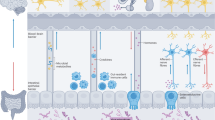
Abstract
Visceral pain is a major clinical problem and one of the most common reasons patients with gastrointestinal disorders seek medical help. Peripheral sensory neurons that innervate the gut can detect noxious stimuli and send signals to the central nervous system that are perceived as pain. There is a bidirectional communication network between the gastrointestinal tract and the nervous system that mediates pain through the gut–brain axis. Sensory neurons detect mechanical and chemical stimuli within the intestinal tissues, and receive signals from immune cells, epithelial cells and the gut microbiota, which results in peripheral sensitization and visceral pain. This Review focuses on molecular communication between these non-neuronal cell types and neurons in visceral pain. These bidirectional interactions can be dysregulated during gastrointestinal diseases to exacerbate visceral pain. We outline the anatomical pathways involved in pain processing in the gut and how cell–cell communication is integrated into this gut–brain axis. Understanding how bidirectional communication between the gut and nervous system is altered during disease could provide new therapeutic targets for treating visceral pain.
Key Points
-
Visceral pain is a substantial clinical issue associated with several visceral diseases and one of the primary reasons why patients with gastrointestinal disorders seek medical assistance.
-
Sensory neurons originating from the dorsal root ganglia innervate the gut and are responsible for detecting noxious stimuli and transmitting signals to the central nervous system, which are perceived as pain.
-
The bidirectional communication network between the gastrointestinal tract and the nervous system is known as the gut–brain axis; this network has a crucial role in mediating pain in the context of visceral disorders.
-
Immune cells, epithelial cells and the gut microbiota all communicate with sensory neurons; this communication can lead to peripheral sensitization and modulation of visceral pain processing.
-
Dysregulation of the interactions between non-neuronal cell types and neurons can contribute to visceral hypersensitivity in gastrointestinal diseases, and understanding these alterations could open new therapeutic targets for the treatment of visceral pain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alemi, F. et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 144, 145–154 (2013).
Cervero, F. & Laird, J. M. Visceral pain. Lancet 353, 2145–2148 (1999).
Collett, B. Visceral pain: the importance of pain management services. Br. J. Pain. 7, 6–7 (2013).
Fass, R. & Navarro-Rodriguez, T. Noncardiac chest pain. J. Clin. Gastroenterol. 42, 636–646 (2008).
Gasbarrini, A. et al. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig. Dis. 25, 237–240 (2007).
Kappelman, M. D. et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin. Gastroenterol. Hepatol. 5, 1424–1429 (2007).
Lee, R. W., Hodgson, L. E., Jackson, M. B. & Adams, N. Problem based review: pleuritic chest pain. Acute Med. 11, 172–182 (2012).
Sikandar, S. & Dickenson, A. H. Visceral pain – the ins and outs, the ups and downs. Curr. Opin. Support. Palliat. Care 6, 17–26 (2012).
Drewes, A. M. et al. Gastrointestinal pain. Nat. Rev. Dis. Prim. 6, 1 (2020).
Grundy, L., Erickson, A. & Brierley, S. M. Visceral pain. Annu. Rev. Physiol. 81, 261–284 (2019).
Beery, A. K. & Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572 (2011).
Cervero, F. Visceral pain: mechanisms of peripheral and central sensitization. Ann. Med. 27, 235–239 (1995).
Basbaum, A. I., Bautista, D. M., Scherrer, G. & Julius, D. Cellular and molecular mechanisms of pain. Cell 139, 267–284 (2009).
Dubin, A. E. & Patapoutian, A. Nociceptors: the sensors of the pain pathway. J. Clin. Invest. 120, 3760–3772 (2010).
Phillips, R. J. & Powley, T. L. Innervation of the gastrointestinal tract: patterns of aging. Auton. Neurosci. 136, 1–19 (2007).
Uesaka, T., Young, H. M., Pachnis, V. & Enomoto, H. Development of the intrinsic and extrinsic innervation of the gut. Dev. Biol. 417, 158–167 (2016).
Altschuler, S. M., Escardo, J., Lynn, R. B. & Miselis, R. R. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104, 502–509 (1993).
Berthoud, H. R. & Neuhuber, W. L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 85, 1–17 (2000).
Grundy, D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut 51, 2–5 (2002).
Sengupta, J. N. in Sensory Nerves. Handbook of Experimental Pharmacology Vol. 194 (eds Canning, B. & Spina, D.) 31–74 (Springer, 2009).
Yu, S., Kollarik, M., Ouyang, A., Myers, A. C. & Undem, B. J. Mast cell-mediated long-lasting increases in excitability of vagal C fibers in guinea pig esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G850–G856 (2007).
Kollarik, M., Ru, F. & Brozmanova, M. Vagal afferent nerves with the properties of nociceptors. Auton. Neurosci. 153, 12–20 (2010).
Yu, S., Undem, B. J. & Kollarik, M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J. Physiol. 563, 831–842 (2005).
Yu, S., Ru, F., Ouyang, A. & Kollarik, M. 5-Hydroxytryptamine selectively activates the vagal nodose C-fibre subtype in the guinea-pig oesophagus. Neurogastroenterol. Motil. 20, 1042–1050 (2008).
Bielefeldt, K. & Davis, B. M. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G130–G138 (2008).
Bielefeldt, K., Zhong, F., Koerber, H. R. & Davis, B. M. Phenotypic characterization of gastric sensory neurons in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G987–G997 (2006).
Kang, Y.-M., Bielefeldt, K. & Gebhart, G. F. Sensitization of mechanosensitive gastric vagal afferent fibers in the rat by thermal and chemical stimuli and gastric ulcers. J. Neurophysiol. 91, 1981–1989 (2004).
Ozaki, N. & Gebhart, G. F. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G1449–G1459 (2001).
Sengupta, J. N., Saha, J. K. & Goyal, R. K. Stimulus-response function studies of esophageal mechanosensitive nociceptors in sympathetic afferents of opossum. J. Neurophysiol. 64, 796–812 (1990).
Sengupta, J. N., Saha, J. K. & Goyal, R. K. Differential sensitivity to bradykinin of esophageal distension-sensitive mechanoreceptors in vagal and sympathetic afferents of the opossum. J. Neurophysiol. 68, 1053–1067 (1992).
Charney, K. J., Juler, G. L. & Comarr, A. E. General surgery problems in patients with spinal cord injuries. Arch. Surg. 110, 1083–1088 (1975).
Finnerup, N. B., Faaborg, P., Krogh, K. & Jensen, T. S. Abdominal pain in long-term spinal cord injury. Spinal Cord. 46, 198–203 (2008).
Levinthal, D. J. & Bielefeldt, K. Pain without nociception? Eur. J. Gastroenterol. Hepatol. 24, 336–339 (2012).
Yung, J. C. & Groah, S. L. Crohn’s disease in a patient with acute spinal cord injury: a case report of diagnostic challenges in the rehabilitation setting. Arch. Phys. Med. Rehabil. 82, 1274–1278 (2001).
Fink, R. Pain assessment: the cornerstone to optimal pain management. Proc. Bayl. Univ. Med. Cent. 13, 236–239 (2000).
Schuligoi, R. et al. Gastric acid-evoked c-fos messenger RNA expression in rat brainstem is signaled by capsaicin-resistant vagal afferents. Gastroenterology 115, 649–660 (1998).
Lamb, K., Kang, Y.-M., Gebhart, G. F. & Bielefeldt, K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology 125, 1410–1418 (2003).
Kamp, E. H., Jones, R. C. W., Tillman, S. R. & Gebhart, G. F. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G434–G444 (2003).
Grundy, D. Speculations on the structure/function relationship for vagal and splanchnic afferent endings supplying the gastrointestinal tract. J. Autonomic Nerv. Syst. 22, 175–180 (1988).
Kirkup, A. J., Brunsden, A. M. & Grundy, D. I. Receptors on visceral afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G787–G794 (2001).
Traub, R. J., Herdegen, T. & Gebhart, G. F. Differential expression of c-fos and c-jun in two regions of the rat spinal cord following noxious colorectal distention. Neurosci. Lett. 160, 121–125 (1993).
Traub, R. J., Lim, F., Sengupta, J. N., Meller, S. T. & Gebhart, G. F. Noxious distention of viscera results in differential c-Fos expression in second order sensory neurons receiving ‘sympathetic’ or ‘parasympathetic’ input. Neurosci. Lett. 180, 71–75 (1994).
Wang, G., Tang, B. & Traub, R. J. Differential processing of noxious colonic input by thoracolumbar and lumbosacral dorsal horn neurons in the rat. J. Neurophysiol. 94, 3788–3794 (2005).
Traub, R. J. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport 11, 2113–2116 (2000).
Morteau, O., Hachet, T., Caussette, M. & Bueno, L. Experimental colitis alters visceromotor response to colorectal distension in awake rats. Dig. Dis. Sci. 39, 1239–1248 (1994).
Hunt, S. & Mantyh, P. The molecular dynamics of pain control. Nat. Rev. Neurosci. 2, 83–91 (2001).
Cervero, F. & Connell, L. A. Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J. Comp. Neurol. 230, 88–98 (1984).
Bester, H., Menendez, L., Besson, J. M. & Bernard, J. F. Spino (trigemino) parabrachiohypothalamic pathway: electrophysiological evidence for an involvement in pain processes. J. Neurophysiol. 73, 568–585 (1995).
Hodge, C. J. & Apkarian, A. The spinothalamic tract. Crit. Rev. Neurobiol. 5, 363–397 (1990).
Nauta, H. J. W., Hewitt, E., Westlund, K. N. & Willis, W. D. Surgical interruption of a midline dorsal column visceral pain pathway: case report and review of the literature. J. Neurosurg. 86, 538–542 (1997).
Palecek, J. & Willis, D. W. The dorsal column pathway facilitates visceromotor responses to colorectal distention after colon inflammation in rats. Pain 104, 501–507 (2003).
Hirshberg, R. M., AI-Chaer, E. D., Lawand, N. B., Westlund, K. N. & Willis, W. D. Is there a pathway in the posterior funiculus that signals visceral pain? Pain 67, 291–305 (1996).
Nauta, H. J. et al. Punctate midline myelotomy for the relief of visceral cancer pain. J. Neurosurg. 92, 125–130 (2000).
Becker, R., Sure, U. & Bertalanffy, H. Punctate midline myelotomy. A new approach in the management of visceral pain. Acta Neurochir. 141, 881–883 (1999).
Kim, Y. S. & Kwon, S. J. High thoracic midline dorsal column myelotomy for severe visceral pain due to advanced stomach cancer. Neurosurgery 46, 85–90 (2000). discussion 90-92.
Angaut-Petit, D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat’s fasciculus gracilis. Exp. Brain Res. 22, 471–493 (1975).
Giesler, G. J. & Cliffer, K. D. Postsynaptic dorsal column pathway of the rat. II. Evidence against an important role in nociception. Brain Res. 326, 347–356 (1985).
Cliffer, K. & Giesler, G. Postsynaptic dorsal column pathway of the rat. III. Distribution of ascending afferent fibers. J. Neurosci. 9, 3146–3168 (1989).
Wang, C.-C., Willis, W. D. & Westlund, K. N. Ascending projections from the area around the spinal cord central canal: a Phaseolus vulgaris leucoagglutinin study in rats. J. Comp. Neurol. 415, 341–367 (1999).
Willis, W. D., Al-Chaer, E. D., Quast, M. J. & Westlund, K. N. A visceral pain pathway in the dorsal column of the spinal cord. Proc. Natl Acad. Sci. USA 96, 7675–7679 (1999).
Al-Chaer, E. D., Lawand, N. B., Westlund, K. N. & Willis, W. D. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J. Neurophysiol. 76, 2661–2674 (1996).
Feng, Y., Cui, M., Al-Chaer, E. D. & Willis, W. D. Epigastric antinociception by cervical dorsal column lesions in rats. Anesthesiology 89, 411–420 (1998).
Houghton, A. K., Wang, C. C. & Westlund, K. N. Do nociceptive signals from the pancreas travel in the dorsal column? Pain 89, 207–220 (2001).
Houghton, A. K., Kadura, S. & Westlund, K. N. Dorsal column lesions reverse the reduction of homecage activity in rats with pancreatitis. Neuroreport 8, 3795–3800 (1997).
Al-Chaer, E. D., Lawand, N. B., Westlund, K. N. & Willis, W. D. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J. Neurophysiol. 76, 2675–2690 (1996).
Al-Chaer, E. D., Westlund, K. N. & Willis, W. D. Nucleus gracilis: an integrator for visceral and somatic information. J. Neurophysiol. 78, 521–527 (1997).
Vizzard, M., Brisson, M. & Groat, W. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res. 299, 9–26 (2000).
Cameron, O. G. Interoception: the inside story – a model for psychosomatic processes. Psychosom. Med. 63, 697–710 (2001).
Critchley, H. D. & Harrison, N. A. Visceral influences on brain and behavior. Neuron 77, 624–638 (2013).
Dunn, B. D. et al. Listening to your heart. How interoception shapes emotion experience and intuitive decision making. Psychol. Sci. 21, 1835–1844 (2010).
Shah, P., Catmur, C. & Bird, G. From heart to mind: linking interoception, emotion, and theory of mind. Cortex 93, 220–223 (2017).
Ghacibeh, G. A., Shenker, J. I., Shenal, B., Uthman, B. M. & Heilman, K. M. The influence of vagus nerve stimulation on memory. Cogn. Behav. Neurol. 19, 119–122 (2006).
Porges, S. W., Doussard-Roosevelt, J. A. & Maiti, A. K. Vagal tone and the physiological regulation of emotion. Monogr. Soc. Res. Child. Dev. 59, 167–186 (1994).
Lai, J. & David, S. V. Short-term effects of vagus nerve stimulation on learning and evoked activity in auditory cortex. eNeuro https://doi.org/10.1523/ENEURO.0522-20.2021 (2021).
Johnson, R. L. & Wilson, C. G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 11, 203–213 (2018).
Shao, P. et al. Role of vagus nerve stimulation in the treatment of chronic pain. Neuroimmunomodulation 30, 167–183 (2023).
Pertovaara, A. Noradrenergic pain modulation. Prog. Neurobiol. 80, 53–83 (2006).
Dogrul, A., Ossipov, M. H. & Porreca, F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 1280, 52–59 (2009).
Miron, D., Duncan, G. H. & Bushnell, C. M. Effects of attention on the intensity and unpleasantness of thermal pain. Pain 39, 345–352 (1989).
Bantick, S. J. et al. Imaging how attention modulates pain in humans using functional MRI. Brain 125, 310–319 (2002).
Schaible, H.-G., Ebersberger, A. & Natura, G. Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res. Ther. 13, 210 (2011).
Cenac, N. et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 149, 433–444.e7 (2015).
Michaelis, M., Häbler, H. J. & Jäenig, W. Silent afferents: a separate class of primary afferents? Clin. Exp. Pharmacol. Physiol. 23, 99–105 (1996).
Stakenborg, N., Viola, M. F. & Boeckxstaens, G. E. Intestinal neuro-immune interactions: focus on macrophages, mast cells and innate lymphoid cells. Curr. Opin. Neurobiol. 62, 68–75 (2020).
Yu, X. et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat. Commun. 11, 264 (2020).
Shaw, T. N. et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med. 215, 1507–1518 (2018).
Muller, P. A. et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014).
De Schepper, S. et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175, 400–415.e13 (2018).
Viola, M. F. et al. Dedicated macrophages organize and maintain the enteric nervous system. Nature 618, 818–826 (2023).
Hughes, P., Brierley, S. & Blackshaw, L. Post-inflammatory modification of colonic afferent mechanosensitivity. Clin. Exp. Pharmacol. Physiol. 36, 1034–1040 (2009).
Hughes, P. A. et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 62, 1456–1465 (2013).
Hughes, P. A. et al. Immune derived opioidergic inhibition of viscerosensory afferents is decreased in irritable bowel syndrome patients. Brain Behav. Immun. 42, 191–203 (2014).
Grubišić, V. et al. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep. 32, 108100 (2020).
Kwon, M. J. et al. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J. Neurosci. 33, 15095–15108 (2013).
Qiao, L. Y. & Tiwari, N. Spinal neuron-glia-immune interaction in cross-organ sensitization. Am. J. Physiol. Gastrointest. Liver Physiol. 319, G748–G760 (2020).
Albert-Bayo, M. et al. Intestinal mucosal mast cells: key modulators of barrier function and homeostasis. Cells 8, 135 (2019).
Forsythe, P. & Bienenstock, J. The mast cell-nerve functional unit: a key component of physiologic and pathophysiologic responses. Chem. Immunol. Allergy 98, 196–221 (2012).
Barbara, G. et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126, 693–702 (2004).
Cremon, C. et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am. J. Gastroenterol. 104, 392–400 (2009).
Akbar, A. et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57, 923–929 (2008).
Bashashati, M. et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol. Motil. 30, e13192 (2018).
Barbara, G. et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132, 26–37 (2007).
Buhner, S. et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137, 1425–1434 (2009).
Cenac, N. et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Invest. 117, 636–647 (2007).
Grabauskas, G. et al. Prostaglandin E2, produced by mast cells in colon tissues from patients with irritable bowel syndrome, contributes to visceral hypersensitivity in mice. Gastroenterology 158, 2195–2207.e6 (2020).
Klooker, T. K. et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 59, 1213–1221 (2010).
Wang, J. et al. Clinical efficacy and safety of ketotifen in treating irritable bowel syndrome with diarrhea. Eur. J. Gastroenterol. Hepatol. 32, 706–712 (2020).
Wouters, M. M. et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 150, 875–887.e9 (2016).
Aguilera-Lizarraga, J. et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 590, 151–156 (2021).
Satitsuksanoa, P., Jansen, K., Głobińska, A., van de Veen, W. & Akdis, M. Regulatory immune mechanisms in tolerance to food allergy. Front. Immunol. 9, 2939 (2018).
Ma, H., Tao, W. & Zhu, S. T lymphocytes in the intestinal mucosa: defense and tolerance. Cell Mol. Immunol. 16, 216–224 (2019).
Rittner, H. L. et al. Opioid peptide-expressing leukocytes: identification, recruitment, and simultaneously increasing inhibition of inflammatory pain. Anesthesiology 95, 500–508 (2001).
Kamphuis, S. et al. Role of endogenous pro-enkephalin A-derived peptides in human T cell proliferation and monocyte IL-6 production. J. Neuroimmunol. 84, 53–60 (1998).
Kavelaars, A., Ballieux, R. E. & Heijnen, C. J. The role of IL-1 in the corticotropin-releasing factor and arginine- vasopressin-induced secretion of immunoreactive β-endorphin by human peripheral blood mononuclear cells. J. Immunol. 142, 2338–2342 (1989).
Smith, E. M., Morrill, A. C., Meyer, W. J. & Blalock, J. E. Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. Nature 321, 881–882 (1986).
Verma–Gandhu, M. et al. CD4+ T-cell modulation of visceral nociception in mice. Gastroenterology 130, 1721–1728 (2006).
Boué, J. et al. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4+ T cells in mice. Gastroenterology 146, 166–175 (2014).
Basso, L. et al. T-lymphocyte-derived enkephalins reduce Th1/Th17 colitis and associated pain in mice. J. Gastroenterol. 53, 215–226 (2018).
Gabanyi, I. et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016).
Matheis, F. et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 180, 64–78.e16 (2020).
Bednarska, O. et al. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology 153, 948–960.e3 (2017).
Seillet, C. et al. The neuropeptide VIP confers anticipatory mucosal immunity by regulating ILC3 activity. Nat. Immunol. 21, 168–177 (2020).
Talbot, J. et al. Feeding-dependent VIP neuron–ILC3 circuit regulates the intestinal barrier. Nature 579, 575–580 (2020).
Wallrapp, A. et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356 (2017).
Cardoso, V. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017).
Klose, C. S. N. et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 (2017).
Moriyama, S. et al. β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359, 1056–1061 (2018).
Hoeffel, G. et al. Sensory neuron-derived TAFA4 promotes macrophage tissue repair functions. Nature 594, 94–99 (2021).
Lu, Y.-Z. et al. CGRP sensory neurons promote tissue healing via neutrophils and macrophages. Nature 628, 604–611 (2024).
Pinho-Ribeiro, F. A. et al. Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell 173, 1083–1097.e22 (2018).
Yang, N. J. et al. Nociceptive sensory neurons mediate inflammation induced by Bacillus anthracis edema toxin. Front. Immunol. 12, 642373 (2021).
Filtjens, J. et al. Nociceptive sensory neurons promote CD8 T cell responses to HSV-1 infection. Nat. Commun. 12, 2936 (2021).
Cohen, J. A. et al. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178, 919–932.e14 (2019).
Kashem, S. W. et al. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43, 515–526 (2015).
Perner, C. et al. Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity 53, 1063–1077.e7 (2020).
Riol-Blanco, L. et al. Nociceptive sensory neurons drive interleukin-23 mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014).
Zhu, Y. et al. A chemogenetic screen reveals that Trpv1-expressing neurons control regulatory T cells in the gut. Science 385, eadk1679 (2024).
Okumura, R. & Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 49, e338 (2017).
Makadia, P. A. et al. Optogenetic activation of colon epithelium of the mouse produces high-frequency bursting in extrinsic colon afferents and engages visceromotor responses. J. Neurosci. 38, 5788–5798 (2018).
Najjar, S. A. et al. Optogenetic inhibition of the colon epithelium reduces hypersensitivity in a mouse model of inflammatory bowel disease. Pain 162, 1126–1134 (2021).
Latorre, R., Sternini, C., De Giorgio, R. & Greenwood-Van Meerveld, B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol. Motil. 28, 620–630 (2016).
Bohórquez, D. V. et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Invest. 125, 782–786 (2015).
Kaelberer, M. M. et al. A gut-brain neural circuit for nutrient sensory transduction. Science 361, eaat5236 (2018).
Bellono, N. W. et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198.e16 (2017).
Gershon, M. D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Bayrer, J. R. et al. Gut enterochromaffin cells drive visceral pain and anxiety. Nature 616, 137–142 (2023).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Coutinho, H. B. et al. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J. Clin. Pathol. 51, 512–514 (1998).
Wehkamp, J. et al. Reduced Paneth cell α-defensins in ileal Crohn’s disease. Proc. Natl Acad. Sci. USA 102, 18129–18134 (2005).
Estienne, M. et al. Maternal deprivation alters epithelial secretory cell lineages in rat duodenum: role of CRF-related peptides. Gut 59, 744–751 (2010).
Riba, A. et al. Paneth cell defects induce microbiota dysbiosis in mice and promote visceral hypersensitivity. Gastroenterology 153, 1594–1606.e2 (2017).
Lu, V. B. et al. Adenosine triphosphate is co-secreted with glucagon-like peptide-1 to modulate intestinal enterocytes and afferent neurons. Nat. Commun. 10, 1029 (2019).
Mihara, H., Uchida, K., Koizumi, S. & Moriyama, Y. Involvement of VNUT-exocytosis in transient receptor potential vanilloid 4-dependent ATP release from gastrointestinal epithelium. PLoS ONE 13, e0206276 (2018).
Uehara, S. et al. Vesicular storage and secretion of L-glutamate from glucagon-like peptide 1-secreting clonal intestinal L cells. J. Neurochem. 96, 550–560 (2006).
Hayashi, M., Morimoto, R., Yamamoto, A. & Moriyama, Y. Expression and localization of vesicular glutamate transporters in pancreatic islets, upper gastrointestinal tract, and testis. J. Histochem. Cytochem. 51, 1375–1390 (2003).
Schütz, B. et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front. Physiol. 6, 87 (2015).
Lai, N. Y. et al. Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate Salmonella host defense. Cell 180, 33–49.e22 (2020).
Yang, D. et al. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell 185, 4190–4205.e25 (2022).
Zhang, W. et al. Gut-innervating nociceptors regulate the intestinal microbiota to promote tissue protection. Cell 185, 4170–4189.e20 (2022).
Shreiner, A. B., Kao, J. Y. & Young, V. B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 31, 69–75 (2015).
Turnbaugh, P. J. et al. The Human Microbiome Project. Nature 449, 804–810 (2007).
Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 (2012).
Duncan, S. H., Louis, P. & Flint, H. J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Env. Microbiol. 70, 5810–5817 (2004).
Long, X. et al. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol. Motil. 30, e13227 (2018).
Bourdu, S. et al. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology 128, 1996–2008 (2005).
Tarrerias, A. L. et al. Short-chain fatty acid enemas fail to decrease colonic hypersensitivity and inflammation in TNBS-induced colonic inflammation in rats. Pain 100, 91–97 (2002).
Lewandowski, K., Kaniewska, M., Karłowicz, K., Rosołowski, M. & Rydzewska, G. The effectiveness of microencapsulated sodium butyrate at reducing symptoms in patients with irritable bowel syndrome. Prz. Gastroenterol. 17, 28–34 (2022).
Vanhoutvin, S. A. L. W. et al. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol. Motil. 21, 952–e76 (2009).
Russo, R. et al. Sodium butyrate and its synthetic amide derivative modulate nociceptive behaviors in mice. Pharmacol. Res. 103, 279–291 (2016).
Nozu, T., Miyagishi, S., Nozu, R., Takakusaki, K. & Okumura, T. Butyrate inhibits visceral allodynia and colonic hyperpermeability in rat models of irritable bowel syndrome. Sci. Rep. 9, 19603 (2019).
Schwab, M. et al. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NFκB signalling. Mol. Immunol. 44, 3625–3632 (2007).
Sukhithasri, V., Nisha, N., Biswas, L., Anil Kumar, V. & Biswas, R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol. Res. 168, 396–406 (2013).
Millet, A. & Jendzjowsky, N. Pathogen recognition by sensory neurons: hypotheses on the specificity of sensory neuron signaling. Front. Immunol. 14, 1184000 (2023).
Liu, T., Gao, Y.-J. & Ji, R.-R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 28, 131–144 (2012).
Ochoa-Cortes, F. et al. Bacterial cell products signal to mouse colonic nociceptive dorsal root ganglia neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G723–G732 (2010).
Meseguer, V. et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 5, 3125 (2014).
Tedesco, L. S., Fuseler, J., Grisham, M., Wolf, R. & Roerig, S. C. Therapeutic administration of nitric oxide synthase inhibitors reverses hyperalgesia but not inflammation in a rat model of polyarthritis. Pain 95, 215–223 (2002).
Stimpson, S. A., Dalldorf, F. G., Otterness, I. G. & Schwab, J. H. Exacerbation of arthritis by IL-1 in rat joints previously injured by peptidoglycan-polysaccharide. J. Immunol. 140, 2964–2969 (1988).
Das, N. et al. HMGB1 activates proinflammatory signaling via TLR5 leading to allodynia. Cell Rep. 17, 1128–1140 (2016).
Maruyama, K. et al. Nociceptors boost the resolution of fungal osteoinflammation via the TRP channel–CGRP–Jdp2 axis. Cell Rep. 19, 2730–2742 (2017).
Rudick, C. N. et al. O-antigen modulates infection-induced pain states. PLoS ONE 7, e41273 (2012).
Nozu, T., Miyagishi, S., Nozu, R., Takakusaki, K. & Okumura, T. Lipopolysaccharide induces visceral hypersensitivity: role of interleukin-1, interleukin-6, and peripheral corticotropin-releasing factor in rats. J. Gastroenterol. 52, 72–80 (2017).
Vergnolle, N. Modulation of visceral pain and inflammation by protease-activated receptors. Br. J. Pharmacol. 141, 1264–1274 (2004).
Kayssi, A., Amadesi, S., Bautista, F., Bunnett, N. W. & Vanner, S. Mechanisms of protease-activated receptor 2-evoked hyperexcitability of nociceptive neurons innervating the mouse colon. J. Physiol. 580, 977–991 (2007).
Cattaruzza, F. et al. Cathepsin S is activated during colitis and causes visceral hyperalgesia by a PAR2-dependent mechanism in mice. Gastroenterology 141, 1864–1874.e3 (2011).
Rolland-Fourcade, C. et al. Epithelial expression and function of trypsin-3 in irritable bowel syndrome. Gut 66, 1767–1778 (2017).
Karanjia, R., Spreadbury, I., Bautista-cruz, F., Tsang, M. E. & Vanner, S. Activation of protease-activated receptor-4 inhibits the intrinsic excitability of colonic dorsal root ganglia neurons. Neurogastroenterol. Motil. 21, 1218–1221 (2009).
Augé, C., Balz-hara, D., Steinhoff, M., Vergnolle, N. & Cenac, N. Protease-activated receptor-4 (PAR4): a role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol. Motil. 21, 1189–e107 (2009).
Asfaha, S., Brussee, V., Chapman, K., Zochodne, D. W. & Vergnolle, N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br. J. Pharmacol. 135, 1101–1106 (2002).
Moloney, R. D. et al. Stress and the microbiota–gut–brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci. Ther. 22, 102–117 (2015).
Greenwood-Van Meerveld, B. & Johnson, A. C. Mechanisms of stress-induced visceral pain. J. Neurogastroenterol. Motil. 24, 7–18 (2018).
Petitfils, C. et al. Identification of bacterial lipopeptides as key players in IBS. Gut 72, 939–950 (2023).
Kassinen, A. et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 133, 24–33 (2007).
Si, J.-M., Yu, Y.-C., Fan, Y.-J. & Chen, S.-J. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J. Gastroenterol. 10, 1802–1805 (2004).
Rajilić-Stojanović, M. et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 141, 1792–1801 (2011).
Saulnier, D. M. et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 141, 1782–1791 (2011).
Botschuijver, S. et al. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology 153, 1026–1039 (2017).
Jeffery, I. B. et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61, 997–1006 (2012).
Zhou, Y. et al. Increased Enterococcus faecalis infection is associated with clinically active Crohn disease. Medicine 95, e5019 (2016).
Castaño-Rodríguez, N., Kaakoush, N. O., Lee, W. S. & Mitchell, H. M. Dual role of Helicobacter and Campylobacter species in IBD: a systematic review and meta-analysis. Gut 66, 235–249 (2017).
Mckernan, D. P., Fitzgerald, P., Dinan, T. G. & Cryan, J. F. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol. Motil. 22, 1029–e268 (2010).
Johnson, A. C., Greenwood-Van Meerveld, B. & McRorie, J. Effects of Bifidobacterium infantis 35624 on post-inflammatory visceral hypersensitivity in the rat. Dig. Dis. Sci. 56, 3179–3186 (2011).
Whorwell, P. J. et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 101, 1581–1590 (2006).
Agostini, S. et al. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol. Motil. 24, 376–e172 (2012).
Pokusaeva, K. et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 29, e12904 (2017).
Kamiya, T. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 55, 191–196 (2006).
Liu, Y.-W. et al. Lactobacillus plantarum PS128 ameliorated visceral hypersensitivity in rats through the gut–brain axis. Probiotics Antimicro. Prot. 12, 980–993 (2020).
Ducrotté, P., Sawant, P. & Jayanthi, V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 18, 4012–4018 (2012).
Pérez-Berezo, T. et al. Identification of an analgesic lipopeptide produced by the probiotic Escherichia coli strain Nissle 1917. Nat. Commun. 8, 1314 (2017).
Vulevic, J., Tzortzis, G., Juric, A. & Gibson, G. R. Effect of a prebiotic galactooligosaccharide mixture (B-GOS®) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol. Motil. 30, e13440 (2018).
Kannampalli, P. et al. Probiotic Lactobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol. Motil. 26, 1694–1704 (2014).
Saneian, H., Pourmoghaddas, Z., Roohafza, H. & Gholamrezaei, A. Synbiotic containing Bacillus coagulans and fructo-oligosaccharides for functional abdominal pain in children. Gastroenterol. Hepatol. Bed Bench 8, 56–65 (2015).
Chang, L. et al. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology 130, 1435–1446 (2006).
Icenhour, A. et al. Are there sex differences in visceral sensitivity in young healthy men and women? Neurogastroenterol. Motil. 31, e13664 (2019).
Posserud, I. et al. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology 133, 1113–1123 (2007).
Almario, C. V. et al. Prevalence and burden of illness of Rome IV irritable bowel syndrome in the United States: results from a nationwide cross-sectional study. Gastroenterology 165, 1475–1487 (2023).
Kim, Y. S. & Kim, N. Sex-gender differences in irritable bowel syndrome. J. Neurogastroenterol. Motil. 24, 544–558 (2018).
Lungaro, L. et al. Impact of female gender in inflammatory bowel diseases: a narrative review. J. Pers. Med. 13, 165 (2023).
Barbara, G. et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology 156, 46–58.e7 (2019).
Park, J. H. et al. Contribution of sex and gender roles to the incidence of post-infectious irritable bowel syndrome in a prospective study. Sci. Rep. 13, 19467 (2023).
Guo, T. et al. Sex differences in zymosan-induced behavioral visceral hypersensitivity and colorectal afferent sensitization. Am. J. Physiol. Gastrointest. Liver Physiol. 326, G133–G146 (2024).
Kozik, A. J., Nakatsu, C. H., Chun, H. & Jones-Hall, Y. L. Age, sex, and TNF associated differences in the gut microbiota of mice and their impact on acute TNBS colitis. Exp. Mol. Pathol. 103, 311–319 (2017).
Madar, J. et al. Piezo2 regulates colonic mechanical sensitivity in a sex specific manner in mice. Nat. Commun. 14, 2158 (2023).
Bábíčková, J. et al. Sex differences in experimentally induced colitis in mice: a role for estrogens. Inflammation 38, 1996–2006 (2015).
Hases, L., Birgersson, M., Indukuri, R., Archer, A. & Williams, C. Colitis induces sex-specific intestinal transcriptomic responses in mice. Int. J. Mol. Sci. 23, 10408 (2022).
Prusator, D. K. & Greenwood-Van Meerveld, B. Sex-related differences in pain behaviors following three early life stress paradigms. Biol. Sex. Differ. 7, 29 (2016).
Louwies, T. & Greenwood-Van Meerveld, B. Sex differences in the epigenetic regulation of chronic visceral pain following unpredictable early life stress. Neurogastroenterol. Motil. 32, e13751 (2020).
Larauche, M. et al. Visceral analgesia induced by acute and repeated water avoidance stress in rats: sex difference in opioid involvement. Neurogastroenterol. Motil. 24, 1031–e547 (2012).
Rosztóczy, A. et al. Influence of sex and experimental protocol on the effect of maternal deprivation on rectal sensitivity to distension in the adult rat. Neurogastroenterol. Motil. 15, 679–686 (2003).
Mason, K. L., Huffnagle, G. B., Noverr, M. C. & Kao, J. Y. Overview of gut immunology. Adv. Exp. Med. Biol. 635, 1–14 (2008).
Eutamene, H. et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol. Motil. 22, 312–e84 (2010).
Castro, J. et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology 145, 1334–1346.e11 (2013).
Black, C. J. et al. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology 155, 1753–1763 (2018).
De Palma, G. et al. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci. Transl. Med. 14, eabj1895 (2022).
Decraecker, L. et al. Treatment of non-constipated irritable bowel syndrome with the histamine 1 receptor antagonist ebastine: a randomised, double-blind, placebo-controlled trial. Gut 73, 459–469 (2024).
Lobo, B. et al. Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: a pilot study. U Eur. Gastroenterol. J. 5, 887–897 (2017).
Mawe, G. M. & Hoffman, J. M. Serotonin signalling in the gut – functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486 (2013).
Black, C. J. et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut 69, 74–82 (2020).
Shah, E. D., Lacy, B. E., Chey, W. D., Chang, L. & Brenner, D. M. Tegaserod for irritable bowel syndrome with constipation in women younger than 65 years without cardiovascular disease: pooled analyses of 4 controlled trials. Am. J. Gastroenterol. 116, 1601–1611 (2021).
Vakil, N. et al. Tegaserod treatment for dysmotility-like functional dyspepsia: results of two randomized, controlled trials. Am. J. Gastroenterol. 103, 1906–1919 (2008).
Lindström, E. et al. The selective 5-hydroxytryptamine 1A antagonist, AZD7371 [3(R)-(N,N-dicyclobutylamino)-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide (R,R)-tartrate monohydrate] (robalzotan tartrate monohydrate), inhibits visceral pain-related visceromotor, but not autonomic cardiovascular, responses to colorectal distension in rats. J. Pharmacol. Exp. Ther. 329, 1048–1055 (2009).
Drossman, D. A. et al. Randomized, double-blind, placebo-controlled trial of the 5-HT1A receptor antagonist AZD7371 tartrate monohydrate (robalzotan tartrate monohydrate) in patients with irritable bowel syndrome. Am. J. Gastroenterol. 103, 2562–2569 (2008).
Loeza-Alcocer, E., McPherson, T. P. & Gold, M. S. Peripheral GABA receptors regulate colonic afferent excitability and visceral nociception. J. Physiol. 597, 3425–3439 (2019).
Saito, Y. A. et al. Randomised clinical trial: pregabalin vs placebo for irritable bowel syndrome. Aliment. Pharmacol. Ther. 49, 389–397 (2019).
Kotikula, I. et al. Randomised clinical trial: the effects of pregabalin vs placebo on functional dyspepsia. Aliment. Pharmacol. Ther. 54, 1026–1032 (2021).
Fioramonti, J., Gaultier, E., Toulouse, M., Sanger, G. J. & Bueno, L. Intestinal anti-nociceptive behaviour of NK3 receptor antagonism in conscious rats: evidence to support a peripheral mechanism of action. Neurogastroenterol. Motil. 15, 363–369 (2003).
Houghton, L. A. et al. Effect of the NK3 receptor antagonist, talnetant, on rectal sensory function and compliance in healthy humans. Neurogastroenterol. Motil. 19, 732–743 (2007).
Acknowledgements
I.M.C. acknowledges funding from the Kenneth Rainin Foundation, NIH/NIDDK grant R01DK127257 and the Food Allergy Science Initiative. K.A.M. was supported by NIH fellowship F32DK137456.
Author information
Authors and Affiliations
Contributions
K.A.M. researched data for the article. Both authors contributed substantially to discussion of the content, wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
I.M.C. is on scientific advisory boards for GSK Pharmaceuticals and Nilo Therapeutics. He also consults for Panther Life Sciences and Fzata. His laboratory has received sponsored research support from GSK, Abbvie/Allergan and Moderna. K.A.M. declares no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Asbjørn Drewes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meerschaert, K.A., Chiu, I.M. The gut–brain axis and pain signalling mechanisms in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 22, 206–221 (2025). https://doi.org/10.1038/s41575-024-01017-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-024-01017-9
This article is cited by
-
Next-generation enteric neuroscience — fostering the future of the field
Nature Reviews Gastroenterology & Hepatology (2025)