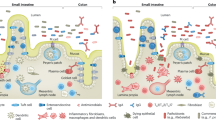
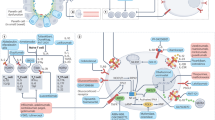
Abstract
The incidence of inflammatory bowel disease (IBD) has risen in the past decades and has emerged as a global health issue. IBD is characterized by chronic inflammation of the gastrointestinal tract. There is compelling evidence for the role of biological sex in IBD epidemiology, pathophysiology, disease progression, symptoms and extra-intestinal diseases. IBD disease course, management and therapies differ between men and women, yet there is a paucity of analysis of sex as a factor. This Review discusses the known influence of sex-linked genetic factors, hormones and hormone receptors in IBD incidence, prevalence, disease burden and clinical manifestation. Furthermore, we review the mechanisms underlying these sex-dependent effects on the dysregulation of gastrointestinal mucosal immunity (immune, epithelial barrier and microbiota) in IBD. To support the progressive inclusion of sex in the study of IBD, we summarize the current standard research methodology that should be implemented to investigate sex as a biological variable in IBD studies. Enhanced comprehension of the influence of sex in IBD pathophysiology will advance the development of targeted therapies and improve patient care.
Key points
-
Biological sex differentially affects inflammatory bowel disease (IBD) subtypes, ulcerative colitis, and Crohn’s disease epidemiology, disease course and symptoms across ages.
-
This article summarizes current knowledge of the effect of biological sex on IBD, highlights research gaps and recommends best practices in research methodology to integrate sex in IBD research.
-
To improve patient care, integration and comprehension of biological sex in IBD, personalized therapeutic strategies and the inclusion of a broader population in preclinical and clinical research are needed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
McGregor, A. J. et al. How to study the impact of sex and gender in medical research: a review of resources. Biol. Sex Differ. 7, 46 (2016).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Gahagan, J., Gray, K. & Whynacht, A. Sex and gender matter in health research: addressing health inequities in health research reporting. Int. J. Equity Health 14, 12–15 (2015).
Shah, S. C. et al. Sex-based differences in incidence of inflammatory bowel diseases — pooled analysis of population-based studies from Western countries. Gastroenterology 155, 1079–1089.e3 (2018).
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2017).
Mauvais-Jarvis, F. et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 396, 565–582 (2020).
Rustgi, S. D., Kayal, M. & Shah, S. C. Sex-based differences in inflammatory bowel diseases: a review. Therap. Adv. Gastroenterol. 13, 1756284820915043 (2020).
Sceats, L. A., Morris, A. M., Bundorf, M. K., Park, K. T. & Kin, C. Sex differences in treatment strategies among patients with ulcerative colitis: a retrospective cohort analysis of privately insured patients. Dis. Colon Rectum 62, 586–594 (2019).
Khan, S. et al. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the United States: a systematic review. J. Clin. Pharm. Ther. 44, 495–507 (2019).
Schiebinger, L. & Klinge, I. Gendered innovation in health and medicine. Z. Geschlecht Kult. Ges. 7, 29–50 (2015).
Hunt, L., Nielsen, M. W. & Schiebinger, L. A framework for sex, gender, and diversity analysis in research. Science 377, 1492–1495 (2022).
Day, S., Mason, R., Lagosky, S. & Rochon, P. A. Integrating and evaluating sex and gender in health research. Health Res. Policy Syst. 14, 75 (2016).
Park, J. & Cheon, J. H. Incidence and prevalence of inflammatory bowel disease across Asia. Yonsei Med. J. 62, 99–108 (2021).
Goodman, W. A., Erkkila, I. P. & Pizarro, T. T. Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 740–754 (2020).
Varatharaj, A. et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 7, 875–882 (2020).
Rich-Edwards, J. W., Kaiser, U. B., Chen, G. L., Manson, J. A. E. & Goldstein, J. M. Sex and gender differences research design for basic, clinical, and population studies: essentials for investigators. Endocr. Rev. 39, 424–439 (2018).
Hammarström, A. & Annandale, E. A conceptual muddle: an empirical analysis of the use of ‘sex’ and ‘gender’ in ‘gender-specific medicine’ journals. PLoS ONE 7, e34193 (2012).
Ainsworth, C. Sex redefined — the idea of two sexes is simplistic. Biologists now think there is a wider spectrum than that. Nature 518, 288–291 (2015).
Witchel Feldman, S. Disorders of sex development. Semin. Reprod. Med. 30, 337 (2012).
Jones, T. Intersex studies: a systematic review of international health literature. SAGE Open https://doi.org/10.1177/2158244017745577 (2018).
Sax, L. How common is intersex? A response to Anne Fausto-Sterling. J. Sex Res. 39, 174–178 (2002).
Tannenbaum, C., Greaves, L. & Graham, I. D. Why sex and gender matter in implementation research Economic, social, and ethical factors affecting the implementation of research. BMC Med. Res. Methodol. 16, 145 (2016).
Madla, C. M. et al. Let’s talk about sex: differences in drug therapy in males and females. Adv. Drug Deliv. Rev. 175, 113804 (2021).
Tannenbaum, C., Ellis, R. P., Eyssel, F., Zou, J. & Schiebinger, L. Sex and gender analysis improves science and engineering. Nature 575, 137–146 (2019).
Anyane-Yeboa, A., Quezada, S., Rubin, D. T. & Balzora, S. The impact of the social determinants of health on disparities in inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 20, 2427–2434 (2022).
World Health Organization. A conceptual framework for action on the social determinants of health (WHO, 2010).
Nguyen, N. H., Khera, R., Ohno-Machado, L., Sandborn, W. J. & Singh, S. Prevalence and effects of food insecurity and social support on financial toxicity in and healthcare use by patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 19, 1377–1386.e5 (2021).
Bernstein, C. N., Walld, R. & Marrie, R. A. Social determinants of outcomes in inflammatory bowel disease. Am. J. Gastroenterol. 115, 2036–2046 (2020).
Shah, R. et al. Medical and social determinants of health as predictors of adverse outcomes in patients with inflammatory bowel disease. Bayl. Univ. Med. Cent. Proc. 36, 165–170 (2023).
Vlassoff, C. Gender differences in determinants and consequences of health and illness. J. Health Popul. Nutr. 25, 47–61 (2007).
Greuter, T., Manser, C., Pittet, V., Vavricka, S. R. & Biedermann, L. Gender differences in inflammatory bowel disease. Digestion 101, 98–104 (2020).
Dale, L. et al. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 148, 1087–1106 (2015).
Varì, R., Scazzocchio, B. & Del Papa, S. Dietary habits and gender difference. J. Gend. Specif. Med. 3, 55–58 (2017).
Küçük, N. et al. Fruit and vegetable consumption across population segments: evidence from a national household survey. J. Heal. Popul. Nutr. 42, 54 (2023).
Fagerli, R. A. & Wandel, M. Gender differences in opinions and practices with regard to a ‘Healthy Diet’. Appetite 32, 171–190 (1999).
Li, K. K. et al. An examination of sex differences in relation to the eating habits and nutrient intakes of university students. J. Nutr. Educ. Behav. 44, 246–250 (2012).
Hou, J. K., Abraham, B. & El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am. J. Gastroenterol. 106, 563–573 (2011).
Lambert, K. et al. Systematic review with meta-analysis: dietary intake in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 54, 742–754 (2021).
Peters, V. et al. Habitual dietary intake of IBD patients differs from population controls: a case–control study. Eur. J. Nutr. 60, 345–356 (2021).
Godala, M., Gaszyńska, E., Walczak, K. & Małecka-Wojciesko, E. Habitual dietary intake and adherence to dietary guidelines of patients with inflammatory bowel diseases. Gastroenterol. Insights 15, 69–86 (2024).
Kamp, K., Pennings, B., Javelli, D., Wyatt, G. & Given, B. Dietary patterns, beliefs and behaviors among individuals with inflammatory bowel disease: a cross-sectional study. J. Hum. Nutr. Diet. 34, 257–264 (2022).
Pueschel, L. et al. Patients with inflammatory bowel disease show fewer sex-related differences in their dietary behavior than the general population: a qualitative analysis. Nutrients 16, 2954 (2024).
Zhou, X. L. et al. Protein intake and risk of inflammatory bowel disease: a meta-analysis. Asia Pac. J. Clin. Nutr. 31, 443–449 (2022).
Xu, F., Park, S., Liu, Y. & Greenlund, K. J. Dietary intake patterns among adults with inflammatory bowel disease in the United States, 2015. PLoS ONE 16, e0250441 (2021).
World Health Organization. Global status report on alcohol and health and treatment of substance use disorders (WHO, 2024).
Griswold, M. G. et al. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392, 1015–1035 (2018).
Goh, C. M. J. et al. Gender differences in alcohol use: a nationwide study in a multiethnic population. Int. J. Ment. Health Addict. 22, 1161–1175 (2024).
White, M. A. Gender differences in the epidemiology of alcohol use and related harms in the United States. Alcohol. Res. 40, 01 (2020).
Park, S. H. & Kim, D. J. Global and regional impacts of alcohol use on public health: emphasis on alcohol policies. Clin. Mol. Hepatol. 26, 652–661 (2020).
White, B. A., Ramos, G. P. & Kane, S. The impact of alcohol in inflammatory bowel diseases. Inflamm. Bowel Dis. 28, 466–473 (2022).
Ramos, G. P. & Kane, S. Alcohol use in patients with inflammatory bowel disease. Gastroenterol. Hepatol. 17, 221–225 (2021).
Dai, X., Gakidou, E. & Lopez, A. D. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob. Control. 31, 129–137 (2022).
Piovani, D. et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 157, 647–659.e4 (2019).
Persson, P. G., Ahlbom, A. & Hellers, G. Inflammatory bowel disease and tobacco smoke — a case-control study. Gut 31, 1377–1381 (1990).
Cosnes, J., Nion-Larmurier, I., Afchain, P., Beaugerie, L. & Gendre, J. P. Gender differences in the response of colitis to smoking. Clin. Gastroenterol. Hepatol. 2, 41–48 (2004).
Karczewski, J., Poniedzialek, B., Piotr, R. & Malgorzata, M. Different effect of smoking on genders in Crohn’s disease. Turk. J. Gastroenterol. 26, 133–139 (2015).
Higuchi, L. M. et al. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am. J. Gastroenterol. 107, 1399–1406 (2012).
Sutherland, L. R., Ramcharan, S., Bryant, H. & Fick, G. Effect of cigarette smoking on recurrence of Crohn’s disease. Gastroenterology 98, 1123–1128 (1990).
Tobin, M. V., Logan, R. F. A., Langman, M. J. S., Mcconnell, R. B. & Gilmore, I. T. Cigarette smoking and inflammatory bowel disease. Gastroenterology 93, 316–321 (1987).
Benoni, C. & Nilsson, Å. Smoking habits in patients with inflammatory bowel disease: a case-control study. Scand. J. Gastroenterol. 22, 1130–1136 (1987).
Lindberg, E., Tysk, C., Andersson, K. & Järnerot, G. Smoking and inflammatory bowel disease. A case control study. Gut 29, 352–357 (1988).
Piovani, D. et al. Ethnic differences in the smoking-related risk of inflammatory bowel disease: a systematic review and meta-analysis. J. Crohns Colitis 15, 1658–1678 (2021).
World Health Organization. WHO guidelines on physical activity and sedentary behaviour (WHO, 2020).
Guthold, R., Stevens, G. A., Riley, L. M. & Bull, F. C. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1.6 million participants. Lancet Child Adolesc. Health 4, 23–35 (2020).
Guthold, R., Stevens, G. A., Riley, L. M. & Bull, F. C. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 6, e1077–e1086 (2018).
Sonnenberg, A. Occupational distribution of inflammatory bowel disease among German employees. Gut 31, 1037–1040 (1990).
Bøggild, H., Tüchsen, F. & Ørhede, E. Occupation, employment status and chronic inflammatory bowel disease in Denmark. Int. J. Epidemiol. 25, 630–637 (1996).
Cucino, C. & Sonnenberg, A. Occupational mortality from inflammatory bowel disease in the United States 1991-1996. Am. J. Gastroenterol. 96, 1101–1105 (2001).
Lautenschlager, S. A. et al. Lifestyle factors associated with inflammatory bowel disease: data from the Swiss IBD cohort study. BMC Gastroenterol. 23, 71 (2023).
Hlavaty, T. et al. Smoking, breastfeeding, physical inactivity, contact with animals, and size of the family influence the risk of inflammatory bowel disease: a Slovak case–control study. United Eur. Gastroenterol. J. 1, 109–119 (2013).
Jones, P. D. et al. Exercise decreases risk of future active disease in inflammatory bowel disease patients in remission. Inflamm. Bowel Dis. 21, 1063–1071 (2015).
Ng, S. C. et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut 64, 1063–1071 (2015).
Khalili, H. et al. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses’ Health Study cohorts. BMJ 347, f6633 (2013).
Melinder, C. et al. Physical fitness in adolescence and subsequent inflammatory bowel disease risk. Clin. Transl. Gastroenterol. 6, e121–e128 (2015).
Wang, R., Li, Z., Liu, S. & Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 13, e065186 (2023).
Xu, L., Huang, G., Cong, Y., Yu, Y. & Li, Y. Sex-related differences in inflammatory bowel diseases: the potential role of sex hormones. Inflamm. Bowel Dis. 28, 1766–1775 (2022).
Zheng, H. B., de la Morena, M. T. & Suskind, D. L. The growing need to understand very early onset inflammatory bowel disease. Front. Immunol. 12, 675186 (2021).
Ouahed, J. et al. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflamm. Bowel Dis. 26, 820–842 (2020).
Heyman, M. B. et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J. Pediatr. 146, 35–40 (2005).
Bequet, E. et al. Incidence and phenotype at diagnosis of very-early-onset compared with later-onset paediatric inflammatory bowel disease: a population-based study [1988–2011]. J. Crohns Colitis 11, 519–526 (2017).
Ruemmele, F. M. et al. Characteristics of inflammatory bowel disease with onset during the first year of life. J. Pediatr. Gastroenterol. Nutr. 43, 603–609 (2006).
Benchimol, E. I. et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology 147, 803–813.e7 (2014).
Shah, S. C. et al. Sex-based differences in the incidence of inflammatory bowel diseases — pooled analysis of population-based studies from the Asia-Pacific region. Aliment. Pharmacol. Ther. 49, 904–911 (2019).
Mak, W. Y., Zhao, M., Ng, S. C. & Burisch, J. The epidemiology of inflammatory bowel disease: East meets West. J. Gastroenterol. Hepatol. 35, 380–389 (2020).
Sýkora, J. et al. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J. Gastroenterol. 24, 2741–2763 (2018).
Kuenzig, M. E. et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology 162, 1147–1159.e4 (2022).
Lee, H. A. et al. Characteristics of pediatric inflammatory bowel disease in Korea: comparison with EUROKIDS data. Gut Liver 9, 756–760 (2015).
Duricova, D., Burisch, J., Jess, T., Gower-Rousseau, C. & Lakatos, P. L. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J. Crohns Colitis 8, 1351–1361 (2014).
Kern, I. et al. Incidence trends of pediatric onset inflammatory bowel disease in the years 2000–2009 in Saxony, Germany–first results of the Saxon Pediatric IBD Registry. PLoS ONE 16, e0243774 (2021).
Wittig, R., Albers, L., Koletzko, S., Saam, J. & Von Kries, R. Pediatric chronic inflammatory bowel disease in a German statutory health INSURANCE-incidence rates from 2009 to 2012. J. Pediatr. Gastroenterol. Nutr. 68, 244–250 (2019).
Zurek, M. et al. Epidemiology and care structures for children and adolescents and young adults up to the 26th year of life with inflammatory bowel diseases (IBD) in Leipzig/Saxony/Germany. J. Public Health 26, 437–442 (2018).
Malaty, H. M., Fan, X., Opekun, A. R., Thibodeaux, C. & Ferry, G. D. Rising incidence of inflammatory bowel disease among children: a 12-year study. J. Pediatr. Gastroenterol. Nutr. 50, 27–31 (2010).
Hong, S. J. et al. Characteristics and incidence trends for pediatric inflammatory bowel disease in Daegu-Kyungpook province in Korea: a multi-center study. J. Korean Med. Sci. 33, e132 (2018).
Carroll, M. W. et al. The impact of inflammatory bowel disease in Canada 2018: children and adolescents with IBD. J. Can. Assoc. Gastroenterol. 2, S49–S67 (2019).
Van Limbergen, J. et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 135, 1114–1122 (2008).
Denise, H. et al. Gender differences in paediatric patients of the Swiss. Pediatr. Gastroenterol. Hepatol. Nutr. 17, 147–154 (2014).
Gupta, N. et al. Gender differences in presentation and course of disease in pediatric patients with Crohn disease. Pediatrics 120, e1418–e1425 (2007).
Park, S. H. et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong District of Seoul, Korea in 1986-2015. J. Crohns Colitis 13, 1410–1417 (2019).
Dotson, J. L., Bricker, J. B., Kappelman, M. D., Chisolm, D. & Crandall, W. V. Assessment of sex differences for treatment, procedures, complications, and associated conditions among adolescents hospitalized with Crohn’s disease. Inflamm. Bowel Dis. 21, 2619–2624 (2015).
Ferguson, A. & Sedgwick, D. M. Juvenile onset inflammatory bowel disease: height and body mass index in adult life. Br. Med. J. 308, 1259–1263 (1994).
Gupta, N., Lustig, R. H., Kohn, M. A. & Vittinghoff, E. Menarche in pediatric patients with Crohn’s disease. Dig. Dis. Sci. 57, 2975–2981 (2012).
DeBoer, M. D. et al. Increases in sex hormones during anti-tumor necrosis factor α therapy in adolescents with Crohn’s disease. J. Pediatr. 171, 146–152.e2 (2016).
Alatab, S. et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30 (2020).
Lungaro, L. et al. Impact of female gender in inflammatory bowel diseases: a narrative review. J. Pers. Med. 13, 165 (2023).
Brant, S. R. & Nguyen, G. C. Is there a gender difference in the prevalence of Crohn’s disease or ulcerative colitis? Inflamm. Bowel Dis. 14, 2–3 (2008).
Severs, M. et al. Sex-related differences in patients with inflammatory bowel disease: results of 2 prospective cohort studies. Inflamm. Bowel Dis. 24, 1298–1306 (2018).
King, D. et al. Changing patterns in the epidemiology and outcomes of inflammatory bowel disease in the United Kingdom: 2000-2018. Aliment. Pharmacol. Ther. 51, 922–934 (2020).
Tragnone, A. et al. Incidence of inflammatory bowel disease in Italy: a nationwide population-based study. Int. J. Epidemiol. 25, 1044–1052 (1996).
Wei, S. C. et al. A nationwide population-based study of the inflammatory bowel diseases between 1998 and 2008 in Taiwan. BMC Gastroenterol. 13, 166 (2013).
Wilson, J. et al. High incidence of inflammatory bowel disease in Australia: a prospective population-based Australian incidence study. Inflamm. Bowel Dis. 16, 1550–1556 (2010).
Leddin, D., Tamim, H. & Levy, A. R. Decreasing incidence of inflammatory bowel disease in Eastern Canada: a population database study. BMC Gastroenterol. 14, 140 (2014).
Ng, S. C. et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and colitis epidemiology study. Gastroenterology 145, 158–165.e2 (2013).
Gordon, H. et al. ECCO guidelines on extraintestinal manifestations in inflammatory bowel disease. J. Crohns Colitis 18, 1–37 (2024).
Bernstein, C. N., Blanchard, J. F., Rawsthorne, P. & Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am. J. Gastroenterol. 96, 1116–1122 (2001).
Vavricka, S. R. et al. Extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1982–1992 (2015).
Wagtmans, M. J., Verspaget, H. W., Lamers, C. B. H. W. & Van Hogezand, R. A. Gender-related differences in the clinical course of Crohn’s disease. Am. J. Gastroenterol. 96, 1541–1546 (2001).
Söderlund, S. et al. Inflammatory bowel disease confers a lower risk of colorectal cancer to females than to males. Gastroenterology 138, 1697–1703.e2 (2010).
Wu, H. et al. Inflammatory bowel disease and cardiovascular diseases. Eur. Heart J. Open 2, oeab029 (2021).
Kirchgesner, J. et al. Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut 67, 1261–1268 (2018).
Macaluso, F. S., Ventimiglia, M. & Orlando, A. Effectiveness and safety of vedolizumab in inflammatory bowel disease: a comprehensive meta-analysis of observational studies. J. Crohns Colitis 17, 1217–1227 (2023).
Lee, L. Y. W., Gardezi, A. S., Santharam, V., Boyd, J. & Lanzon-Miller, S. Effect of azathioprine intolerance on outcomes of inflammatory bowel disease: a cross-sectional study. Frontline Gastroenterol. 5, 40–43 (2014).
Fidder, H. et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut 58, 501–508 (2009).
Peyrin-Biroulet, L. et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin. Gastroenterol. Hepatol. 14, 348–354.e17 (2016).
Kishi, M. et al. A review on the current status and definitions of activity indices in inflammatory bowel disease: how to use indices for precise evaluation. J. Gastroenterol. 57, 246–266 (2022).
Shaoul, R. & Day, A. S. An overview of tools to score severity in pediatric inflammatory bowel disease. Front. Pediatr. 9, 615216 (2021).
Ishige, T. et al. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J. Gastroenterol. 45, 911–917 (2010).
Pigneur, B. et al. Natural history of Crohn’s disease: comparison between childhood- and adult-onset disease. Inflamm. Bowel Dis. 16, 953–961 (2010).
Aloi, M. et al. Presenting features and disease course of pediatric ulcerative colitis. J. Crohns Colitis 7, e509–e515 (2013).
Benchimol, E. I., To, T., Griffiths, A. M., Rabeneck, L. & Guttmann, A. Outcomes of pediatric inflammatory bowel disease: socioeconomic status disparity in a universal-access healthcare system. J. Pediatr. 158, 960–967.e4 (2011).
Gupta, N. et al. Risk factors for initial surgery in pediatric patients with Crohn’s disease. Gastroenterology 130, 1069–1077 (2006).
Gupta, N. et al. Presentation and disease course in early- compared to later-onset pediatric Crohn’s disease. Am. J. Gastroenterol. 103, 2092–2098 (2008).
Polito, J. M. et al. Crohn’s disease: influence of age at diagnosis on site and clinical type of disease. Gastroenterology 111, 580–586 (1996).
Sempere, L. et al. Gender biases and diagnostic delay in inflammatory bowel disease: multicenter observational study. Inflamm. Bowel Dis. 29, 1886–1894 (2023).
Blumenstein, I. et al. Female patients suffering from inflammatory bowel diseases are treated less frequently with immunosuppressive medication and have a higher disease activity. A subgroup analysis of a large multi-centre, prospective, internet-based study. J. Crohns Colitis 5, 203–210 (2011).
Allison, J., Herrinton, L. J., Liu, L., Yu, J. & Lowder, J. Natural history of severe ulcerative colitis in a community-based health plan. Clin. Gastroenterol. Hepatol. 6, 999–1003 (2008).
Coskun, M. Intestinal epithelium in inflammatory bowel disease. Front. Med. 1, 24 (2014).
Lefèvre, N. et al. The number of X chromosomes influences inflammatory cytokine production following Toll-like receptor stimulation. Front. Immunol. 10, 1052 (2019).
Uhlig, H. H. & Muise, A. M. Clinical genomics in inflammatory bowel disease. Trends Genet. 33, 629–641 (2017).
Nameirakpam, J., Rikhi, R., Rawat, S. S., Sharma, J. & Suri, D. Genetics on early onset inflammatory bowel disease: an update. Genes Dis. 7, 93–106 (2020).
Chandrakasan, S., Venkateswaran, S. & Kugathasan, S. Nonclassic inflammatory bowel disease in young infants: immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome, and other disorders. Pediatr. Clin. North Am. 64, 139–160 (2017).
Nambu, R. et al. A systematic review of monogenic inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 20, e653–e663 (2022).
Cui, J., Shen, Y., & Li, R. Estrogen synthesis and signaling pathways during ageing: from periphery to brain. Trends Mol. Med. 19, 197–209 (2012).
Bouguen, G., Dubuquoy, L., Desreumaux, P., Brunner, T. & Bertin, B. Intestinal steroidogenesis. Steroids 103, 64–71 (2015).
Geremia, A., Biancheri, P., Allan, P., Corazza, G. R. & Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 13, 3–10 (2014).
Cooke, P. S., Nanjappa, M. K., Ko, C., Prins, G. S. & Hess, R. A. Estrogens in male physiology. Physiol. Rev. 97, 995–1043 (2017).
Oettel, M. & Mukhopadhyay, A. K. Progesterone: the forgotten hormone in men? Aging Male 7, 236–257 (2004).
Bianchi, V. E. et al. The role of androgens in women’s health and wellbeing. Pharmacol. Res. 171, 105758 (2021).
Igarashi, M. et al. Female-dominant estrogen production in healthy children before adrenarche. Endocr. Connect. 10, 1221–1226 (2021).
Zec, I. et al. Reference intervals for reproductive hormones in prepubertal children on the automated Roche Cobas E 411 analyzer. Clin. Biochem. 45, 1206–1212 (2012).
Courant, F. et al. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. J. Clin. Endocrinol. Metab. 95, 82–92 (2010).
Leifke, E. et al. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin. Endocrinol. 53, 689–695 (2000).
Greenblatt, R. B., Oettinger, M. & Bohler, C. S. Estrogen‐androgen levels in aging men and women: therapeutic considerations. J. Am. Geriatr. Soc. 24, 173–178 (1976).
Erbay, G., Senol, G., Anar, C., Meral, A. R. & Tuzel, O. Relationship between tuberculosis and female hormone levels in post-menopausal women. Southeast Asian J. Trop. Med. Public Health 47, 78–83 (2016).
Mohamad, N. V. et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 22, 129–140 (2019).
Bini, E. I. et al. The implication of pro-inflammatory cytokines in the impaired production of gonadal androgens by patients with pulmonary tuberculosis. Tuberculosis 95, 701–706 (2015).
Demirtaş Şahin, T. et al. TNF-α antagonism with etanercept enhances penile NOS expression, cavernosal reactivity, and testosterone levels in aged rats. Can. J. Physiol. Pharmacol. 96, 200–207 (2018).
Lahat, A., Falach-Malik, A., Haj, O., Shatz, Z. & Ben-Horin, S. Change in bowel habits during menstruation: are IBD patients different? Therap. Adv. Gastroenterol. 13, 1756284820929806 (2020).
Lim, S. M. et al. The effect of the menstrual cycle on inflammatory bowel disease: a prospective study. Gut Liver 7, 51–57 (2013).
Saha, S. et al. Menstrual cycle changes in women with inflammatory bowel disease: a study from the Ocean State Crohn’s and Colitis Area Registry. Inflamm. Bowel Dis. 20, 534–540 (2014).
Rolston, V. S. et al. The influence of hormonal fluctuation on inflammatory bowel disease symptom severity — a cross-sectional cohort study. Inflamm. Bowel Dis. 24, 387–393 (2018).
Bharadwaj, S., Barber, M. D., Graff, L. A. & Shen, B. Symptomatology of irritable bowel syndrome and inflammatory bowel disease during the menstrual cycle. Gastroenterol. Rep. 3, 185–193 (2015).
Crittenden, S. et al. Prostaglandin E2 promotes intestinal inflammation via inhibiting microbiota-dependent regulatory T cells. Sci. Adv. 7, eabd7954 (2021).
Khalili, H. et al. Endogenous levels of circulating androgens and risk of Crohn’s disease and ulcerative colitis among women: a nested case-control study from the nurses’ health study cohorts. Inflamm. Bowel Dis. 21, 1378–1385 (2015).
Ortizo, R. et al. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: a meta-analysis of case-controlled and cohort studies. Eur. J. Gastroenterol. Hepatol. 29, 1064–1070 (2017).
Wang, X. et al. Use of oral contraceptives and risk of ulcerative colitis – a systematic review and meta-analysis. Pharmacol. Res. 139, 367–374 (2019).
Khalili, H. et al. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut 62, 1153–1159 (2013).
Pasvol, T. J., Bloom, S., Segal, A. W., Rait, G. & Horsfall, L. Use of contraceptives and risk of inflammatory bowel disease: a nested case–control study. Aliment. Pharmacol. Ther. 55, 318–326 (2022).
Khalili, H. et al. Hormone therapy increases risk of ulcerative colitis but not Crohn’s disease. Gastroenterology 143, 1199–1206 (2012).
Chiaffarino, F. et al. Endometriosis and inflammatory bowel disease: a systematic review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 252, 246–251 (2020).
Jess, T., Frisch, M., Jørgensen, K. T., Pedersen, B. V. & Nielsen, N. M. Increased risk of inflammatory bowel disease in women with endometriosis: a nationwide Danish cohort study. Gut 61, 1279–1283 (2012).
Kane, S. V. & Reddy, D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am. J. Gastroenterol. 103, 1193–1196 (2008).
Tremellen, K., McPhee, N. & Pearce, K. Metabolic endotoxaemia related inflammation is associated with hypogonadism in overweight men. Basic Clin. Androl. 27, 5 (2017).
Nasser, M. et al. Testosterone therapy in men with Crohn’s disease improves the clinical course of the disease: data from long-term observational registry study. Horm. Mol. Biol. Clin. Investig. 22, 111–117 (2015).
Klil-Drori, A. J. et al. Androgen deprivation therapy and the incidence of inflammatory bowel disease in patients with prostate cancer. Am. J. Epidemiol. 184, 15–22 (2016).
Zucker, K. J. Epidemiology of gender dysphoria and transgender identity. Sex. Health 14, 404–411 (2017).
Nolan, I. T., Kuhner, C. J. & Dy, G. W. Demographic and temporal trends in transgender identities and gender confirming surgery. Transl. Androl. Urol. 8, 184–190 (2019).
Statistics Canada. Canada is the first country to provide census data on transgender and non-binary people. StatCan https://www150.statcan.gc.ca/n1/daily-quotidien/220427/dq220427b-eng.htm (2022).
Abramovich, A. et al. Assessment of health conditions and health service use among transgender patients in Canada. JAMA Netw. Open 3, e2015036 (2020).
Hassan, B. et al. The impact of hormone therapy on inflammatory bowel disease in transgender and nonbinary individuals. Inflamm. Bowel Dis. https://doi.org/10.1093/ibd/izae236 (2024).
Habbema, J. D. F. et al. Towards less confusing terminology in reproductive medicine: a proposal. Hum. Reprod. 19, 1497–1501 (2004).
Zegers-Hochschild, F. et al. The international glossary on infertility and fertility care, 2017. Hum. Reprod. 32, 1786–1801 (2017).
Tavernier, N., Fumery, M., Peyrin-Biroulet, L., Colombel, J. F. & Gower-Rousseau, C. Systematic review: fertility in non-surgically treated inflammatory bowel disease. Aliment. Pharmacol. Ther. 38, 847–853 (2013).
Druvefors, E., Landerholm, K., Hammar, U., Myrelid, P. & Andersson, R. E. Impaired fertility in women with inflammatory bowel disease: a national cohort study from Sweden. J. Crohns Colitis 15, 383–390 (2021).
Bonthala, N. & Kane, S. Updates on women’s health issues in patients with inflammatory bowel disease. Curr. Treat. Options Gastroenterol. 16, 86–100 (2018).
Baird, D. D., Narendranathan, M. & Sandler, R. S. Increased risk of preterm birth for women with inflammatory bowel disease. Gastroenterology 99, 987–994 (1990).
Laube, R. et al. Knowledge and attitudes towards pregnancy in females with inflammatory bowel disease: an international, multi-centre study. J. Crohns Colitis 14, 1248–1255 (2020).
Winter, R. W., Boyd, T., Chan, W. W., Levy, A. N. & Friedman, S. Risk factors for voluntary childlessness in men and women with inflammatory bowel disease. Inflamm. Bowel Dis. 28, 1927–1931 (2022).
Marri, S. R., Ahn, C. & Buchman, A. L. Voluntary childlessness is increased in women with inflammatory bowel disease. Inflamm. Bowel Dis. 13, 591–599 (2007).
Mountifield, R., Bampton, P., Prosser, R., Muller, K. & Andrews, J. M. Fear and fertility in inflammatory bowel disease: a mismatch of perception and reality affects family planning decisions. Inflamm. Bowel Dis. 15, 720–725 (2009).
Frolkis, A. D. et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 145, 996–1006 (2013).
Lee, S. et al. Surgery for inflammatory bowel disease has unclear impact on female fertility: a Cochrane collaboration systematic review. J. Can. Assoc. Gastroenterol. 4, 115–124 (2021).
Waljee, A., Waljee, J., Morris, A. M. & Higgins, P. D. R. Threefold increased risk of infertility: a meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis. Gut 55, 1575–1580 (2006).
Cornish, J. A. et al. The effect of restorative proctocolectomy on sexual function, urinary function, fertility, pregnancy and delivery: a systematic review. Dis. Colon Rectum 50, 1128–1138 (2007).
Rajaratnam, S. G., Eglinton, T. W., Hider, P. & Fearnhead, N. S. Impact of ileal pouch-anal anastomosis on female fertility: meta-analysis and systematic review. Int. J. Colorectal Dis. 26, 1365–1374 (2011).
Gorgun, E. et al. Fertility is reduced after restorative proctocolectomy with ileal pouch anal anastomosis: a study of 300 patients. Surgery 136, 795–803 (2004).
Hudson, M. et al. Fertility and pregnancy in inflammatory bowel disease. Int. J. Gynecol. Obstet. 58, 229–237 (1997).
Carini, F. et al. Inflammatory bowel disease and infertility: analysis of literature and future perspectives. Acta Biomed. 92, 5–9 (2021).
Bharadwaj, S., Philpott, J. R., Barber, M. D., Graff, L. A. & Shen, B. Women’s health issues after ileal pouch surgery. Inflamm. Bowel Dis. 20, 2470–2482 (2014).
Druvefors, E., Andersson, R. E., Hammar, U., Landerholm, K. & Myrelid, P. Minor impact on fertility in men with inflammatory bowel disease: a National Cohort Study from Sweden. Aliment. Pharmacol. Ther. 56, 292–300 (2022).
Narendranathan, M., Sandler, R. S., Chirayath, S. M. & Savitz, D. A. Male infertility in inflammatory bowel disease. J. Clin. Gastroenterol. 11, 403–406 (1989).
Palomba, S. et al. Inflammatory bowel diseases and human reproduction: a comprehensive evidence-based review. World J. Gastroenterol. 20, 7123–7136 (2014).
Rossato, M. & Foresta, C. Antisperm antibodies in inflammatory bowel disease. Arch. Intern. Med. 164, 2283 (2004).
Dimitrova, D., Kalaydjiev, S., Mendizova, A., Piryova, E. & Nakov, L. Circulating antibodies to human spermatozoa in patients with ulcerative colitis. Fertil. Steril. 84, 1533–1535 (2005).
Cosentino, M. J., Chey, W. Y., Takihara, H. & Cockett, A. T. K. The effects of sulfasalazine on human male fertility potential and seminal prostaglandins. J. Urol. 132, 682–686 (1984).
Fukushima, T. et al. Effects of sulfasalazine on sperm acrosome reaction and gene expression in the male reproductive organs of rats. Toxicol. Sci. 85, 675–682 (2005).
Alonso, V. et al. Sulfasalazine induced oxidative stress: a possible mechanism of male infertility. Reprod. Toxicol. 27, 35–40 (2009).
Gubatan, J. et al. Paternal medications in inflammatory bowel disease and male fertility and reproductive outcomes: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 21, 2222–2238 (2023).
French, A. E., Koren, G. & Motherisk Team. Effect of methotrexate on male fertility. Can. Fam. Physician 49, 577–578 (2003).
Sussman, A. & Leonard, J. M. Psoriasis, methotrexate, and oligospermia. Arch. Dermatol. 116, 215–217 (1980).
Haberman, J. et al. Male fertility in cyclosporine-treated renal transplant patients. J. Urol. 145, 294–296 (1991).
Bouloux, P. M. G., Wass, J. A. H., Parslow, J. M., Hendry, W. F. & Besser, G. M. Effect of cyclosporin A in male autoimmune infertility. Fertil. Steril. 46, 81–85 (1986).
Siervo, G. E. M. et al. Low dose of cyclosporine A disrupts sperm parameters and testosterone levels reversibly in mice. Toxicol. Appl. Pharmacol. 460, 116374 (2023).
Park, Y. E. & Kim, T. O. Sexual dysfunction and fertility problems in men with inflammatory bowel disease. World J. Mens. Health 37, 285–297 (2019).
Peyrin-Biroulet, L. et al. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970–2004). Am. J. Gastroenterol. 107, 1693–1701 (2012).
Samuel, S. et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm. Bowel Dis. 19, 1858–1866 (2013).
Farouk, R. et al. Functional outcomes after ileal pouch-anal anastomosis for chronic ulcerative colitis. Ann. Surg. 231, 919–926 (2000).
Heuthorst, L. et al. Ileal pouch-anal anastomosis complications and pouch failure: a systematic review and meta-analysis. Ann. Surg. 23, 2062–2074 (2021).
Robinson, D. P. & Klein, S. L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 63, 263–271 (2012).
Druckmann, R. & Druckmann, M. A. Progesterone and the immunology of pregnancy. J. Steroid Biochem. Mol. Biol. 97, 389–396 (2005).
Jee, H. L., Benjamin, U., Jungyoon, C., Jeongho, P. & Chang, H. K. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J. Immunol. 187, 1778–1787 (2011).
Khan, D. & Ansar Ahmed, S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 6, 635 (2016).
Harding, A. T. & Heaton, N. S. The impact of estrogens and their receptors on immunity and inflammation during infection. Cancers 14, 909 (2022).
Draca, S. Estriol and progesterone: a new role for sex hormones. Int. J. Biomed. Sci. 2, 305–307 (2006).
Nekrasova, I. & Shirshev, S. Estriol in regulation of cell-mediated immune reactions in multiple sclerosis. J. Neuroimmunol. 349, 577421 (2020).
Van Der Giessen, J., Huang, V. W., Janneke Van Der Woude, C. & Fuhler, G. M. Modulatory effects of pregnancy on inflammatory bowel disease. Clin. Transl. Gastroenterol. 10, e00009 (2019).
Ding, N. et al. Management of biologics in pregnant, lactating patients with inflammatory bowel disease and the impact on neonatal vaccination: a systematic review of clinical practice guidelines and consensus statements. J. Clin. Pharm. Ther. 47, 1952–1965 (2022).
Wierman, M. E. Sex steroid effects at target tissues: mechanisms of action. Adv. Physiol. Educ. 31, 26–33 (2007).
Laurentino, S. S. et al. Identification of androgen receptor variants in testis from humans and other vertebrates. Andrologia 45, 187–194 (2013).
Kadel, S. & Kovats, S. Sex hormones regulate innate immune cells and promote sex differences in respiratory virus infection. Front. Immunol. 9, 1653 (2018).
Wilkenfeld, S. R., Lin, C. & Frigo, D. E. Events coordinate steroid hormone actions. Steroids 133, 2–7 (2019).
Asavasupreechar, T. et al. Systemic distribution of progesterone receptor subtypes in human tissues. J. Steroid Biochem. Mol. Biol. 199, 105599 (2020).
Rastelli, D. et al. Diminished androgen levels are linked to irritable bowel syndrome and cause bowel dysfunction in mice. J. Clin. Invest. 132, e150789 (2022).
Winborn, W. B., Sheridan, P. J. & McGill, H. C. Sex steroid receptors in the stomach, liver, pancreas, and gastrointestinal tract of the baboon. Gastroenterology 92, 23–32 (1987).
Kawano, N. et al. Identification and localization of estrogen receptor α- and β-positive cells in adult male and female mouse intestine at various estrogen levels. Histochem. Cell Biol. 121, 399–405 (2004).
Salih, M. A., Sims, S. H. & Kalu, D. N. Putative intestinal estrogen receptor: evidence for regional differences. Mol. Cell. Endocrinol. 121, 47–55 (1996).
Verdú, E. F., Deng, Y., Bercik, P. & Collins, S. M. Modulatory effects of estrogen in two murine models of experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G27–G36 (2002).
Campbell-Thompson, M., Lynch, I. J. & Bhardwaj, B. Expression of estrogen receptor (ER) subtypes and ERβ isoforms in colon cancer. Cancer Res. 61, 632–640 (2001).
Mähler, M. et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 274, 6–9 (1998).
Bábíčková, J. et al. Sex differences in experimentally induced colitis in mice: a role for estrogens. Inflammation 38, 1996–2006 (2015).
Armstrong, C. M., Allred, K. F., Weeks, B. R., Chapkin, R. S. & Allred, C. D. Estradiol has differential effects on acute colonic inflammation in the presence and absence of estrogen receptor β expression. Dig. Dis. Sci. 62, 1977–1984 (2017).
Song, C. H. et al. Effects of 17β-estradiol on colonic permeability and inflammation in an azoxymethane/dextran sulfate sodium-induced colitis mouse model. Gut Liver 12, 682–693 (2018).
Jacenik, D. et al. Sex- and age-related estrogen signaling alteration in inflammatory bowel diseases: modulatory role of estrogen receptors. Int. J. Mol. Sci. 20, 3175 (2019).
Goodman, W. A. et al. Loss of estrogen-mediated immunoprotection underlies female gender bias in experimental Crohn’s-like ileitis. Mucosal Immunol. 7, 1255–1265 (2014).
Goodman, W. A. et al. Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine. Proc. Natl. Acad. Sci. USA 117, 17166–17176 (2020).
van Langen, M. L. et al. Estrogen receptor-β signaling modulates epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 300, 621–626 (2011).
Goodman, W. A. et al. Estrogen receptor α loss-of-function protects female mice from DSS-induced experimental colitis. Cell. Mol. Gastroenterol. Hepatol. 5, 630–633.e1 (2018).
Hases, L. et al. Intestinal estrogen receptor beta suppresses colon inflammation and tumorigenesis in both sexes. Cancer Lett. 492, 54–62 (2020).
Fan, W. et al. Estrogen receptor β activation inhibits colitis by promoting NLRP6-mediated autophagy. Cell Rep. 41, 111454 (2022).
Jacenik, D. et al. G protein-coupled estrogen receptor mediates anti-inflammatory action in Crohn’s disease. Sci. Rep. 9, 6749 (2019).
Cook, L. C. et al. The role of estrogen signaling in a mouse model of inflammatory bowel disease: a Helicobacter hepaticus model. PLoS ONE 9, e94209 (2014).
Karatepe, O. et al. The effect of progesterone in the prevention of the chemically induced experimental colitis in rats TT - Efeito da progesterona na prevenção de colite experimental induzida quimicamente em ratos. Acta Cir. Bras. 27, 23–29 (2012).
Hou, Q., Huang, J., Ayansola, H., Masatoshi, H. & Zhang, B. Intestinal stem cells and immune cell relationships: potential therapeutic targets for inflammatory bowel diseases. Front. Immunol. 11, 623691 (2021).
Lee, S. H. et al. Anti-microbial antibody response is associated with future onset of Crohn’s disease independent of biomarkers of altered gut barrier function, subclinical inflammation, and genetic risk. Gastroenterology 161, 1540–1551 (2021).
Sciarra, F., Campolo, F., Franceschini, E., Carlomagno, F. & Venneri, M. A. Gender-specific impact of sex hormones on the immune system. Int. J. Mol. Sci. 24, 6302 (2023).
Ahluwalia, B., Moraes, L., Magnusson, M. K. & Öhman, L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand. J. Gastroenterol. 53, 379–389 (2018).
Michele, F. H., Vladimir, B. B. & Sabra, L. K. Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav. Immun. 22, 503–516 (2008).
Sugimoto, K. Role of STAT3 in inflammatory bowel disease. World J. Gastroenterol. 14, 5110–5114 (2008).
Hedl, M., Proctor, D. D. & Abraham, C. JAK2 disease-risk variants are gain of function and JAK signaling threshold determines innate receptor-induced proinflammatory cytokine secretion in macrophages. J. Immunol. 197, 3695–3704 (2016).
Marriott, I., Bost, K. L. & Huet-Hudson, Y. M. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J. Reprod. Immunol. 71, 12–27 (2006).
Rettew, J. A., Huet-Hudson, Y. M. & Marriott, I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol. Reprod. 78, 432–437 (2008).
Powrie, F. et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1, 553–562 (1994).
Mohammad, I. et al. Estrogen receptor contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci. Signal. 11, eaap9415 (2018).
Dosiou, C. et al. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J. Endocrinol. 196, 67–77 (2008).
Pierdominici, M. et al. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget 6, 40443–40451 (2015).
Guo, D. et al. Estrogen receptor β activation ameliorates DSS-induced chronic colitis by inhibiting inflammation and promoting Treg differentiation. Int. Immunopharmacol. 77, 105971 (2019).
Eichele, D. D. & Kharbanda, K. K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 23, 6016–6029 (2017).
Yu, X. et al. Androgen maintains intestinal homeostasis by inhibiting BMP signaling via intestinal stromal cells. Stem Cell Rep. 15, 912–925 (2020).
Homma, H. et al. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am. J. Physiol. Gastrointest. Liver Physiol. 288, 466–472 (2005).
Wada-Hiraike, O. et al. Role of estrogen receptor β in colonic epithelium. Proc. Natl. Acad. Sci. USA 103, 2959–2964 (2006).
Braniste, V. et al. Oestradiol decreases colonic permeability through oestrogen receptor β-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J. Physiol. 587, 3317–3328 (2009).
van der Giessen, J., van der Woude, C., Peppelenbosch, M. & Fuhler, G. A direct effect of sex hormones on epithelial barrier function in inflammatory bowel disease models. Cells 8, 261 (2019).
Harris, H. A. et al. Evaluation of an estrogen receptor-β agonist in animal models of human disease. Endocrinology 144, 4241–4249 (2003).
Thursby, E. & Juge, N. Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836 (2017).
Natividad, J. M. M. & Verdu, E. F. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol. Res. 69, 42–51 (2013).
Amy, M. S., Alyssa, K. W. & Tiffany, L. W. Cancer-promoting effects of microbial dysbiosis. Curr. Oncol. Rep. 16, 406 (2014).
Santana, P. T., Rosas, S. L. B., Ribeiro, B. E., Marinho, Y. & de Souza, H. S. P. Dysbiosis in inflammatory bowel disease: pathogenic role and potential therapeutic targets. Int. J. Mol. Sci. 23, 1–25 (2022).
Leibovitzh, H. et al. Altered gut microbiome composition and function are associated with gut barrier dysfunction in healthy relatives of patients with Crohn’s disease. Gastroenterology 163, 1364–1376.e10 (2022).
Raygoza Garay, J. A. et al. Gut microbiome composition is associated with future onset of Crohn’s disease in healthy first-degree relatives. Gastroenterology 165, 670–681 (2023).
Forbes, J. D., Van Domselaar, G. & Bernstein, C. N. Microbiome survey of the inflamed and noninflamed gut at different compartments within the gastrointestinal tract of inflammatory bowel disease patients. Inflamm. Bowel Dis. 22, 817–825 (2016).
Nishino, K. et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 53, 95–106 (2018).
Lawal, S. A., Voisin, A., Olof, H., Bording-Jorgensen, M. & Armstrong, H. Diversity of the microbiota communities found in the various regions of the intestinal tract in healthy individuals and inflammatory bowel diseases. Front. Immunol. 14, 1242242 (2023).
Org, E. et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7, 313–322 (2016).
Mueller, S. et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 72, 1027–1033 (2006).
He, S. et al. The gut microbiome and sex hormone-related diseases. Front. Microbiol. 12, 711137 (2021).
Markle, J. G. M. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013).
Colldén, H. et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab. 317, E1182–E1192 (2019).
Haro, C. et al. Intestinal microbiota is influenced by gender and body mass index. PLoS ONE 11, e0154090 (2016).
Zhang, X. et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat. Aging 1, 87–100 (2021).
Yurkovetskiy, L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412 (2013).
Yang, Y. et al. Estrogen inhibits the overgrowth of Escherichia coli in the rat intestine under simulated microgravity. Mol. Med. Rep. 17, 2313–2320 (2018).
Koren, O. et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480 (2012).
Nuriel-Ohayon, M. et al. Progesterone increases bifidobacterium relative abundance during late pregnancy. Cell Rep. 27, 730–736.e3 (2019).
Arruvito, L., Sanz, M., Banham, A. H. & Fainboim, L. Expansion of CD4+CD25+ and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J. Immunol. 178, 2572–2578 (2007).
Lee, G. S. et al. Estrogen enhances female small intestine epithelial organoid regeneration. J. BioX Res. 2, 9–15 (2019).
Miller, L. R. et al. Considering sex as a biological variable in preclinical research. FASEB J. 31, 29–34 (2017).
Garcia-Sifuentes, Y. & Maney, D. L. Reporting and misreporting of sex differences in the biological sciences. eLife 10, e70817 (2021).
Nieuwenhuis, S., Forstmann, B. U. & Wagenmakers, E. J. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat. Neurosci. 14, 1105–1107 (2011).
Buch, T. et al. Benefits of a factorial design focusing on inclusion of female and male animals in one experiment. J. Mol. Med. 97, 871–877 (2019).
Hines, M. Sex-related variation in human behavior and the brain. Trends Cogn. Sci. 14, 448–456 (2010).
Breehl, L. & Caban, O. Physiology, Puberty (StatPearls, 2023).
Morton, A. & Teasdale, S. Physiological changes in pregnancy and their influence on the endocrine investigation. Clin. Endocrinol. 96, 3–11 (2022).
Jiang, L. Q. et al. Hormonal and reproductive factors in relation to the risk of rheumatoid arthritis in women: a prospective cohort study with 223 526 participants. RMD Open 10, e003338 (2024).
Arnold, A. P. & Chen, X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 30, 1–9 (2009).
Joshi, A., Soni, A. & Acharya, S. In vitro models and ex vivo systems used in inflammatory bowel disease. Vitr. Model. 1, 213–227 (2022).
Swanson, K. D., Theodorou, E. & Kokkotou, E. Reproducing the human mucosal environment ex vivo: inflammatory bowel disease as a paradigm. Curr. Opin. Gastroenterol. 34, 384–391 (2018).
Bailoo, J. D., Reichlin, T. S. & Würbel, H. Refinement of experimental design and conduct in laboratory animal research. ILAR J. 55, 383–391 (2014).
Barnett, A. G. & Glasziou, P. Target and actual sample sizes for studies from two trial registries from 1999 to 2020: an observational study. BMJ Open 11, 1–13 (2021).
Patel, M. X., Doku, V. & Tennakoon, L. Challenges in recruitment of research participants. Adv. Psychiatr. Treat. 9, 229–238 (2003).
Beltz, A. M., Beery, A. K. & Becker, J. B. Analysis of sex differences in pre-clinical and clinical data sets. Neuropsychopharmacology 44, 2155–2158 (2019).
Diester, C. M., Banks, M. L., Neigh, G. N. & Negus, S. S. Experimental design and analysis for consideration of sex as a biological variable. Neuropsychopharmacology 44, 2159–2162 (2019).
DeCasien, A. R., Guma, E., Liu, S. & Raznahan, A. Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biol. Sex. Differ. 13, 43 (2022).
Usselman, C. W. et al. Guidelines on the use of sex and gender in cardiovascular research. Am. J. Physiol. Heart Circ. Physiol. 326, H238–H255 (2024).
Maté-Jimenez, J., Muñoz, S., Vicent, D. & Pajares, J. M. Incidence and prevalence of ulcerative colitis and Crohn’s disease in urban and rural areas of Spain from 1981 to 1988. J. Clin. Gastroenterol. 18, 27–31 (1994).
Hauser, G., Tkalcić, M., Stimac, D., Milić, S. & Sincić, B. M. Gender related differences in quality of life and affective status in patients with inflammatory bowel disease. Coll. Antropol. 35, 203–207 (2011).
Zelinkova, Z. et al. Sex-dimorphic adverse drug reactions to immune suppressive agents in inflammatory bowel disease. World J. Gastroenterol. 18, 6967–6973 (2012).
Heath, E. M., Kim, R. B. & Wilson, A. A comparative analysis of drug therapy, disease phenotype, and health care outcomes for men and women with inflammatory bowel disease. Dig. Dis. Sci. 67, 4287–4294 (2022).
McCarthy, L. et al. Sex and gender-based analysis in pharmacy practice research: a scoping review. Res. Soc. Adm. Pharm. 13, 1045–1054 (2017).
Colineaux, H., Soulier, A., Lepage, B. & Kelly-Irving, M. Considering sex and gender in Epidemiology: a challenge beyond terminology. From conceptual analysis to methodological strategies. Biol. Sex. Differ. 13, 23 (2022).
Geerling, B. J., Van Marken Lichtenbelt, W. D., Stockbrügger, R. W. & Brummer, R. J. M. Gender specific alterations of body composition in patients with inflammatory bowel disease compared with controls. Eur. J. Clin. Nutr. 53, 479–485 (1999).
Bommena, S., Goldberg, A., Amini, M. & Alishahi, Y. Depression in women with inflammatory bowel disease: a multifaceted approach for a multidimensional problem. Inflamm. Bowel Dis. 29, 1957–1970 (2023).
Tso, V. K. et al. Metabolomic profiles are gender, disease and time specific in the interleukin-10 gene-deficient mouse model of inflammatory bowel disease. PLoS ONE 8, e67654 (2013).
Metge, C. J., Blanchard, J. F., Peterson, S. & Bernstein, C. N. Use of pharmaceuticals by inflammatory bowel disease patients: a population-based study. Am. J. Gastroenterol. 96, 3348–3355 (2001).
Fägerstam, J. P. & Whiss, P. A. Higher platelet P-selectin in male patients with inflammatory bowel disease compared to healthy males. World J. Gastroenterol. 12, 1270–1272 (2006).
Agrawal, M. et al. Gender-based differences in response to tumor necrosis factor inhibitor therapies for ulcerative colitis: individual participant data meta-analyses of clinical trials. Inflamm. Bowel Dis. 29, 1–8 (2023).
Maunder, R., Toner, B., De Rooy, E. & Moskovitz, D. Influence of sex and disease on illness-related concerns in inflammatory bowel disease. Can. J. Gastroenterol. 13, 728–732 (1999).
Son, H. J. et al. Sex-related alterations of gut microbiota in the C57BL/6 mouse model of inflammatory bowel disease. J. Cancer Prev. 24, 173–182 (2019).
d’Aldebert, E. et al. Characterization of human colon organoids from inflammatory bowel disease patients. Front. Cell Dev. Biol. 8, 363 (2020).
Armstrong, H. K. et al. Unfermented β-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology 164, 228–240 (2023).
Bedke, T. et al. Protective function of sclerosing cholangitis on IBD. Gut 73, 1292–1301 (2024).
Zhang, Z. et al. Sex-specific differences in the gut microbiome in response to dietary fiber supplementation in IL-10-deficient mice. Nutrients 12, 2088 (2020).
Brand, E. C. et al. Healthy cotwins share gut microbiome signatures with their inflammatory bowel disease twins and unrelated patients. Gastroenterology 160, 1970–1985 (2021).
Zou, F. et al. Associations between sex hormones, receptors, binding proteins and inflammatory bowel disease: a Mendelian randomization study. Front. Endocrinol. 15, 1272746 (2024).
Hoffmann, J. P., Liu, J. A., Seddu, K. & Klein, S. L. Sex hormone signaling and regulation of immune function. Immunity 56, 2472–2491 (2023).
Homo-Delarche, F. et al. Sex steroids, glucocorticoids, stress and autoimmunity. J. Steroid Biochem. Molec. Biol. 40, 619–637 (1991).
Chuffa, L. G., Lupi-Júnior, L. A., Costa, A. B., Amorim, J. P. & Seiva, F. R. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids 118, 93–108 (2017).
Pardridge, M. W. Serum bioavailability of sex steroid hormones. Clin. Endocrinol. Metab. 15, 259–278 (1986).
Gao, X., Loggie, B. W. & Nawaz, Z. The roles of sex steroid receptor coregulators in cancer. Mol. Cancer 1, 7 (2002).
Thakur, M. K. & Paramanik, V. Role of steroid hormone coregulators in health and disease. Horm. Res. 71, 194–200 (2009).
Blumenstein, I. & Sonnenberg, E. Sex- and gender-related differences in inflammatory bowel diseases. Front. Gastroenterol. 2, 1199687 (2023).
Golder, S., O’Connor, K., Wang, Y., Stevens, R. & Gonzalez-Hernandez, G. Best practices on big data analytics to address sex-specific biases in our understanding of the etiology, diagnosis, and prognosis of diseases. Annu. Rev. Biomed. Data Sci. 5, 251–267 (2022).
Cremin, C. J., Dash, S. & Huang, X. Big data: historic advances and emerging trends in biomedical research. Curr. Res. Biotechnol. 4, 138–151 (2022).
Dash, S., Shakyawar, S. K., Sharma, M. & Kaushik, S. Big data in healthcare: management, analysis and future prospects. J. Big Data 6, 54 (2019).
Acknowledgements
D.M.T. is supported by the Children’s Hospital Research Institute of Manitoba/Research Manitoba PhD Studentship Award and the Mindel & Tom Olenick Research Studentships in Medicine. C.N.B. is supported in part by the Bingham Chair in Gastroenterology. J.-E.G. is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). H.K.A. is supported by the University of Manitoba and Canada Research Chair. S.M. is supported by the Canadian Institutes of Health Research and NSERC.
Author information
Authors and Affiliations
Contributions
D.M.T. and H.K.A. researched data for the article. C.N.B., D.M.T., J.-E.G. and H.K.A. contributed substantially to discussion of the content. D.M.T., C.N.B., S.M. and H.K.A. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.N.B. has consulted for Abbvie Canada, Amgen Canada, Bristol Myers Squibb Canada, Eli Lilly, Janssen Canada, Pfizer Canada, Ferring Canada, Pendopharm Canada, Sandoz Canada and Takeda Canada. He has received unrestricted educational grants from Abbvie Canada, Boston Scientific, Fresenius-Kabi Canada, Bristol Myers Squibb Canada, Janssen Canada, Pfizer Canada, Organon Canada and Takeda Canada and research grants from Abbvie, Janssen, Bristol Myers Squibb, Pfizer, Sandoz and Takeda. He has been on speaker’s bureaus of Abbvie Canada, Eli Lilly, Fresenius-Kabi, Takeda Canada, Pfizer Canada and Janssen Canada. J.-E.G. has served as a member of the scientific medical advisory committee for Crohn’s and Colitis Canada and as a member and chair of the research advisory committee for Research Manitoba. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Gionata Fiorino, Alessandro Armuzzi, Giuseppe Privitera and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tshikudi, D.M., Bernstein, C.N., Mishra, S. et al. Influence of biological sex in inflammatory bowel diseases. Nat Rev Gastroenterol Hepatol 22, 415–437 (2025). https://doi.org/10.1038/s41575-025-01038-y
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41575-025-01038-y