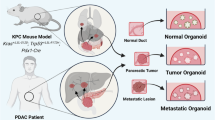
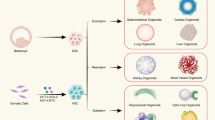
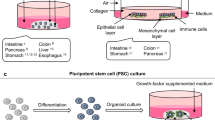
Abstract
Over the past decade, advances in organoid culturing methods have enabled the growth of three-dimensional cellular cultures in vitro with increasing fidelity with respect to the cellular composition, architecture and function of in vivo organs. The increased accessibility and ability to manipulate organoids as an in vitro system have led to a shift in the landscape of experimental biology. Whether derived from stem cells or tissue-resident cells, organoids are now routinely used in studies of development, homeostasis, regeneration and disease modelling, including viral infection and cancer. These applications of organoids are highly relevant for gastrointestinal tissues, including the liver and pancreas. In this Review, we explore the current and emerging advances in liver and pancreas organoid technologies for both discovery and clinical translation research and provide an outlook on the challenges ahead.
Key points
-
Modelling the liver and pancreas using organoid technology provides an accessible, often human-based system for research into fundamental questions regarding their embryonic development, function, disease modelling and clinical applications.
-
Liver and pancreas organoids are generated utilizing multiple sources as starting material, including pluripotent stem cells, embryonic, fetal and adult stem cells, and adult differentiated cells, each with specific advantages and disadvantages.
-
Current liver and pancreas organoid models have enabled a greater understanding of both acquired and inborn diseases that are not possible with in vivo models.
-
Advances in co-culture technologies are leading to the generation of organoids with multiple interacting cell populations found within the in vivo liver or pancreas enabling the production of higher fidelity models with more mature cell types.
-
Standardization of protocols, improvement of organoid architecture accuracy and transition towards chemically defined extracellular matrices will drive advances in the liver and pancreas organoid field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Lancaster, M. A. & Huch, M. Disease modelling in human organoids. Dis. Model. Mech. 12, dmm039347 (2019).
Marsee, A. et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 28, 816–832 (2021).
Treyer, A. & Müsch, A. Hepatocyte polarity. Compr. Physiol. 3, 243–287 (2013).
Gissen, P. & Arias, I. M. Structural and functional hepatocyte polarity and liver disease. J. Hepatol. 63, 1023–1037 (2015).
Campana, L., Esser, H., Huch, M. & Forbes, S. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat. Rev. Mol. Cell Biol. 22, 608–624 (2021).
Jamieson, J. D. & Palade, G. E. Synthesis, intracellular transport, and discharge of secretory proteins in stimulated pancreatic exocrine cells. J. Cell Biol. 50, 135–158 (1971).
Kopp, J. L. et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22, 737–750 (2012).
Logsdon, C. D. & Ji, B. The role of protein synthesis and digestive enzymes in acinar cell injury. Nat. Rev. Gastroenterol. Hepatol. 10, 362–370 (2013).
Reichert, M. & Rustgi, A. K. Pancreatic ductal cells in development, regeneration, and neoplasia. J. Clin. Invest. 121, 4572–4578 (2011).
Delous, M. et al. Sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS Genet. 8, e1002754 (2012).
Gu, G., Dubauskaite, J. & Melton, D. A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002).
Romer, A. I. & Sussel, L. Pancreatic islet cell development and regeneration. Curr. Opin. Endocrinol. Diabetes Obes. 22, 255–264 (2015).
Kisseleva, T. & Brenner, D. A. Hepatic stellate cells and the reversal of fibrosis. J. Gastroenterol. Hepatol. 21, S84–S87 (2006).
Fallowfield, J. A. et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 178, 5288–5295 (2007).
Wisse, E., De Zanger, R. B., Charels, K., Van Der Smissen, P. & McCuskey, R. S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology 5, 683–692 (1985).
Coelho, I., Duarte, N., Macedo, M. P. & Penha-Gonçalves, C. Insights into macrophage/monocyte-endothelial cell crosstalk in the liver: a role for Trem-2. J. Clin. Med. 10, 1248 (2021).
Braet, F. & Wisse, E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp. Hepatol. 1, 1 (2002).
Tuchweber, B., Desmoulière, A., Bochaton-Piallat, M. L., Rubbia-Brandt, L. & Gabbiani, G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab. Invest. 74, 265–278 (1996).
Beaussier, M. et al. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab. Invest. 87, 292–303 (2007).
Mass, E. et al. Specification of tissue-resident macrophages during organogenesis. Science 353, aaf4238 (2016).
Nairz, M., Theurl, I., Swirski, F. K. & Weiss, G. ‘Pumping iron’ – how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflug. Arch. 469, 397–418 (2017).
Hwang, R. F. et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68, 918–926 (2008).
Apte, M. V., Wilson, J. S., Lugea, A. & Pandol, S. J. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 144, 1210–1219 (2013).
Olsson, R. & Carlsson, P.-O. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int. J. Biochem. Cell Biol. 38, 710–714 (2006).
Kao, D.-I. et al. Endothelial cells control pancreatic cell fate at defined stages through EGFL7 signaling. Stem Cell Rep. 4, 181–189 (2015).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335–1347 (2023).
Devarbhavi, H. et al. Global Burden of Liver Disease: 2023 update. J. Hepatol. 79, 516–537 (2023).
Li, T. et al. Global and regional burden of pancreatitis: epidemiological trends, risk factors, and projections to 2050 from the Global Burden of Disease Study 2021. BMC Gastroenterol. 24, 398 (2024).
Li, X., Zhang, Y., Yan, Z., Jiang, W. & Rui, S. Global, regional and national burden of pancreatic cancer and its attributable risk factors from 2019 to 2021, with projection to 2044. Front. Oncol. 14, 1521788 (2024).
NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 404, 2077–2093 (2024).
Prior, N., Inacio, P. & Huch, M. Liver organoids: from basic research to therapeutic applications. Gut 68, 2228–2237 (2019).
Zhao, Z. et al. Organoids. Nat. Rev. Methods Primers 2, 94 (2022).
Afonso, M. B., Marques, V., van Mil, S. W. C. & Rodrigues, C. M. P. Human liver organoids: from generation to applications. Hepatology 79, 1432–1451 (2024).
Liu, S. et al. Liver organoids: updates on generation strategies and biomedical applications. Stem Cell Res. Ther. 15, 244 (2024).
Hu, Y. et al. Liver organoid culture methods. Cell Biosci. 13, 197 (2023).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Takebe, T. et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 21, 2661–2670 (2017).
Sekine, K. et al. Generation of human induced pluripotent stem cell-derived liver buds with chemically defined and animal origin-free media. Sci. Rep. 10, 17937 (2020).
Ogawa, S. et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat. Biotechnol. 33, 853 (2015).
Sampaziotis, F. et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat. Biotechnol. 33, 845 (2015).
Godoy, P. et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Archives of Toxicol. 87, 1315–1530 (2013).
Wang, S. et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 29, 1009–1026 (2019).
Saiki, N. et al. Self-organization of sinusoidal vessels in pluripotent stem cell-derived human liver bud organoids. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-025-01416-6 (2025).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Rezania, A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133 (2014).
Russ, H. A. et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 34, 1759–1772 (2015).
Gonçalves, C. A. et al. A 3D system to model human pancreas development and its reference single-cell transcriptome atlas identify signaling pathways required for progenitor expansion. Nat. Commun. 12, 3144 (2021).
Huang, L. et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med. 21, 1364–1371 (2015).
Breunig, M. et al. Modeling plasticity and dysplasia of pancreatic ductal organoids derived from human pluripotent stem cells. Cell Stem Cell 28, 1105–1124.e19 (2021).
Wiedenmann, S. et al. Single-cell-resolved differentiation of human induced pluripotent stem cells into pancreatic duct-like organoids on a microwell chip. Nat. Biomed. Eng. 5, 897–913 (2021).
Huang, L. et al. Commitment and oncogene-induced plasticity of human stem cell-derived pancreatic acinar and ductal organoids. Cell Stem Cell 28, 1090–1104.e6 (2021).
Darrigrand, J. F., Isaacson, A. & Spagnoli, F. M. Generation of human iPSC-derived pancreatic organoids to study pancreas development and disease. F1000Research 14, 575 (2025).
Desai, R., Huang, L., Gonzalez, R. S. & Muthuswamy, S. K. Oncogenic GNAS uses PKA-dependent and independent mechanisms to induce cell proliferation in human pancreatic ductal and acinar organoids. Mol. Cancer Res. 22, 440–451 (2024).
Godoy, P. et al. Gene networks and transcription factor motifs defining the differentiation of stem cells into hepatocyte-like cells. J. Hepatol. 63, 934–942 (2015).
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Blokzijl, F. et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538, 260–264 (2016).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013).
Huch, M. et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32, 2708–2721 (2013).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Georgakopoulos, N. et al. Long-term expansion, genomic stability and in vivo safety of adult human pancreas organoids. BMC Dev. Biol. 20, 4 (2020).
Aloia, L. et al. Epigenetic remodelling licences adult cholangiocytes for organoid formation and liver regeneration. Nat. Cell Biol. 21, 1321–1333 (2019).
Edgar, R. D. et al. Culture-associated DNA methylation changes impact on cellular function of human intestinal organoids. Cell. Mol. Gastroenterol. Hepatol. 14, 1295–1310 (2022).
Tapia, N. & Schöler, H. R. Molecular obstacles to clinical translation of iPSCs. Cell Stem Cell 19, 298–309 (2016).
Antoniou, A. et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology 136, 2325–2333 (2009).
Wesley, B. T. et al. Single-cell atlas of human liver development reveals pathways directing hepatic cell fates. Nat. Cell Biol. 24, 1487–1498 (2022).
George, J., Goodwin, B., Liddle, C., Tapner, M. & Farrell Westmead, G. C. Time-dependent expression of cytochrome P450 genes in primary cultures of well-differentiated human hepatocytes. J. Lab. Clin. Med. 129, 638–648 (1997).
Hu, H. et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175, 1591–1606 (2018).
Hendriks, D., Artegiani, B., Hu, H., Chuva de Sousa Lopes, S. & Clevers, H. Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat. Protoc. 16, 182–217 (2021).
Prior, N. et al. Lgr5+ stem and progenitor cells reside at the apex of a heterogeneous embryonic hepatoblast pool. Development 146, dev174557 (2019).
Sugiyama, T. et al. Reconstituting pancreas development from purified progenitor cells reveals genes essential for islet differentiation. Proc. Natl Acad. Sci. USA 110, 12691–12696 (2013).
Greggio, C. et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140, 4452–4462 (2013).
Bonfanti, P. et al. Ex vivo expansion and differentiation of human and mouse fetal pancreatic progenitors are modulated by epidermal growth factor. Stem Cell Dev. 24, 1766–1778 (2015).
Andersson-Rolf, A. et al. Long-term in vitro expansion of a human fetal pancreas stem cell that generates all three pancreatic cell lineages. Cell 187, 7394–7413.e22 (2024).
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 (2004).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Bonner-Weir, S. et al. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl Acad. Sci. USA 97, 7999–8004 (2000).
Nantasanti, S. et al. Disease modeling and gene therapy of copper storage disease in canine hepatic organoids. Stem Cell Rep. 5, 895–907 (2015).
Soroka, C. J. et al. Bile-derived organoids from patients with primary sclerosing cholangitis recapitulate their inflammatory immune profile. Hepatology 70, 871–882 (2019).
Roos, F. J. M. et al. Human branching cholangiocyte organoids recapitulate functional bile duct formation. Cell Stem Cell 29, 776–794.e13 (2022).
Peng, W. C. et al. Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell 175, 1607–1619.e15 (2018).
Dowbaj, A. M. et al. Mouse liver assembloids model periportal architecture and biliary fibrosis. Nature 644, 473–482 (2025).
Igarashi, R. et al. Generation of human adult hepatocyte organoids with metabolic functions. Nature 641, 1248–1257 (2025).
Yuan, L. et al. Human assembloids recapitulate periportal liver tissue in vitro. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-5314788/v1 (2025).
Georgia, S. & Bhushan, A. β cell replication is the primary mechanism for maintaining postnatal β cell mass. J. Clin. Investig. 114, 963–968 (2004).
Kopp, J. L. et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138, 653–665 (2011).
Solar, M. et al. Pancreatic exocrine duct cells give rise to insulin-producing β cells during embryogenesis but not after birth. Dev. Cell 17, 849–860 (2009).
Larsen, H. L. & Grapin-Botton, A. The molecular and morphogenetic basis of pancreas organogenesis. Semin. Cell Dev. Biol. 66, 51–68 (2017).
Grapin-Botton, A. & Kim, Y. H. Pancreas organoid models of development and regeneration. Development 149, dev201004 (2022).
Rovira, M. et al. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc. Natl Acad. Sci. USA 107, 75–80 (2010).
Su, K. H., Cuthbertson, C. & Christophi, C. Review of experimental animal models of acute pancreatitis. HPB 8, 264–286 (2006).
Schuijers, J. & Clevers, H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 31, 2685–2696 (2012).
Barker, N. et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010).
Bhushan, A. et al. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 128, 5109–5117 (2001).
Fernández, Á. et al. A single-cell atlas of the murine pancreatic ductal tree identifies novel cell populations with potential implications in pancreas regeneration and exocrine pathogenesis. Gastroenterology 167, 944–960.e15 (2024).
Boj, S. F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
Loomans, C. J. M. et al. Expansion of adult human pancreatic tissue yields organoids harboring progenitor cells with endocrine differentiation potential. Stem Cell Rep. 10, 712–724 (2018).
Cherubini, A. et al. Exploring human pancreatic organoid modelling through single-cell RNA sequencing analysis. Commun. Biol. 7, 1527 (2024).
Wang, D. et al. Long-term expansion of pancreatic islet organoids from resident Procr+ progenitors. Cell 180, 1198–1211.e19 (2020).
Heidenreich, A. C., Bacigalupo, L., Rossotti, M. & Rodríguez-Seguí, S. A. Identification of mouse and human embryonic pancreatic cells with adult Procr+ progenitor transcriptomic and epigenomic characteristics. Front. Endocrinol. 16, 1543960 (2025).
Lee, J. et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. eLife 2, e00940 (2013).
Xu, X. et al. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 (2008).
Gribben, C. et al. Ductal Ngn3-expressing progenitors contribute to adult β cell neogenesis in the pancreas. Cell Stem Cell 28, 2000–2008.e4 (2021).
Shao, W. et al. Advances in liver organoids: replicating hepatic complexity for toxicity assessment and disease modeling. Stem Cell Res. Ther. 16, 27 (2025).
Wills, E. S. et al. Chromosomal abnormalities in hepatic cysts point to novel polycystic liver disease genes. Eur. J. Hum. Genet. 24, 1707–1714 (2016).
Guan, Y. et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight 2, e94954 (2017).
Mun, S. J. et al. Efficient and reproducible generation of human induced pluripotent stem cell-derived expandable liver organoids for disease modeling. Sci. Rep. 13, 22935 (2023).
Ortuño-Costela, M. C., Pinzani, M. & Vallier, L. Cell therapy for liver disorders: past, present and future. Nat. Rev. Gastroenterol. Hepatol. 22, 329–342 (2025).
Ouchi, R. et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. https://doi.org/10.1016/J.CMET.2019.05.007 (2019).
Belenguer, G. et al. RNF43/ZNRF3 loss predisposes to hepatocellular-carcinoma by impairing liver regeneration and altering the liver lipid metabolic ground-state. Nat. Commun. 13, 334 (2022).
Hendriks, D. et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat. Biotechnol. 41, 1567–1581 (2023).
Jansen, P. L. M. et al. The ascending pathophysiology of cholestatic liver disease. Hepatology 65, 722–738 (2017).
Banales, J. M. et al. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 16, 269–281 (2019).
Hirschfield, G. M. et al. The genetics of complex cholestatic disorders. Gastroenterology 144, 1357–1374 (2013).
Mayer, C. et al. Apical bulkheads accumulate as adaptive response to impaired bile flow in liver disease. EMBO Rep. 24, e57181 (2023).
Amarachintha, S. P. et al. Biliary organoids uncover delayed epithelial development and barrier function in biliary atresia. Hepatology 75, 89–103 (2022).
Nie, Y. Z. et al. Recapitulation of hepatitis B virus–host interactions in liver organoids from human induced pluripotent stem cells. eBioMedicine 35, 114–123 (2018).
Zhao, B. et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 11, 771–775 (2020).
Lui, V. C. H. et al. Human liver organoid derived intra-hepatic bile duct cells support SARS-CoV-2 infection and replication. Sci. Rep. 12, 5375 (2022).
Broutier, L. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435 (2017).
Nuciforo, S. et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 24, 1363–1376 (2018).
De Crignis, E. et al. Application of human liver organoids as a patient-derived primary model for HBV infection and related hepatocellular carcinoma. eLife 10, e60747 (2021).
Yang, H. et al. Pharmacogenomic profiling of intra-tumor heterogeneity using a large organoid biobank of liver cancer. Cancer Cell 42, 535–551.e8 (2024).
van Tienderen, G. S. et al. Hepatobiliary tumor organoids for personalized medicine: a multicenter view on establishment, limitations, and future directions. Cancer Cell. 40, 226–230 (2022).
Artegiani, B. et al. Probing the tumor suppressor function of BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell 24, 927–943.e6 (2019).
Hosein, A. N., Dougan, S. K., Aguirre, A. J. & Maitra, A. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat. Cancer 3, 272–286 (2022).
Halbrook, C. J., Lyssiotis, C. A., Pasca di Magliano, M. & Maitra, A. Pancreatic cancer: advances and challenges. Cell 186, 1729–1754 (2023).
Niger, M. et al. One size does not fit all for pancreatic cancers: a review on rare histologies and therapeutic approaches. World J. Gastrointest. Oncol. 12, 833–849 (2020).
Asa, S. L. Pancreatic endocrine tumors. Mod. Pathol. 24, S66–S77 (2011).
Tiriac, H. et al. Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment. Gastrointest. Endosc. 87, 1474–1480 (2018).
Bhalerao, N. et al. ST6GAL1 sialyltransferase promotes acinar to ductal metaplasia and pancreatic cancer progression. JCI Insight 8, e161563 (2023).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988.e16 (2018).
Seino, T. et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell 22, 454–467.e6 (2018).
Tamagawa, H. et al. Wnt-deficient and hypoxic environment orchestrates squamous reprogramming of human pancreatic ductal adenocarcinoma. Nat. Cell Biol. https://doi.org/10.1038/s41556-024-01498-5 (2024).
Papargyriou, A. et al. Heterogeneity-driven phenotypic plasticity and treatment response in branched-organoid models of pancreatic ductal adenocarcinoma. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-024-01273-9 (2024).
Ooi, C. Y. & Durie, P. R. Cystic fibrosis from the gastroenterologist’s perspective. Nat. Rev. Gastroenterol. Hepatol. 13, 175–185 (2016).
Hohwieler, M. et al. Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 66, 473–486 (2017).
O’Malley, Y. et al. Pancreatic duct organoid swelling is chloride-dependent. J. Cyst. Fibros. 23, 169–171 (2024).
Shapiro, A. M. J. et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep. Med. 2, 100466 (2021).
Vegas, A. J. et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 22, 306–311 (2016).
Aghazadeh, Y. et al. Microvessels support engraftment and functionality of human islets and hESC-derived pancreatic progenitors in diabetes models. Cell Stem Cell 28, 1936–1949.e8 (2021).
Du, Y. et al. Human pluripotent stem-cell-derived islets ameliorate diabetes in non-human primates. Nat. Med. 28, 272–282 (2022).
Wang, S. et al. Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient. Cell 187, 6152–6164.e18 (2024).
Choi, J. et al. Light-stimulated insulin secretion from pancreatic islet-like organoids derived from human pluripotent stem cells. Mol. Ther. 31, 1480–1495 (2023).
Huang, X. et al. Stomach-derived human insulin-secreting organoids restore glucose homeostasis. Nat. Cell Biol. 25, 778–786 (2023).
Zhang, X., Ma, Z., Song, E. & Xu, T. Islet organoid as a promising model for diabetes. Protein Cell 13, 239–257 (2022).
Beydag-Tasöz, B. S., Yennek, S. & Grapin-Botton, A. Towards a better understanding of diabetes mellitus using organoid models. Nat. Rev. Endocrinol. 19, 232–248 (2023).
Meng, H. et al. FGF7 enhances the expression of ACE2 in human islet organoids aggravating SARS-CoV-2 infection. Signal. Transduct. Target. Ther. 9, 104 (2024).
Maxwell, K. G. et al. Gene-edited human stem cell-derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med. 12, eaax9106 (2020).
Ilegems, E. et al. HIF-1α inhibitor PX-478 preserves pancreatic β cell function in diabetes. Sci. Transl. Med. 14, eaba9112 (2022).
Ludwig, T. E. et al. ISSCR standards for the use of human stem cells in basic research. Stem Cell Rep. 18, 1744–1752 (2023).
Elci, B. S., Nikolaev, M., Rezakhani, S. & Lutolf, M. P. Bioengineered tubular biliary organoids. Adv. Healthc. Mater. 13, e2302912 (2024).
Ben-Moshe, S. et al. Spatial sorting enables comprehensive characterization of liver zonation. Nat. Metab. 1, 899–911 (2019).
Panday, R., Monckton, C. P. & Khetani, S. R. The role of liver zonation in physiology, regeneration, and disease. Semin. Liver Dis. 42, 1–16 (2022).
Kietzmann, T. Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol. 11, 622–630 (2017).
Zhang, Y. et al. hESCs-derived organoids achieve liver zonation features through LSEC modulation. Adv. Sci. 12, e2411667 (2025).
Al Reza, H. et al. Multi-zonal liver organoids from human pluripotent stem cells. Nature 641, 1258–1267 (2025).
Mallanna S. K. et al. Expandable, functional hepatocytes derived from primary cells enable liver therapeutics. Preprint at https://www.biorxiv.org/content/10.1101/2024.12.28.630269v1 (2024).
Lee, B. H. et al. Control of lumen geometry and topology by the interplay between pressure and cell proliferation rate in pancreatic organoids. Preprint at https://www.biorxiv.org/content/10.1101/2024.05.29.596462v2 (2024).
Deguchi, S. & Takayama, K. State-of-the-art liver disease research using liver-on-a-chip. Inflamm. Regener. 42, 62 (2022).
Deng, J. et al. Engineered liver-on-a-chip platform to mimic liver functions and its biomedical applications: a review. Micromachines 10, 676 (2019).
Hassan, S. et al. Liver-on-a-chip models of fatty liver disease. Hepatology 71, 733–740 (2020).
Ang, L. T. et al. A roadmap for human liver differentiation from pluripotent stem cells. Cell Rep. 22, 2190–2205 (2018).
Wu, F. et al. Generation of hepatobiliary organoids from human induced pluripotent stem cells. J. Hepatol. 70, 1145–1158 (2019).
Koike, H. et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut–midgut boundary. Nature 574, 112–116 (2019).
Akbari, S. et al. Robust, long-term culture of endoderm-derived hepatic organoids for disease modeling. Stem Cell Rep. 13, 627–641 (2019).
Tadokoro, T. et al. Human iPSC-liver organoid transplantation reduces fibrosis through immunomodulation. Sci. Transl. Med. 16, eadg0338 (2024).
Marsee, A. et al. Mass generation and long-term expansion of hepatobiliary organoids from adult primary human hepatocytes. Preprint at bioRxiv https://doi.org/10.1101/2024.06.10.598262 (2024).
Kanton, S. & Pasça, S. P. Human assembloids. Development 149, dev201120 (2022).
Hebb, D. O. The Organization of Behavior: A Neuropsychological Theory (Wiley, 1949).
Andersen, J. et al. Generation of functional human 3D cortico-motor assembloids. Cell 183, 1913–1929.e26 (2020).
Bagley, J. A., Reumann, D., Bian, S., Lévi-Strauss, J. & Knoblich, J. A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751 (2017).
Cordero-Espinoza, L. et al. Dynamic cell contacts between periportal mesenchyme and ductal epithelium act as a rheostat for liver cell proliferation. Cell Stem Cell https://doi.org/10.1016/j.stem.2021.07.002 (2021).
Dowbaj, A. M., Kohler, T. N., Cordero-Espinoza, L., Hollfelder, F. & Huch, M. Generation of liver mesenchyme and ductal cell organoid co-culture using cell self-aggregation and droplet microfluidics. Star. Protoc. 4, 102333 (2023).
Yang, L. et al. Human vascularized macrophage-islet organoids to model immune-mediated pancreatic β cell pyroptosis upon viral infection. Cell Stem Cell 31, 1612–1629.e8 (2024).
Hughes, C. S., Postovit, L. M. & Lajoie, G. A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890 (2010).
Gan, Z., Qin, X., Liu, H., Liu, J. & Qin, J. Recent advances in defined hydrogels in organoid research. Bioactive Mater. 28, 386–401 (2023).
Cao, Y. et al. Inclusion of cross-linked elastin in gelatin/PEG hydrogels favourably influences fibroblast phenotype. Polymers 12, 670 (2020).
Kapp, T. G. et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep. 7, 39805 (2017).
Sorrentino, G. et al. Mechano-modulatory synthetic niches for liver organoid derivation. Nat. Commun. 11, 3416 (2020).
Garreta, E. et al. Rethinking organoid technology through bioengineering. Nat. Mater. 20, 145–155 (2021).
Belicova, L. et al. Anisotropic expansion of hepatocyte lumina enforced by apical bulkheads. J. Cell Biol. 220, e202103003 (2021).
Box, G. E. P. Science and statistics. J. Am. Stat. Assoc. 71, 791–799 (1976).
Box, G. E. & Draper, N. R. Empirical Model-Building and Response Surfaces (Wiley, 1987).
Acknowledgements
N.P. is supported by the Academy of Medical Sciences Springboard Award (SBF007/100092) and Wessex Medical Research. J.G.J. is funded by the Gerald Kerkut Trust PhD studentship and Institute for Life Sciences, University of Southampton. M.H. is supported by the Max Planck Gessellschaft, and is funded by the European Research Council under the European Union’s Horizon Europe research and innovation programme (grant agreement no. 101088869), which also supports A.S. and A.N. The views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for the views and opinions expressed.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
M.H. is inventor in several patents related to organoid technology. M.H and A.S. are inventors in a patent on assembloids. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Giuseppe Pettinato, Hasan Rashidi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sljukic, A., Green Jenkinson, J., Niksic, A. et al. Advances in liver and pancreas organoids: how far we have come and where we go next. Nat Rev Gastroenterol Hepatol 23, 44–64 (2026). https://doi.org/10.1038/s41575-025-01116-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-025-01116-1
This article is cited by
-
Metabolic dysfunction-associated steatotic liver disease and steatohepatitis-associated hepatocarcinoma preclinical models
Nature Reviews Gastroenterology & Hepatology (2026)