, Terns, and Theratechnologies and research grants from Gilead Sciences outside of the submitted work. J.V.L. acknowledges grants and speaker fees from AbbVie, Gilead Sciences, MSD, Novo Nordisk and Roche Diagnostics to his institution, grants from Boehringer Ingelheim, GSK and Madrigal to his institution, speaker fees from Echosens, Janssen, Novo Nordisk and ViiV, and consulting fees from Echosens, Gilead Sciences, GSK, Novavax and Roche Diagnostics outside of the submitted work.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Fatiha Nassir, Tokushige Katsutoshi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Allen, A.M., Younossi, Z.M., Diehl, A.M. et al. Envisioning how to advance the MASH field. Nat Rev Gastroenterol Hepatol 21, 726–738 (2024). https://doi.org/10.1038/s41575-024-00938-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-024-00938-9
This article is cited by
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicle in nonalcoholic fatty liver disease: a systematic review and meta-analysis of preclinical evidence
Lipids in Health and Disease (2025)
-
Genomic medicine in hepatology: mechanisms and liver treatment strategies
Molecular Medicine (2025)
-
A new treatment and updated clinical practice guidelines for MASLD
Nature Reviews Gastroenterology & Hepatology (2025)
-
Immunopathogenic mechanisms and immunoregulatory therapies in MASLD
Cellular & Molecular Immunology (2025)
-
Large language models for clinical decision support in gastroenterology and hepatology
Nature Reviews Gastroenterology & Hepatology (2025)