Abstract
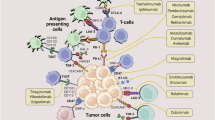
Hepatocellular carcinoma (HCC) represents a global health challenge, and ranks among one of the most prevalent and deadliest cancers worldwide. Therapeutic advances have expanded the treatment armamentarium for patients with advanced HCC, but obstacles remain. Precision oncology, which aims to match specific therapies to patients who have tumours with particular features, holds great promise. However, its implementation has been hindered by the existence of numerous ‘HCC influencers’ that contribute to the high inter-patient heterogeneity. HCC influencers include tumour-related characteristics, such as genetic alterations, immune infiltration, stromal composition and aetiology, and patient-specific factors, such as sex, age, germline variants and the microbiome. This Review delves into the intricate world of HCC, describing the most innovative model systems that can be harnessed to identify precision and/or personalized therapies. We provide examples of how different models have been used to nominate candidate biomarkers, their limitations and strategies to optimize such models. We also highlight the importance of reproducing distinct HCC influencers in a flexible and modular way, with the aim of dissecting their relative contribution to therapy response. Next-generation HCC models will pave the way for faster discovery of precision therapies for patients with advanced HCC.
Key points
-
Precision therapies in hepatocellular carcinoma (HCC) are difficult to establish owing to high inter-patient heterogeneity.
-
Several factors, or ‘HCC influencers’, contribute to HCC inter-patient heterogeneity.
-
Current in vitro and in vivo models recapitulate some but not all of the HCC influencers.
-
Improvements in the establishment success rate of in vitro HCC models could help the implementation of precision or personalized therapies for HCC, but they might not be ideal for immunotherapy studies.
-
Next-generation in vivo models, recapitulating multiple HCC influencers as well as including human cellular components, could help the implementation of precision therapies for HCC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Llovet, J. M. et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 3, 386–401 (2022).
Simon, S. M. Fighting rare cancers: lessons from fibrolamellar hepatocellular carcinoma. Nat. Rev. Cancer 23, 335–346 (2023).
Ilyas, S. I. et al. Cholangiocarcinoma — novel biological insights and therapeutic strategies. Nat. Rev. Clin. Oncol. 20, 470–486 (2023).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
Zhu, A. X. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 282–296 (2019).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Kelley, R. K. et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J. Clin. Oncol. 39, 2991–3001 (2021).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Zhu, A. X. et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 19, 940–952 (2018).
Yau, T. et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from checkmate 040. J. Clin. Oncol. 37, 4012 (2019).
Zanella, E. R., Grassi, E. & Trusolino, L. Towards precision oncology with patient-derived xenografts. Nat. Rev. Clin. Oncol. 19, 719–732 (2022).
Mulero-Sanchez, A., Pogacar, Z. & Vecchione, L. Importance of genetic screens in precision oncology. ESMO Open 4, e000505 (2019).
Llovet, J. M. Exploring a new pathway for biomarker-based approval of immunotherapies. Nat. Rev. Clin. Oncol. 20, 279–280 (2023).
Barcena-Varela, M. & Lujambio, A. The endless sources of hepatocellular carcinoma heterogeneity. Cancers 13, 2621 (2021).
Limousin, W. et al. Molecular-based targeted therapies in patients with hepatocellular carcinoma and hepato-cholangiocarcinoma refractory to atezolizumab/bevacizumab. J. Hepatol. 79, 1450–1458 (2023).
Sang, Y. B., Kim, G., Hwang, S., Kang, H. & Chon, H. J. Dramatic response to cabozantinib in a patient with refractory hepatocellular carcinoma with c-MET amplification. J. Clin. Transl. Hepatol. 11, 747–750 (2023).
Schulze, K. et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 47, 505–511 (2015).
Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341.e23 (2017).
Totoki, Y. et al. High-resolution characterization of a hepatocellular carcinoma genome. Nat. Genet. 43, 464–469 (2011).
Hoshida, Y. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 69, 7385–7392 (2009).
Boyault, S. et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 45, 42–52 (2007).
Sia, D. et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 153, 812–826 (2017).
Hoshida, Y. et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N. Engl. J. Med. 359, 1995–2004 (2008).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556 (2023).
Schwabe, R. F. & Greten, T. F. Gut microbiome in HCC — mechanisms, diagnosis and therapy. J. Hepatol. 72, 230–238 (2020).
Trepo, E. et al. Common genetic variation in alcohol-related hepatocellular carcinoma: a case-control genome-wide association study. Lancet Oncol. 23, 161–171 (2022).
Sangro, B. et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 73, 1460–1469 (2020).
Haber, P. K. et al. Molecular markers of response to anti-PD1 therapy in advanced hepatocellular carcinoma. Gastroenterology 164, 72–88.e15 (2022).
Zhu, A. X. et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 28, 1599–1611 (2022).
Pfister, D. et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592, 450–456 (2021).
Ruiz de Galarreta, M. et al. β-Catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 9, 1124–1141 (2019).
Magen, A. et al. Intratumoral dendritic cell-CD4+ T helper cell niches enable CD8+ T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. 29, 1389–1399 (2023).
Harding, J. J. et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next generation sequencing for matching patients to targeted and immune therapies. Clin. Cancer Res. 25, 2116–2126 (2018).
Brown, T. J., Mamtani, R., Gimotty, P. A., Karasic, T. B. & Yang, Y.-X. Outcomes of hepatocellular carcinoma by etiology with first-line atezolizumab and bevacizumab: a real-world analysis. J. Cancer Res. Clin. Oncol. 149, 2345–2354 (2023).
Kudo, M. Selection of systemic treatment regimen for unresectable hepatocellular carcinoma: does etiology matter? Liver Cancer 11, 283–289 (2022).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Gracia-Sancho, J., Caparrós, E., Fernández-Iglesias, A. & Francés, R. Role of liver sinusoidal endothelial cells in liver diseases. Nat. Rev. Gastroenterol. Hepatol. 18, 411–431 (2021).
Cogliati, B., Yashaswini, C. N., Wang, S., Sia, D. & Friedman, S. L. Friend or foe? The elusive role of hepatic stellate cells in liver cancer. Nat. Rev. Gastroenterol. Hepatol. 20, 647–661 (2023).
Qiu, Z. et al. Hepatocellular carcinoma cell lines retain the genomic and transcriptomic landscapes of primary human cancers. Sci. Rep. 6, 27411 (2016).
Hirschfield, H. et al. In vitro modeling of hepatocellular carcinoma molecular subtypes for anti-cancer drug assessment. Exp. Mol. Med. 50, e419 (2018).
Caruso, S. et al. Analysis of liver cancer cell lines identifies agents with likely efficacy against hepatocellular carcinoma and markers of response. Gastroenterology 157, 760–776 (2019).
Qiu, Z. et al. A pharmacogenomic landscape in human liver cancers. Cancer Cell 36, 179–193.e11 (2019).
Marasco, G. et al. Non-invasive tests for the prediction of primary hepatocellular carcinoma. World J. Gastroenterol. 26, 3326–3343 (2020).
Levrero, M. & Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 64, S84–S101 (2016).
Ye, H. et al. Gene network analysis of hepatocellular carcinoma identifies modules associated with disease progression, survival, and chemo drug resistance. Int. J. Gen. Med. 14, 9333–9347 (2021).
Reiberger, T. et al. An orthotopic mouse model of hepatocellular carcinoma with underlying liver cirrhosis. Nat. Protoc. 10, 1264–1274 (2015).
Hage, C. et al. Characterizing responsive and refractory orthotopic mouse models of hepatocellular carcinoma in cancer immunotherapy. PLoS ONE 14, e0219517 (2019).
Torrens, L. et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology 74, 2652–2669 (2021).
Zabransky, D. J. et al. Profiling of syngeneic mouse HCC tumor models as a framework to understand anti-PD-1 sensitive tumor microenvironments. Hepatology 77, 1566–1579 (2023).
Darlington, G. J., Bernhard, H. P., Miller, R. A. & Ruddle, F. H. Expression of liver phenotypes in cultured mouse hepatoma cells. J. Natl Cancer Inst. 64, 809–819 (1980).
Kress, S. et al. p53 mutations are absent from carcinogen-induced mouse liver tumors but occur in cell lines established from these tumors. Mol. Carcinog. 6, 148–158 (1992).
Zender, L. et al. Generation and analysis of genetically defined liver carcinomas derived from bipotential liver progenitors. Cold Spring Harb. Symp. Quant. Biol. 70, 251–261 (2005).
Molina-Sanchez, P. et al. Cooperation between distinct cancer driver genes underlies intertumor heterogeneity in hepatocellular carcinoma. Gastroenterology 159, 2203–2220.e14 (2020).
Marsee, A. et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 28, 816–832 (2021).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
Dutta, D., Heo, I. & Clevers, H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med. 23, 393–410 (2017).
Dong, R., Zhang, B. & Zhang, X. Liver organoids: an in vitro 3D model for liver cancer study. Cell Biosci. 12, 152 (2022).
Broutier, L. et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743 (2016).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013).
Broutier, L. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435 (2017).
Nuciforo, S. et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 24, 1363–1376 (2018).
Li, L. et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight 4, e121490 (2019).
Li, L. et al. Protein synthesis inhibitor omacetaxine is effective against hepatocellular carcinoma. JCI Insight 6, e138197 (2021).
Xian, L. et al. Heterogeneity, inherent and acquired drug resistance in patient-derived organoid models of primary liver cancer. Cell Oncol. 45, 1019–1036 (2022).
Tiriac, H. et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 8, 1112–1129 (2018).
Ganesh, K. et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 25, 1607–1614 (2019).
Ooft, S. N. et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 11, eaay2574 (2019).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Meier, M. A. et al. Patient-derived tumor organoids for personalized medicine in a patient with rare hepatocellular carcinoma with neuroendocrine differentiation: a case report. Commun. Med. 2, 80 (2022).
Sun, L. et al. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat. Cell Biol. 21, 1015–1026 (2019).
Thng, D. K. H. et al. Splice-switch oligonucleotide-based combinatorial platform prioritizes synthetic lethal targets CHK1 and BRD4 against MYC-driven hepatocellular carcinoma. Bioeng. Transl. Med. 8, e10363 (2023).
Nie, Y. Z. et al. Recapitulation of hepatitis B virus-host interactions in liver organoids from human induced pluripotent stem cells. eBioMedicine 35, 114–123 (2018).
De Crignis, E. et al. Application of human liver organoids as a patient-derived primary model for HBV infection and related hepatocellular carcinoma. eLife 10, e60747 (2021).
Wang, S. et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 29, 1009–1026 (2019).
Ouchi, R. et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 30, 374–384.e6 (2019).
Ramli, M. N. B. et al. Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology 159, 1471–1486.e12 (2020).
McCarron, S. et al. Functional characterization of organoids derived from irreversibly damaged liver of patients with NASH. Hepatology 74, 1825–1844 (2021).
Hu, H. et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175, 1591–1606.e19 (2018).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Hendriks, D. et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat. Biotechnol. 41, 1567–1581 (2023).
Cao, W. et al. Modeling liver cancer and therapy responsiveness using organoids derived from primary mouse liver tumors. Carcinogenesis 40, 145–154 (2019).
Liu, J. et al. Cancer-associated fibroblasts provide a stromal niche for liver cancer organoids that confers trophic effects and therapy resistance. Cell. Mol. Gastroenterol. Hepatol. 11, 407–431 (2021).
Rialdi, A. et al. WNTinib is a multi-kinase inhibitor with specificity against β-catenin mutant hepatocellular carcinoma. Nat. Cancer 4, 1157–1175 (2023).
van Tienderen, G. S. et al. Hepatobiliary tumor organoids for personalized medicine: a multicenter view on establishment, limitations, and future directions. Cancer Cell 40, 226–230 (2022).
Kong, J. C. H. et al. Tumor-infiltrating lymphocyte function predicts response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. JCO Precis. Oncol. 2, 1–15 (2018).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988.e16 (2018).
Zou, F. et al. The CD39+ HBV surface protein-targeted CAR-T and personalized tumor-reactive CD8+ T cells exhibit potent anti-HCC activity. Mol. Ther. 29, 1794–1807 (2021).
Liu, T. et al. High-affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma (HCC) activity by activating CD39+CD8+ T cells. Gut 70, 1965–1977 (2021).
Zhou, Z. et al. Evaluation of the tumoricidal efficacy of adoptive cell transfer using hepatocellular carcinoma-derived organoids. J. Gastrointest. Oncol. 13, 732–743 (2022).
Daniel, V. C. et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 69, 3364–3373 (2009).
Sun, F. X. et al. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int. J. Cancer 66, 239–243 (1996).
Gao, Y. S., Chen, X. P., Li, K. Y. & Wu, Z. D. Nude mice model of human hepatocellular carcinoma via orthotopic implantation of histologically intact tissue. World J. Gastroenterol. 10, 3107–3111 (2004).
Armengol, C. et al. Orthotopic implantation of human hepatocellular carcinoma in mice: analysis of tumor progression and establishment of the BCLC-9 cell line. Clin. Cancer Res. 10, 2150–2157 (2004).
Huynh, H., Soo, K. C., Chow, P. K., Panasci, L. & Tran, E. Xenografts of human hepatocellular carcinoma: a useful model for testing drugs. Clin. Cancer Res. 12, 4306–4314 (2006).
Gu, Q. et al. Genomic characterization of a large panel of patient-derived hepatocellular carcinoma xenograft tumor models for preclinical development. Oncotarget 6, 20160–20176 (2015).
He, S. et al. PDXliver: a database of liver cancer patient derived xenograft mouse models. BMC Cancer 18, 550 (2018).
Blumer, T. et al. Hepatocellular carcinoma xenografts established from needle biopsies preserve the characteristics of the originating tumors. Hepatol. Commun. 3, 971–986 (2019).
Tischfield, D. J. et al. Establishment of hepatocellular carcinoma patient-derived xenografts from image-guided percutaneous biopsies. Sci. Rep. 9, 10546 (2019).
Hu, B. et al. Establishment of a hepatocellular carcinoma patient-derived xenograft platform and its application in biomarker identification. Int. J. Cancer 146, 1606–1617 (2020).
Zhu, M. et al. Uncovering biological factors that regulate hepatocellular carcinoma growth using patient-derived xenograft assays. Hepatology 72, 1085–1101 (2020).
Xu, W. et al. Comprehensive comparison of patient-derived xenograft models in hepatocellular carcinoma and metastatic liver cancer. Int. J. Med. Sci. 17, 3073–3081 (2020).
Sun, F., Tang, Z. & Liu, K. Highly metastatic model of human hepatocellular carcinoma established in nude mice using orthotopic organ selection of metastatic variant from patient specimens. Zhonghua Zhong Liu Za Zhi 18, 109–112 (1996).
Aruga, A., Takasaki, K. & Hanyu, F. Establishment and characterization of liver metastatic model of human hepatoma in nude mice. Int. Hepatol. Commun. 1, 138–145 (1993).
Zhao, Y. et al. Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut 67, 1845–1854 (2018).
Zhao, Y. et al. Analysis and validation of human targets and treatments using a hepatocellular carcinoma-immune humanized mouse model. Hepatology 74, 1395–1410 (2021).
Tan, Z. et al. Isoformic PD-1-mediated immunosuppression underlies resistance to PD-1 blockade in hepatocellular carcinoma patients. Gut 72, 1568–1580 (2023).
Xiong, Z. et al. Targeting PPAR-γ counteracts tumour adaptation to immune-checkpoint blockade in hepatocellular carcinoma. Gut 72, 1758–1773 (2023).
Yang, Z. et al. Tumor-derived peptidoglycan recognition protein 2 predicts survival and antitumor immune responses in hepatocellular carcinoma. Hepatology 71, 1626–1642 (2020).
Wu, H. et al. Hepatic interferon regulatory factor 8 expression suppresses hepatocellular carcinoma progression and enhances the response to anti-programmed cell death protein-1 therapy. Hepatology 76, 1602–1616 (2022).
Fang, Y. et al. Monocarboxylate transporter 4 inhibition potentiates hepatocellular carcinoma immunotherapy through enhancing T cell infiltration and immune attack. Hepatology 77, 109–123 (2023).
Chen, Y. et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 61, 1591–1602 (2015).
Becher, O. J. & Holland, E. C. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res. 66, 3355–3358 (2006).
Cho, K. et al. Genetically engineered mouse models for liver cancer. Cancers 12, 14 (2019).
Casey, S. C. et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 352, 227–231 (2016).
Xu, Y. et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat. Med. 25, 301–311 (2019).
Dhanasekaran, R. et al. MYC overexpression drives immune evasion in hepatocellular carcinoma that is reversible through restoration of proinflammatory macrophages. Cancer Res. 83, 626–640 (2023).
Srivastava, J. et al. Astrocyte elevated gene-1 and c-Myc cooperate to promote hepatocarcinogenesis in mice. Hepatology 61, 915–929 (2015).
Brown, Z. J., Heinrich, B. & Greten, T. F. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat. Rev. Gastroenterol. Hepatol. 15, 536–554 (2018).
Xue, W. et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514, 380–384 (2014).
Breinig, M. et al. Multiplexed orthogonal genome editing and transcriptional activation by Cas12a. Nat. Methods 16, 51–54 (2019).
Zafra, M. P. et al. Optimized base editors enable efficient editing in cells, organoids and mice. Nat. Biotechnol. 36, 888–893 (2018).
Yang, P. L., Althage, A., Chung, J. & Chisari, F. V. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc. Natl Acad. Sci. USA 99, 13825–13830 (2002).
Huang, L. R., Wu, H. L., Chen, P. J. & Chen, D. S. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl Acad. Sci. USA 103, 17862–17867 (2006).
McCaffrey, A. P. et al. Determinants of hepatitis C translational initiation in vitro, in cultured cells and mice. Mol. Ther. 5, 676–684 (2002).
Chang, J., Sigal, L. J., Lerro, A. & Taylor, J. Replication of the human hepatitis delta virus genome Is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. J. Virol. 75, 3469–3473 (2001).
Huang, M., Sun, R., Huang, Q. & Tian, Z. Technical improvement and application of hydrodynamic gene delivery in study of liver diseases. Front. Pharmacol. 8, 591 (2017).
Chen, X. & Calvisi, D. F. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am. J. Pathol. 184, 912–923 (2014).
Monga, S. P. Inside-out or outside-in: choosing the right model of hepatocellular cancer. Gene Expr. 20, 139–145 (2020).
Fan, B. et al. Cholangiocarcinomas can originate from hepatocytes in mice. J. Clin. Invest. 122, 2911–2915 (2012).
Hu, S. et al. NOTCH-YAP1/TEAD-DNMT1 axis drives hepatocyte reprogramming into intrahepatic cholangiocarcinoma. Gastroenterology 163, 449–465 (2022).
Shang, R. et al. Cabozantinib-based combination therapy for the treatment of hepatocellular carcinoma. Gut 70, 1746–1757 (2021).
Adebayo Michael, A. O. et al. Inhibiting glutamine-dependent mTORC1 activation ameliorates liver cancers driven by β-catenin mutations. Cell Metab. 29, 1135–1150.e6 (2019).
Tang, M. et al. Liver cancer heterogeneity modeled by in situ genome editing of hepatocytes. Sci. Adv. 8, eabn5683 (2022).
Yuen, V. W. et al. Using mouse liver cancer models based on somatic genome editing to predict immune checkpoint inhibitor responses. J. Hepatol. 78, 376–389 (2023).
Liu, Y. T. et al. A novel spontaneous hepatocellular carcinoma mouse model for studying T-cell exhaustion in the tumor microenvironment. J. Immunother. Cancer 6, 144 (2018).
DuPage, M. et al. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell 19, 72–85 (2011).
Ochoa, M. C. et al. Synergistic effects of combined immunotherapy strategies in a model of multifocal hepatocellular carcinoma. Cell Rep. Med. 4, 101009 (2023).
Hsu, C. L. & Loomba, R. From NAFLD to MASLD: implications of the new nomenclature for preclinical and clinical research. Nat. Metab. 6, 600–602 (2024).
Febbraio, M. A. et al. Preclinical models for studying NASH-driven HCC: how useful are they? Cell Metab. 29, 18–26 (2019).
Asgharpour, A. et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 65, 579–588 (2016).
Tsuchida, T. et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 69, 385–395 (2018).
Dow, M. et al. Integrative genomic analysis of mouse and human hepatocellular carcinoma. Proc. Natl Acad. Sci. USA 115, E9879–E9888 (2018).
Pinyol, R. et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J. Hepatol. 75, 865–878 (2021).
Dudek, M. et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature 592, 444–449 (2021).
Shalapour, S. et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017).
Wong, A. M. et al. Unique molecular characteristics of NAFLD-associated liver cancer accentuate β-catenin/TNFRSF19-mediated immune evasion. J. Hepatol. 77, 410–423 (2022).
Leslie, J. et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut 71, 2093–2106 (2022).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Ma, W. et al. Gut microbiota shapes the efficiency of cancer therapy. Front. Microbiol. 10, 1050 (2019).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Nguyen, P. H. D. et al. Intratumoural immune heterogeneity as a hallmark of tumour evolution and progression in hepatocellular carcinoma. Nat. Commun. 12, 227 (2021).
Losic, B. et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat. Commun. 11, 291 (2020).
Moeini, A. et al. Mixed hepatocellular cholangiocarcinoma tumors: cholangiolocellular carcinoma is a distinct molecular entity. J. Hepatol. 66, 952–961 (2017).
Ma, L. et al. Single-cell atlas of tumor cell evolution in response to therapy in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Hepatol. 75, 1397–1408 (2021).
Ma, L. et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell 36, 418–430.e6 (2019).
Ho, D. W. et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat. Commun. 12, 3684 (2021).
Zhou, P. Y. et al. Single-cell and spatial architecture of primary liver cancer. Commun. Biol. 6, 1181 (2023).
Zhang, W. et al. The zinc finger protein Miz1 suppresses liver tumorigenesis by restricting hepatocyte-driven macrophage activation and inflammation. Immunity 54, 1168–1185.e8 (2021).
Wang, L. et al. Inhibition of Arid1a increases stem/progenitor cell-like properties of liver cancer. Cancer Lett. 546, 215869 (2022).
Chen, W. S. et al. Single-cell transcriptomics reveals opposing roles of Shp2 in Myc-driven liver tumor cells and microenvironment. Cell Rep. 37, 109974 (2021).
Zhou, L. et al. Lineage tracing and single-cell analysis reveal proliferative Prom1+ tumour-propagating cells and their dynamic cellular transition during liver cancer progression. Gut 71, 1656–1668 (2022).
Craig, A. J., von Felden, J., Garcia-Lezana, T., Sarcognato, S. & Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 17, 139–152 (2020).
Trefts, E., Gannon, M. & Wasserman, D. H. The liver. Curr. Biol. 27, R1147–R1151 (2017).
Verma, B. & Wesa, A. Establishment of humanized mice from peripheral blood mononuclear cells or cord blood CD34+ hematopoietic stem cells for immune-oncology studies evaluating new therapeutic agents. Curr. Protoc. Pharmacol. 89, e77 (2020).
De La Rochere, P. et al. Humanized mice for the study of immuno-oncology. Trends Immunol. 39, 748–763 (2018).
Rongvaux, A. et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 32, 364–372 (2014).
Yen, C. J. et al. Hepatitis B virus X protein (HBx) enhances centrosomal P4.1-associated protein (CPAP) expression to promote hepatocarcinogenesis. J. Biomed. Sci. 26, 44 (2019).
Chisari, F. V. et al. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc. Natl Acad. Sci. USA 84, 6909–6913 (1987).
Chisari, F. V. et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 59, 1145–1156 (1989).
Kim, C. M., Koike, K., Saito, I., Miyamura, T. & Jay, G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature 351, 317–320 (1991).
Koike, K. et al. High-level expression of hepatitis B virus HBx gene and hepatocarcinogenesis in transgenic mice. Hepatology 19, 810–819 (1994).
Yu, D. Y. et al. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J. Hepatol. 31, 123–132 (1999).
Zhu, H., Wang, Y., Chen, J., Cheng, G. & Xue, J. Transgenic mice expressing hepatitis B virus X protein are more susceptible to carcinogen induced hepatocarcinogenesis. Exp. Mol. Pathol. 76, 44–50 (2004).
Moriya, K. et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat. Med. 4, 1065–1067 (1998).
Inokuchi, S. et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc. Natl Acad. Sci. USA 107, 844–849 (2010).
Tsai, W. C. et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J. Clin. Invest. 122, 2884–2897 (2012).
Smit, J. J. et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75, 451–462 (1993).
Acknowledgements
S.P.M. and A.L. were supported by NIH grant RO1CA251155 awarded to the University of Pittsburgh and Icahn School of Medicine at Mount Sinai.
Author information
Authors and Affiliations
Contributions
A.L. researched the data for the article, made a substantial contribution to the discussion of content, wrote the article, and reviewed and/or edited the manuscript before submission. M.B.-V. researched the data for the article. S.P.M. made a substantial contribution to the discussion of content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Xin Chen; Markus Heim, who co-reviewed with Sandro Nuciforo; Divya Kumar; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barcena-Varela, M., Monga, S.P. & Lujambio, A. Precision models in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 22, 191–205 (2025). https://doi.org/10.1038/s41575-024-01024-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-024-01024-w
This article is cited by
-
Prognostic model for unresectable hepatocellular carcinoma treated with transarterial therapy plus TKIs and anti-PD-1 antibodies
World Journal of Surgical Oncology (2025)
-
WNT–β-catenin signalling in hepatocellular carcinoma: from bench to clinical trials
Nature Reviews Gastroenterology & Hepatology (2025)
-
Increased expression of DNAJC7 promotes the progression of hepatocellular carcinoma by influencing the cell cycle and immune microenvironment
Journal of Cancer Research and Clinical Oncology (2025)
-
Computational discovery and validation of 4′-O-methylochnaflavone as a novel HSP90AB1 inhibitor for hepatocellular carcinoma treatment
Molecular Diversity (2025)
-
EIF4A3-Mediated downregulation of circPTEN promotes hepatocellular carcinoma progression through the miR-1289/RBM38 Axis
Journal of Molecular Histology (2025)