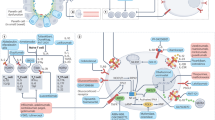
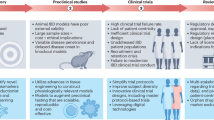
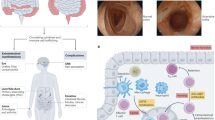
Abstract
Treatment options for the medical management of inflammatory bowel disease (IBD) have expanded substantially over the past decade. Multiple classes of advanced therapies, including both monoclonal antibodies and novel oral small molecules, are now available for the treatment of moderately-to-severely active Crohn’s disease and ulcerative colitis, highlighted by the approvals of the first IL23p19 antagonists, selective Janus kinase inhibitors and sphingosine-1-phosphate receptor modulators. These advances have been accompanied by the identification of novel targets and the rapid growth in both the number and size of IBD clinical trials. Over a dozen landmark randomized controlled trials (RCTs) have been completed in the past 5 years, including the first head-to-head biologic trials, the first combination biologic studies, and multiple phase III registrational trials of novel compounds with new co-primary and composite end points that will change the treatment landscape for years to come. Importantly, the methodology of RCTs in IBD has evolved substantially, with new trial designs, evaluation of unique patient populations, and different types of efficacy and safety end points being key innovations. In this Review, we provide a comprehensive evaluation of how modern RCTs of IBD medical therapies have evolved and the implications for their appraisal that will help guide the application of these data to clinical practice.
Key points
-
Evidence generated from high-quality, randomized controlled trials is essential for the approval of new drugs for the treatment of inflammatory bowel disease (IBD); over time, these trials have increased in size, scope and complexity.
-
There have been substantial advancements in the design of IBD trials, including re-evaluations of which participants are enrolled, how participants are qualified, and how disease activity is measured.
-
For trials in Crohn’s disease, the co-primary end points include both clinical and endoscopic assessment; for trials in ulcerative colitis, the composite primary outcome of clinical remission includes patient-reported and endoscopic measures.
-
Direct comparisons of advanced agents will inform treatment positioning and relative efficacy and safety; some head-to-head trials have now been conducted, and others with active comparator arms are soon to be completed.
-
The long-term sustainability of trials in IBD is threatened by poor recruitment, prolonged study durations and increased costs; future innovations using Bayesian approaches, adaptive trial designs and master protocols might improve trial efficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Targan, S. R. et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. N. Engl. J. Med. 337, 1029–1035 (1997).
Torres, J. et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J. Crohns Colitis 14, 4–22 (2020).
Raine, T. et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J. Crohns Colitis 16, 2–17 (2022).
D’Haens, G. et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 371, 660–667 (2008).
Khanna, R. et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet 386, 1825–1834 (2015).
Colombel, J. F. et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 362, 1383–1395 (2010).
Panaccione, R. et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 146, 392–400.e3 (2014).
Colombel, J. F. et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 390, 2779–2789 (2017).
Sandborn, W. J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736 (2017).
D’Haens, G. et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet 399, 2015–2030 (2022).
Danese, S. et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet 399, 2113–2128 (2022).
Ferrante, M. et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet 399, 2031–2046 (2022).
Loftus, E. V. Jr. et al. Upadacitinib induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 388, 1966–1980 (2023).
Sandborn, W. J. et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 385, 1280–1291 (2021).
Sandborn, W. J. et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet 401, 1159–1171 (2023).
Sands, B. E. et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 381, 1215–1226 (2019).
Danese, S. et al. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (GARDENIA): a randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol. Hepatol. 7, 118–127 (2022).
Rubin, D. T. et al. Etrolizumab versus adalimumab or placebo as induction therapy for moderately to severely active ulcerative colitis (HIBISCUS): two phase 3 randomised, controlled trials. Lancet Gastroenterol. Hepatol. 7, 17–27 (2022).
Feagan, B. G. et al. Guselkumab plus golimumab combination therapy versus guselkumab or golimumab monotherapy in patients with ulcerative colitis (VEGA): a randomised, double-blind, controlled, phase 2, proof-of-concept trial. Lancet Gastroenterol. Hepatol. 8, 307–320 (2023).
Peyrin-Biroulet, L. et al. Risankizumab versus ustekinumab for moderate-to-severe Crohn’s disease. N. Engl. J. Med. 391, 213–223 (2024).
Sands, B. E. et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 399, 2200–2211 (2022).
Wasmann, K. A. et al. Treatment of perianal fistulas in Crohn’s disease, seton versus anti-TNF versus surgical closure following anti-TNF [PISA]: a randomised controlled trial. J. Crohns Colitis 14, 1049–1056 (2020).
Meima-van Praag, E. M. et al. Short-term anti-TNF therapy with surgical closure versus anti-TNF therapy in the treatment of perianal fistulas in Crohn’s disease (PISA-II): a patient preference randomised trial. Lancet Gastroenterol. Hepatol. 7, 617–626 (2022).
Ponsioen, C. Y. et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: a randomised controlled, open-label, multicentre trial. Lancet Gastroenterol. Hepatol. 2, 785–792 (2017).
Luglio, G. et al. Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn’s disease: the SuPREMe-CD study – a randomized clinical trial. Ann. Surg. 272, 210–217 (2020).
Hanauer, S. B. et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 130, 323–333 (2006).
Reinisch, W. et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 60, 780–787 (2011).
Hanauer, S. B. et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 359, 1541–1549 (2002).
Colombel, J. F. et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 132, 52–65 (2007).
Schreiber, S. et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N. Engl. J. Med. 357, 239–250 (2007).
Sandborn, W. J. et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 142, 257–265.e1–3 (2012).
Rutgeerts, P. et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 353, 2462–2476 (2005).
Sandborn, W. J. et al. Certolizumab pegol for the treatment of Crohn’s disease. N. Engl. J. Med. 357, 228–238 (2007).
Feagan, B. G. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369, 699–710 (2013).
Sandborn, W. J. et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 369, 711–721 (2013).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375, 1946–1960 (2016).
Sands, B. E. et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 381, 1201–1214 (2019).
Ghosh, S. et al. Interpreting registrational clinical trials of biological therapies in adults with inflammatory bowel diseases. Inflamm. Bowel Dis. 22, 2711–2723 (2016).
Uzzan, M. et al. Declining enrolment and other challenges in IBD clinical trials: causes and potential solutions. J. Crohns Colitis 17, 1066–1078 (2023).
Johnson, C., Barnes, E. L., Zhang, X. & Long, M. D. Trends and characteristics of clinical trials participation for inflammatory bowel disease in the United States: a report from IBD Partners. Crohns Colitis 2, otaa023 (2020).
Harris, M. S., Wichary, J., Zadnik, M. & Reinisch, W. Competition for clinical trials in inflammatory bowel diseases. Gastroenterology 157, 1457–1461.e2 (2019).
Sandborn, W. J. et al. Etrolizumab for the treatment of ulcerative colitis and Crohn’s disease: an overview of the phase 3 clinical program. Adv. Ther. 37, 3417–3431 (2020).
Sands, B. E. et al. Characterisation of patients with delayed response to ustekinumab for Crohn’s disease [abstract P317]. J. Crohns Colitis 13 (Suppl. 1), 259–260 (2019).
Dubinsky, M. C. et al. Additional risankizumab treatment is effective in patients with moderately to severely active Crohn’s disease who did not achieve cdai clinical response with the initial 12-week induction treatment: results from the advance, motivate, and fortify studies [abstract Tu1710]. Gastroenterology 164 (Suppl. 6), 1088 (2023).
Vermeire, S. et al. Efficacy of etrasimod at week 52 among subjects who reached clinical response at week 12: post hoc analysis of the phase 3 ELEVATE UC 52 trial [abstract P582]. J. Crohns Colitis 17 (Suppl. 1), i709–i711 (2023).
Vermeire, S. et al. Etrasimod for the treatment of ulcerative colitis: up to 2.5 years of pooled safety data from global clinical trials [abstract P490]. J. Crohns Colitis 17 (Suppl. 1), i619–i620 (2023).
D’Haens, G. et al. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 388, 2444–2455 (2023).
US Food and Drug Administration. Demonstrating substantial evidence of effectiveness with one adequate and well-controlled clinical investigation and confirmatory evidence: guidance for industry. FDA www.fda.gov/media/172166/download (2023).
Christensen, E. Methodology of superiority vs. equivalence trials and non-inferiority trials. J. Hepatol. 46, 947–954 (2007).
Lasa, J. S., Zubiaurre, I., Rausch, A. & Olivera, P. Placebo use in the context of inflammatory bowel disease clinical trials. Arq. Gastroenterol. 57, 87–90 (2020).
Crowley, E. et al. Impact of drug approval pathways for paediatric inflammatory bowel disease. J. Crohns Colitis 16, 331–335 (2022).
Almradi, A. et al. Clinical, endoscopic, and safety placebo rates in induction and maintenance trials of Crohn’s disease: meta-analysis of randomised controlled trials. J. Crohns Colitis 16, 717–736 (2022).
Wong, E. C. L. et al. Predictors of placebo induction response and remission in ulcerative colitis. Clin. Gastroenterol. Hepatol. 21, 1050–1060.e9 (2023).
Wong, E. C. L. et al. Predictors of clinical remission to placebo in clinical trials of Crohn’s disease. Inflamm. Bowel Dis. 29, 1390–1398 (2023).
European Medicines Agency. Guideline on the development of new medicinal products for the treatment of ulcerative colitis – revision 1. EMA www.ema.europa.eu/en/development-new-medicinal-products-treatment-ulcerative-colitis-scientific-guideline (2019).
European Medicines Agency. Clinical investigation of medicinal products for the management of Crohn’s disease – scientific guideline. EMA www.ema.europa.eu/en/clinical-investigation-medicinal-products-management-crohns-disease-scientific-guideline (2018).
Ma, C., Guizzetti, L. & Jairath, V. Improving clinical trial efficiency in gastroenterology. Gastroenterology 157, 892–893 (2019).
Sandborn, W. J. et al. Once-daily budesonide MMX(R) extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology 143, 1218–1226.e12 (2012).
Sandborn, W. J. et al. Guselkumab for the treatment of Crohn’s disease: induction results from the phase 2 GALAXI-1 study. Gastroenterology 162, 1650–1664l.e8 (2022).
Panaccione, R. et al. Efficacy and safety of guselkumab therapy in patients with moderately to severely active Crohn’s disease: results of the Galaxi 2 & 3 phase 3 studies. Gastroenterology 166, 1057b2 (2024).
Ferrante, M. et al. Primary efficacy and safety of mirikizumab in moderate to severe Crohn’s disease: results of the treat-through VIVID 1 study [abstract OP05]. J. Crohns Colitis 18 (Suppl. 1), i7–i9 (2024).
George, J. et al. Corticosteroid-free remission vs overall remission in clinical trials of moderate-severe ulcerative colitis and Crohn’s disease. Inflamm. Bowel Dis. 26, 515–523 (2020).
Khanna, R. et al. Endoscopic assessment of inflammatory bowel disease activity in clinical trials. Clin. Gastroenterol. Hepatol. 20, 727–736.e2 (2022).
Peyrin-Biroulet, L. et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 63, 88–95 (2014).
Lewis, J. D. et al. Correlation of stool frequency and abdominal pain measures with simple endoscopic score for Crohn’s disease. Inflamm. Bowel Dis. 26, 304–313 (2020).
Colombel, J. F. et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut 66, 2063–2068 (2017).
Ma, C. et al. Discordance between patient-reported outcomes and mucosal inflammation in patients with mild to moderate ulcerative colitis. Clin. Gastroenterol. Hepatol. 18, 1760–1768.e1 (2020).
Yacyshyn, B. et al. A randomized, double-masked, placebo-controlled study of alicaforsen, an antisense inhibitor of intercellular adhesion molecule 1, for the treatment of subjects with active Crohn’s disease. Clin. Gastroenterol. Hepatol. 5, 215–220 (2007).
Panes, J. et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 66, 1049–1059 (2017).
Gottlieb, K. et al. Endoscopy and central reading in inflammatory bowel disease clinical trials: achievements, challenges and future developments. Gut 70, 418–426 (2021).
Feagan, B. G. et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology 145, 149–157.e2 (2013).
Vuyyuru, S. K. et al. Endoscopic and histological placebo rates in Crohn’s disease clinical trials: a systematic review and meta-analysis. Inflamm. Bowel Dis. 30, 651–659 (2023).
Sedano, R. et al. Systematic review and meta-analysis: clinical, endoscopic, histological and safety placebo rates in induction and maintenance trials of ulcerative colitis. J. Crohns Colitis 16, 224–243 (2022).
Reinisch, W. et al. Impact of various central endoscopy reading models on treatment outcome in Crohn’s disease using data from the randomized, controlled, exploratory cohort arm of the BERGAMOT trial. Gastrointest. Endosc. 93, 174–182.e2 (2021).
Schreiber, S. et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology 129, 807–818 (2005).
Jairath, V. et al. A cluster-randomised controlled trial of an enhanced treatment algorithm for the management of Crohn’s disease: React-2 [abstract OP110]. United Eur. Gastroenterol. J. 10 (Suppl. 8), 89 (2022).
Ha, C., Ullman, T. A., Siegel, C. A. & Kornbluth, A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin. Gastroenterol. Hepatol. 10, 1002–1007 (2012).
Sedano, R. et al. Underrepresentation of minorities and underreporting of race and ethnicity in Crohn’s disease clinical trials. Gastroenterology 162, 338–340.e2 (2022).
Cohen, N. A., Silfen, A. & Rubin, D. T. Inclusion of under-represented racial and ethnic minorities in randomized clinical trials for inflammatory bowel disease. Gastroenterology 162, 17–21 (2022).
Barnes, E. L., Loftus, E. V. Jr. & Kappelman, M. D. Effects of race and ethnicity on diagnosis and management of inflammatory bowel diseases. Gastroenterology 160, 677–689 (2021).
Liu, Y. et al. Associations between the NUDT15 R139C polymorphism and susceptibility to thiopurine-induced leukopenia in Asians: a meta-analysis. Onco Targets Ther. 11, 8309–8317 (2018).
Winthrop, K. L. et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 66, 2675–2684 (2014).
GBD 2017 Inflammatory Bowel Disease Collaborators The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30 (2020).
Ben-Horin, S. et al. Efficacy of biologic drugs in short-duration versus long-duration inflammatory bowel disease: a systematic review and an individual-patient data meta-analysis of randomized controlled trials. Gastroenterology 162, 482–494 (2022).
Pariente, B. et al. Development of the Lemann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology 148, 52–63.e3 (2015).
Danese, S., Fiorino, G., Fernandes, C. & Peyrin-Biroulet, L. Catching the therapeutic window of opportunity in early Crohn’s disease. Curr. Drug. Targets 15, 1056–1063 (2014).
Burisch, J., Safroneeva, E., Laoun, R. & Ma, C. Lack of benefit for early escalation to advanced therapies in ulcerative colitis: critical appraisal of current evidence. J. Crohns Colitis 17, 2002–2011 (2023).
Singh, S. et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: a systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 6, 1002–1014 (2021).
Lasa, J. S., Olivera, P. A., Danese, S. & Peyrin-Biroulet, L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 7, 161–170 (2022).
US Food and Drug Administration. Guidance document. Ulcerative colitis: developing drugs for treatment. FDA www.fda.gov/regulatory-information/search-fda-guidance-documents/ulcerative-colitis-developing-drugs-treatment (2022).
US Food and Drug Administration. Guidance document. Crohn’s disease: developing drugs for treatment. FDA www.fda.gov/regulatory-information/search-fda-guidance-documents/crohns-disease-developing-drugs-treatment (2022).
Yanai, H. & Hanauer, S. B. Assessing response and loss of response to biological therapies in IBD. Am. J. Gastroenterol. 106, 685–698 (2011).
Travis, S. et al. Vedolizumab for the treatment of chronic pouchitis. N. Engl. J. Med. 388, 1191–1200 (2023).
Hanzel, J. et al. Recommendations for standardizing clinical trial design and endoscopic assessment in postoperative Crohn’s disease. Inflamm. Bowel Dis. 28, 1321–1331 (2022).
Kotze, P. G. et al. Modern management of perianal fistulas in Crohn’s disease: future directions. Gut 67, 1181–1194 (2018).
Hindryckx, P. et al. Development and validation of a magnetic resonance index for assessing fistulas in patients with Crohn’s disease. Gastroenterology 157, 1233–1244.e5 (2019).
Vuyyuru, S. K. et al. Patients with Crohn’s disease and permanent ileostomy are universally excluded from clinical trials: a systematic review. Am. J. Gastroenterol. 118, 1285–1288 (2023).
Rieder, F., Zimmermann, E. M., Remzi, F. H. & Sandborn, W. J. Crohn’s disease complicated by strictures: a systematic review. Gut 62, 1072–1084 (2013).
Rieder, F. et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment. Pharmacol. Ther. 48, 347–357 (2018).
Bettenworth, D. et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut 68, 1115–1126 (2019).
Hanzel, J., Ma, C. & Jairath, V. Design of clinical trials for mild to moderate Crohn’s disease. Gastroenterology 162, 1800–1814.e1 (2022).
Sedano, R., Jairath, V. & Ma, C. Design of clinical trials for mild to moderate ulcerative colitis. Gastroenterology 162, 1005–1018 (2022).
Ma, C. et al. Heterogeneity in definitions of efficacy and safety endpoints for clinical trials of Crohn’s disease: a systematic review. Clin. Gastroenterol. Hepatol. 16, 1407–1419.e22 (2018).
Ma, C. et al. Heterogeneity in definitions of endpoints for clinical trials of ulcerative colitis: a systematic review for development of a core outcome set. Clin. Gastroenterol. Hepatol. 16, 637–647.e13 (2018).
Jairath, V. et al. Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment. Pharmacol. Ther. 42, 1200–1210 (2015).
Khanna, R. et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment. Pharmacol. Ther. 41, 77–86 (2015).
Dubinsky, M. C. et al. Incorporating patient experience into drug development for ulcerative colitis: development of the Urgency Numeric Rating Scale, a patient-reported outcome measure to assess bowel urgency in adults. J. Patient Rep. Outcomes 6, 31 (2022).
Daperno, M. et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest. Endosc. 60, 505–512 (2004).
Narula, N. et al. Comparative effectiveness of biologics for endoscopic healing of the ileum and colon in Crohn’s disease. Am. J. Gastroenterol. 117, 1106–1117 (2022).
Feagan, B. G. et al. Early improvement of endoscopic outcomes with risankizumab is associated with reduced hospitalisation and surgery rates in patients with Crohn’s disease [abstract P380]. J. Crohns Colitis 16 (Suppl. 1), i381–i382 (2022).
Ma, C. et al. Methods for handling missing segments in Crohn’s disease clinical trials: analysis from the EXTEND trial. Gut 71, 447–448 (2022).
Geyl, S. et al. Transmural healing as a therapeutic goal in Crohn’s disease: a systematic review. Lancet Gastroenterol. Hepatol. 6, 659–667 (2021).
Goodsall, T. M. et al. Standardisation of intestinal ultrasound scoring in clinical trials for luminal Crohn’s disease. Aliment. Pharmacol. Ther. 53, 873–886 (2021).
Danese, S. et al. Treat to target versus standard of care for patients with Crohn’s disease treated with ustekinumab (STARDUST): an open-label, multicentre, randomised phase 3b trial. Lancet Gastroenterol. Hepatol. 7, 294–306 (2022).
Naegeli, A. N. et al. Full, partial, and modified permutations of the Mayo score: characterizing clinical and patient-reported outcomes in ulcerative colitis patients. Crohns Colitis 3, otab007 (2021).
Schroeder, K. W., Tremaine, W. J. & Ilstrup, D. M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 317, 1625–1629 (1987).
Scherl, E. J. et al. Safety and efficacy of a new 3.3 g b.i.d. tablet formulation in patients with mild-to-moderately-active ulcerative colitis: a multicenter, randomized, double-blind, placebo-controlled study. Am. J. Gastroenterol. 104, 1452–1459 (2009).
The CORE-IBD Collaborators et al.CORE-IBD: a multidisciplinary international consensus initiative to develop a core outcome set for randomized controlled trials in inflammatory bowel disease. Gastroenterology 163, 950–964 (2022).
Travis, S. P. et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology 145, 987–995 (2013).
Travis, S. P. et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 61, 535–542 (2012).
Gatto, N. M. et al. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J. Natl Cancer Inst. 95, 230–236 (2003).
Colombel, J. F. et al. Agreement between rectosigmoidoscopy and colonoscopy analyses of disease activity and healing in patients with ulcerative colitis. Gastroenterology 150, 389–395.e3 (2016).
Magro, F. et al. ECCO position paper: harmonization of the approach to ulcerative colitis histopathology. J. Crohns Colitis 14, 1503–1511 (2020).
Ma, C. et al. An international consensus to standardize integration of histopathology in ulcerative colitis clinical trials. Gastroenterology 160, 2291–2302 (2021).
Mosli, M. H. et al. Development and validation of a histological index for UC. Gut 66, 50–58 (2017).
Marchal-Bressenot, A. et al. Development and validation of the Nancy histological index for UC. Gut 66, 43–49 (2017).
Geboes, K. et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 47, 404–409 (2000).
Peyrin-Biroulet, L. et al. A comparative evaluation of the measurement properties of three histological indices of mucosal healing in ulcerative colitis: Geboes Score, Robarts Histopathology Index, and Nancy Index. J. Crohns Colitis 17, 1733–1743 (2023).
Yoon, H. et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta-analysis. Gastroenterology 159, 1262–1275.e7 (2020).
Ytterberg, S. R. et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 386, 316–326 (2022).
US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death (2021).
Sandborn, W. J. et al. Tofacitinib for the treatment of ulcerative colitis: an integrated summary of up to 7.8 years of safety data from the global clinical programme. J. Crohns Colitis 17, 338–351 (2023).
Panés, J. et al. Analysis of tofacitinib safety in ulcerative colitis from the completed global clinical developmental program up to 9.2 years of drug exposure. United Eur. Gastroenterol. J. 12, 793–801 (2024).
Feagan, B. G. et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 397, 2372–2384 (2021).
Fleischmann, R. et al. Safety profile of upadacitinib in patients at risk of cardiovascular disease: integrated post hoc analysis of the SELECT phase III rheumatoid arthritis clinical programme. Ann. Rheum. Dis. 82, 1130–1141 (2023).
European Medicines Agency. EMA recommends measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. EMA www.ema.europa.eu/en/news/ema-recommends-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic-inflammatory-disorders (2022).
Herfarth, H. H. et al. Investigator-initiated IBD trials in the United States: facts, obstacles, and answers. Inflamm. Bowel Dis. 23, 14–22 (2017).
Buhl, S. et al. Discontinuation of infliximab therapy in patients with Crohn’s disease. NEJM Evid. 1, EVIDoa2200061 (2022).
Louis, E. et al. Withdrawal of infliximab or concomitant immunosuppressant therapy in patients with Crohn’s disease on combination therapy (SPARE): a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol. Hepatol. 8, 215–227 (2023).
Noor, N. M. et al. A biomarker-stratified comparison of top-down versus accelerated step-up treatment strategies for patients with newly diagnosed Crohn’s disease (PROFILE): a multicentre, open-label randomised controlled trial. Lancet Gastroenterol. Hepatol. 9, 415–427 (2024).
Fantini, M. C. et al. Pragmatic trial design to compare real-world effectiveness of different treatments for inflammatory bowel diseases: the PRACTICE-IBD European consensus. J. Crohns Colitis 18, 1222–1231 (2024).
Williams, J. G. et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol. Hepatol. 1, 15–24 (2016).
van Linschoten, R. C. A. et al. Increased versus conventional adalimumab dose interval for patients with Crohn’s disease in stable remission (LADI): a pragmatic, open-label, non-inferiority, randomised controlled trial. Lancet Gastroenterol. Hepatol. 8, 343–355 (2023).
Jairath, V. et al. Efficacy of mirikizumab in comparison to ustekinumab in patients with moderate to severe Crohn’s disease: results from the phase 3 VIVID 1 study [abstract OP35]. J. Crohns Colitis 18 (Suppl. 1), i62–i64 (2024).
Dubinsky, M. C., Collins, R. & Abreu, M. T. International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) Challenges and opportunities in IBD clinical trial design. Gastroenterology 161, 400–404 (2021).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Colombel, J. F. et al. Vedolizumab, adalimumab, and methotrexate combination therapy in Crohn’s disease (EXPLORER). Clin. Gastroenterol. Hepatol. 22, 1487–1496.e12 (2023).
Pallmann, P. et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med. 16, 29 (2018).
Woodcock, J. & LaVange, L. M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 377, 62–70 (2017).
Sandborn, W. J. et al. Oral ritlecitinib and brepocitinib for moderate-to-severe ulcerative colitis: results from a randomized, phase 2b study. Clin. Gastroenterol. Hepatol. 21, 2616–2628.e7 (2023).
Le Berre, C. & Peyrin-Biroulet, L. Selecting end points for disease-modification trials in inflammatory bowel disease: the SPIRIT consensus from the IOIBD. Gastroenterology 160, 1452–1460.e21 (2021).
Author information
Authors and Affiliations
Contributions
C.M. researched data for the article, made a substantial contribution to discussion of the content, wrote the article, and reviewed and/or edited the manuscript before submission. V.J., B.G.F., B.E.S. and R.P. made a substantial contribution to discussion of the content, and reviewed and/or edited the manuscript before submission. L.P.-B. and S.D. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.M. has received consulting fees from AbbVie, Alimentiv, Amgen, AVIR Pharma Inc, Bristol-Myers Squibb, Celltrion, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Pendopharm, Pfizer, Prometheus Biosciences Inc., Roche, Sanofi, Takeda and Tillotts Pharma; speaker’s fees from AbbVie, Amgen, AVIR Pharma Inc., Alimentiv, Bristol-Myers Squibb, Ferring, Fresenius Kabi, Janssen, Organon, Pendopharm, Pfizer, Sanofi and Takeda; royalties from Springer Publishing; and research support from AbbVie, Ferring and Pfizer. V.J. has received consulting/advisory board fees from AbbVie, Alimentiv Inc., Arena pharmaceuticals, Asahi Kasei Pharma, Asieris, Astra Zeneca, Avoro Capital, Bristol-Myers Squibb, Celltrion, Eli Lilly, Endpoint Health, Enthera, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, Gilde Healthcare, GlaxoSmithKline, Genentech, Gilead, Innomar, JAMP, Janssen, Merck, Metacrine, Mylan, Pandion, Pendopharm, Pfizer, Protagonist, Prometheus Biosciences, Reistone Biopharma, Roche, Roivant, Sandoz, SCOPE, Second Genome, Sorriso, Takeda, TD Securities, Teva, Topivert, Ventyx and Vividion; and speaker’s fees from, Abbvie, Ferring, Bristol Myers Squibb, Galapagos, Janssen Pfizer Shire, Takeda and Fresenius Kabi. B.G.F. is a scientific advisory board member for AbbVie, Allergan, Amgen, AstraZeneca, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Elan, Biogen, Ferring, Genentech-Roche, Janssen–Johnson & Johnson, Merck, Millennium, Nestlé, Novo Nordisk, Novartis, Pfizer, Prometheus, Protagonist, Receptos, Salix, Sigmoid Pharma, Takeda, Teva, TiGenix, Tillotts Pharma and UCB Pharma; has received consulting fees from AbbVie, Actogenix, Akros, Albireo Pharma, Allergan, Amgen, AstraZeneca, Avaxia Biologics, Avir Pharma, Axcan, Baxter Healthcare, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan-Biogen, EnGene, Ferring, Genentech-Roche, GiCare Pharma, Gilead Sciences, Given Imaging, GlaxoSmithKline, Ironwood, Janssen Biotech-Centocor, Janssen–Johnson & Johnson, Kyowa Hakko Kirin, Eli Lilly, Merck, Mesoblast Pharma, Millennium, Nestlé, Novo Nordisk, Novartis, Pfizer, Prometheus, Protagonist, Receptos Salix, Sanofi, Shire, Sigmoid Pharma, Synergy Pharma, Takeda, Teva, TiGenix, Tillotts Pharma, UCB Pharma, Vertex, VHsquared, Wyeth, Zealand and Zygenia; has received lecture fees from AbbVie, Janssen–Johnson & Johnson, Takeda and UCB Pharma; has received grant support from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Janssen Biotech-Centocor, Janssen-Johnson & Johnson, Pfizer, Receptos, Sanofi and Takeda; and is the Senior Scientific Officer of Alimentiv Inc. L.P.-B. has received personal fees from AbbVie, Adacyte, Alimentiv, Alma Bio Therapeutics, Amgen, Applied Molecular Transport, Arena, Biogen, BMS, Celltrion, CONNECT Biopharm, Cytoki Pharma, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead, Gossamer Bio, GSK, HAC-Pharma, IAG Image Analysis, Index Pharmaceuticals, Inotrem, Janssen, Lilly, Medac, Mopac, Morphic, MSD, Norgine, Nordic Pharma, Novartis, OM Pharma, ONO Pharma, OSE Immunotherapeutics, Pandion Therapeutics, Par’Immune, Pfizer, Prometheus, Protagonist, Roche, Sanofi, Sandoz, Takeda, Theravance, Thermo Fisher, Tigenix, Tillots, Viatris, Vifor, Ysopia, Abivax, Samsung, Ventyx, Roivant and Vectivbio. S.D. reports consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, Applied Molecular Transport, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen-Johnson & Johnson, Morphic, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, Teladoc Health, TiGenix, UCB Inc., Vial and Vifor; and reports lecture fees from Abbvie, Amgen, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer and Takeda. B.E.S. has served as a consultant or received speaker’s fees from AbbVie, Abivax, Adiso Therapeutics, Alimentiv, Amgen, Arena Pharmaceuticals, Artizan Biosciences, Artugen Therapeutics, AstraZeneca, Bacainn Therapeutics, Biora Therapeutics, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calibr, Celltrion, ClostraBio, Connect Biopharm, Cytoki Pharma, Eli Lilly and Company, Enthera, Evommune, Ferring, Fresenius Kabi, Galapagos, Gilead Sciences, Genentech, Glaxo SmithKline, Gossamer Bio, HMP Acquisition, Imhotex, Immunic, InDex Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Ironwood Pharmaceuticals, Janssen–Johnson & Johnson, Kaleido, Kalyope, Merck, MiroBio, Morphic Therapeutic, MRM Health, OSE Immunotherapeutics, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, RedHill Biopharma, Sun Pharma Global, Surrozen, Synlogic Operating Company, Takeda, Target RWE, Theravance Biopharma R&D, TLL Pharmaceutical, USWM Enterprises, Ventyx Biosciences and Viela Bio; and had stock options from Ventyx Biosciences. R.P. has served as a consultant for Abbott, AbbVie, Abbivax, Alimentiv Inc. (formerly Robarts Clinical Trials), Amgen, Arena Pharmaceuticals, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Fresenius Kabi, Genentech, Gilead Sciences, Glaxo-Smith Kline, JAMP Bio, Janssen, Merck, Mylan, Novartis, Oppilan Pharma, Organon, Pandion Pharma, Pendopharm, Pfizer, Progenity, Prometheus Biosciences, Protagonist Therapeutics, Roche, Sandoz, Satisfai Health, Shire, Sublimity Therapeutics, Takeda Pharmaceuticals, Theravance Biopharma, Trellus, Viatris, Ventyx and UCB; received speaker’s fees from AbbVie, Amgen, Arena Pharmaceuticals, Bristol-Myers Squibb, Celgene, Eli Lilly, Ferring, Fresenius Kabi, Gilead Sciences, Janssen, Merck, Organon, Pfizer, Roche, Sandoz, Shire, and Takeda Pharmaceuticals; and served on advisory boards for AbbVie, Alimentiv Inc. (formerly Robarts Clinical Trials), Amgen, Arena Pharmaceuticals, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Ferring, Fresenius Kabi, Genentech, Gilead Sciences, Glaxo-Smith Kline, JAMP Bio, Janssen, Merck, Mylan, Novartis, Oppilan Pharma, Organon, Pandion Pharma, Pfizer, Progenity, Protagonist Therapeutics, Roche, SandozShire, Sublimity Therapeutics, Takeda Pharmaceuticals and Ventyx.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Peter Irving, Natalia Queiroz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Active comparators
-
Treatment or intervention used as a comparison group in a clinical trial, which is known to be effective for the condition being studied or is already used as a standard treatment.
- Adaptive trial
-
Type of clinical trial that allows for modifications to certain aspects of the trial protocol during its conduct, typically based on accumulating data from the study, making trials more flexible and responsive to emerging evidence while maintaining scientific validity.
- Adverse events
-
Any untoward medical occurrence or undesirable experience that happens in the course of a clinical trial, regardless of whether it is related to the treatment being studied. Adverse events are categorized by their frequency, severity and nature.
- Basket trials
-
Type of clinical trial that evaluates efficacy of a targeted therapy across multiple different diseases or conditions (for example, testing a novel therapy in both Crohn’s disease and ulcerative colitis within the same protocol).
- Bayesian approaches
-
Bayesian statistics use a framework for probabilistic reasoning and inference that treats unknown parameters as random variables with probability distributions and uses observed data to update the prior probability of a hypothesis. Bayesian approaches incorporate prior information, and not just data observed within a trial.
- Blinded
-
Method used in clinical trials to reduce bias by ensuring that certain individuals are unaware of key information (typically treatment assignment); investigators, site personnel, trial sponsors or patients may be blinded during the course of a trial.
- Cluster randomization
-
A method of randomization where groups (or clusters) are randomly assigned to different intervention arms, rather than randomization of individual patients.
- Equivalence trials
-
Type of clinical trial designed to demonstrate that two or more treatments/interventions are equivalent in terms of efficacy within a prespecified equivalence margin.
- Explanatory
-
Type of clinical trial designed to assess efficacy of interventions under ideal or controlled conditions.
- Feeder cohorts
-
A secondary group of patients in a trial, separate from those involved in the primary treatment comparison, that is used to increase participants that can enrol into later phases of a clinical trial.
- Frequentist inference
-
Frequentist (classical) statistics is a traditional approach to statistical inference that estimates unknown parameters solely based on observed data within a clinical trial. Unknown parameters are treated as fixed values, inference is based on hypothesis testing against a null hypothesis to calculate the probability of observing the data (or more extreme data) under the assumption that the null hypothesis is true.
- Master trial protocols
-
A comprehensive clinical trial framework designed to evaluate multiple research questions, interventions or treatment strategies within the same overarching protocol.
- Non-inferiority trials
-
Type of clinical trial designed to demonstrate that a new treatment or intervention is not significantly worse than the comparator treatment, by a prespecified non-inferiority margin (conclusions from non-inferiority trials are that the new treatment is not clinically worse than the comparator, by an acceptable margin).
- Null hypothesis
-
The null hypothesis is the statement/assumption that there is no treatment effect with the intervention being studied and serves as the reference point for statistical testing for significance (that is, if the observed trial data are not consistent with the null hypothesis, then statistical testing is performed to reject the null hypothesis and accept the alternative hypothesis that there is a true treatment effect).
- Open-label
-
Type of trial in which the participant and investigators are aware of the treatments being administered.
- Patient-reported outcome
-
A patient-reported outcome is a measure of patient health status that is directly reported by the patient, without interpretation by a health-care professional or anyone else.
- Placebo
-
A substance or treatment that has no therapeutic effect used as a control treatment in randomized controlled trials.
- Post hoc
-
A post hoc analysis refers to analyses conducted after the data have been collected and is not part of the original planned analyses, typically to explore unexpected findings to identify other potential relationships or patterns in the data.
- Power
-
Power in a clinical trial refers to the probability that the study will detect a true effect of the intervention being tested, if such an effect exists. Studies that are under-powered do not have enough participants to show a true treatment effect, whereas over-powered studies enrol more patients than necessary to show the same treatment effect.
- Pragmatic trials
-
Type of clinical trial designed to assess effectiveness of interventions in more routine or real-world clinical practice settings.
- Primary outcomes
-
The main measure of interest in a clinical trial designed to assess efficacy of an intervention, corresponding to the primary research question/objective.
- Proof-of-concept
-
Demonstration that a specific therapeutic approach is feasible and has potential for efficacy; typically relates to preliminary studies that show a novel treatment has the desired effect or works via the purported mechanism.
- Randomization
-
Process for assigning participants to different treatment groups within a clinical trial.
- Registrational trial
-
(Also known as pivotal trial). Clinical trial designed to provide primary evidence to support efficacy and safety claims for regulatory approval.
- Regulatory approval
-
Process by which governmental regulatory agencies (for example, the FDA and EMA) review and evaluate the efficacy, safety and quality of new medical products before they can be marketed and distributed for use in patients.
- Reliability
-
Extent to which a measurement tool consistently produces the same results when used repeatedly under consistent conditions (degree of stability in the measurements); inter-rater reliability evaluates consistency of measurements obtained by different raters, intra-rater reliability evaluates consistency within the same observer.
- Rescue arms
-
Predefined component of a trial designed to allow participants who have not responded or cannot tolerate the assigned treatment to be offered an alternative treatment or intervention (typically, allows participants randomized to placebo to end participation in a blinded component of the trial and receive the investigational product open-label).
- Responder re-randomization
-
Trial design wherein participants who improved during induction therapy are re-randomized to different study groups in a maintenance trial.
- Responsiveness
-
Ability of a measurement instrument to detect meaningful change in a particular outcome over time or in response to an effective intervention (sensitivity to change in the condition being measured).
- Secondary outcomes
-
Secondary outcomes are additional variables or measures that provide supplementary information about other aspects of an intervention’s efficacy or impact on patient health outcomes.
- Significance
-
Statistical significance is a measure of the likelihood that an observed treatment effect is not due to random chance, but reflects a true effect of the treatment. Significance level (denoted by α) represents the threshold for deciding if results are significant and represents the maximum probability of making a type I error. The commonly used significance level in trials is α = 0.05.
- Superiority trials
-
Type of clinical trial designed to test whether one treatment/intervention is better than another with statistical significance. This is the most common type of trial for obtaining regulatory approval for a novel therapy.
- Synthetic control arms
-
A statistical method used to estimate the outcomes of a treatment group in the absence of a traditional control group, wherein participants are not randomized to an active comparator or placebo, but data from such a group is constructed using either historical or external sources to closely resemble those undergoing treatment.
- Treat-through designs
-
A trial design wherein participants are randomized at the beginning of treatment and continue in the same treatment assignment through to the end of the study.
- Umbrella trials
-
Type of clinical trial that evaluates multiple treatments or interventions simultaneously within the same overarching trial framework (for example, testing multiple novel therapies in a single trial in patients with Crohn’s disease).
- Withdrawal
-
Decision to discontinue trial participation before study completion, which may be voluntary or related to factors such as developing adverse events or lack of efficacy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, C., Jairath, V., Feagan, B.G. et al. Interpreting modern randomized controlled trials of medical therapy in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 21, 792–808 (2024). https://doi.org/10.1038/s41575-024-00989-y
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41575-024-00989-y
This article is cited by
-
Differentiating IL-23 Inhibitors in Crohn’s Disease
Drugs (2025)
-
Etrasimod in moderately to severely active ulcerative colitis: a profile of its use in the EU
Drugs & Therapy Perspectives (2025)