Abstract
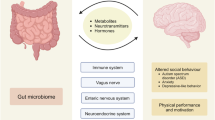
Microglia, the resident immune cells of the brain, are now recognized as being active participants in the onset and progression of many neurological and neuropsychiatric disorders. As a result, substantial effort has been made in finding ways to target, deplete or modulate the aberrant phenotypes of the microglia that are present in these different disease states, albeit with varied levels of success. The gut microbiota has recently emerged as a master regulator of microglia throughout the lifespan; here, we propose that this microbiota–microglia cross-talk may have major implications for our understanding of neurological disorders and neuropsychiatric diseases. We focus on the latest advances in understanding gut–microglia communication in the context of microglial heterogeneity and microglia-related functions, as well as considering the evidence for effects of these pathways on diseases and disorders of the central nervous system. We also address the challenges, opportunities and clinical implications of this emerging area of research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boullerne, A. I. & Feinstein, D. L. History of neuroscience I. Pío del Río-Hortega (1882–1945): the discoverer of microglia and oligodendroglia. ASN Neuro 12, 1759091420953259 (2020).
Wolf, S. A., Boddeke, H. W. G. M. & Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 79, 619–643 (2017).
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014).
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013).
Escoubas, C. C. et al. Type-I-interferon-responsive microglia shape cortical development and behavior. Cell 187, 1936–1954.e24 (2024).
Lawrence, A. R. et al. Microglia maintain structural integrity during fetal brain morphogenesis. Cell 187, 962–980.e19 (2024).
Cheray, M. & Joseph, B. Epigenetics control microglia plasticity. Front. Cell. Neurosci. 12, 1–13 (2018).
Ransohoff, R. M. & Perry, V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 (2009).
Tay, T. L., Savage, J. C., Hui, C. W., Bisht, K. & Tremblay, M. È. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J. Physiol. 595, 1929–1945 (2017).
Camarillo-Guerrero, L. F., Almeida, A., Rangel-Pineros, G., Finn, R. D. & Lawley, T. D. Massive expansion of human gut bacteriophage diversity. Cell 184, 1098–1109.e9 (2021).
Hou, K. et al. Microbiota in health and diseases. Signal. Transduct. Target. Ther. 7, 135 (2022).
Jandhyala, S. M. et al. Role of the normal gut microbiota. World J. Gastroenterol. 21, 8836–8847 (2015).
Cryan, J. F. & Dinan, T. G. Gut microbiota: microbiota and neuroimmune signalling—Metchnikoff to microglia. Nat. Rev. Gastroenterol. Hepatol. 12, 494–496 (2015).
Ratsika, A., Cruz Pereira, J. S., Lynch, C. M. K., Clarke, G. & Cryan, J. F. Microbiota–immune–brain interactions: a lifespan perspective. Curr. Opin. Neurobiol. 78, 102652 (2023).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Erny, D. et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 33, 2260–2276.e7 (2021).
Matcovitch-Natan, O. et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016).
Scheperjans, F. et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 30, 350–358 (2015).
Thion, M. S. & Garel, S. On place and time: microglia in embryonic and perinatal brain development. Curr. Opin. Neurobiol. 47, 121–130 (2017).
Vogt, N. M. et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7, 13537 (2017).
Lynch, C. M. K., Clarke, G. & Cryan, J. F. Powering up microbiome–microglia interactions. Cell Metab. 33, 2097–2099 (2021).
Dodiya, H. B. et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 216, 1542–1560 (2019).
Thion, M. S. et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516.e16 (2018).
Xia, Y. et al. Bacteroides fragilis in the gut microbiomes of Alzheimer’s disease activates microglia and triggers pathogenesis in neuronal C/EBPβ transgenic mice. Nat. Commun. 14, 5471 (2023).
Kipnis, J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 353, 766–771 (2016).
Mazzitelli, J. A. et al. Skull bone marrow channels as immune gateways to the central nervous system. Nat. Neurosci. 26, 2052–2062 (2023).
Fung, T. C., Olson, C. A. & Hsiao, E. Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155 (2017).
Thaiss, C. A., Zmora, N., Levy, M. & Elinav, E. The microbiome and innate immunity. Nature 535, 65–74 (2016).
Cryan, J. F. et al. The microbiota–gut–brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Kasarello, K., Cudnoch-Jedrzejewska, A. & Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 14, 1118529 (2023).
Mayer, E. A., Tillisch, K. & Gupta, A. Gut/brain axis and the microbiota. J. Clin. Invest. 125, 926–938 (2015).
O’Riordan, K. J. et al. Short chain fatty acids: microbial metabolites for gut–brain axis signalling. Mol. Cell. Endocrinol. 546, 111572 (2022).
Mossad, O. & Erny, D. The microbiota–microglia axis in central nervous system disorders. Brain Pathol. 30, 1159–1177 (2020).
Kim, Y. J., Mun, B. R., Choi, K. Y. & Choi, W. S. Oral administration of probiotic bacteria alleviates tau phosphorylation, Aβ accumulation, microglia activation, and memory loss in 5×FAD Mice. Brain Sci. 14, 208 (2024).
Lee, H. J., Hwang, Y. H. & Kim, D. H. Lactobacillus plantarum C29-fermented soybean (DW2009) alleviates memory impairment in 5×FAD transgenic mice by regulating microglia activation and gut microbiota composition. Mol. Nutr. Food Res. 62, e1800359 (2018).
Caetano-Silva, M. E. et al. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 13, 2819 (2023).
Moffett, J. R., Puthillathu, N., Vengilote, R., Jaworski, D. M. & Namboodiri, A. M. Acetate revisited: a key biomolecule at the nexus of metabolism, epigenetics and oncogenesis—part 1: acetyl-CoA, acetogenesis and acyl-CoA short-chain synthetases. Front. Physiol. 11, 580171 (2020).
Keane, L., Cheray, M., Blomgren, K. & Joseph, B. Multifaceted microglia—key players in primary brain tumour heterogeneity. Nat. Rev. Neurol. 17, 243–259 (2021).
Stratoulias, V., Venero, J. L., Tremblay, M. & Joseph, B. Microglial subtypes: diversity within the microglial community. EMBO J. 38, e101997 (2019).
Bisht, K. et al. Dark microglia: a new phenotype predominantly associated with pathological states. Glia 64, 826–839 (2016).
Hammond, L. A. et al. Phase I and pharmacokinetic study of temozolomide on a daily-for-5-days schedule in patients with advanced solid malignancies. J. Clin. Oncol. 17, 2604–2613 (1999).
Masuda, T., Sankowski, R., Staszewski, O. & Prinz, M. Microglia heterogeneity in the single-cell era. Cell Rep. 30, 1271–1281 (2020).
Hove, H. et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019).
Gabandé-Rodríguez, E., Keane, L. & Capasso, M. Microglial phagocytosis in aging and Alzheimer’s disease. J. Neurosci. Res. 2, 284–298 (2020).
Keane, L. et al. mTOR-dependent translation amplifies microglia priming in aging mice. J. Clin. Invest. 131, 1–16 (2021).
Grabert, K. et al. Microglial brain region dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
Guneykaya, D. et al. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 24, 2773–2783.e6 (2018).
Villa, A. et al. Sex-specific features of microglia from adult mice. Cell Rep. 23, 3501–3511 (2018).
Easley-Neal, C., Foreman, O., Sharma, N., Zarrin, A. A. & Weimer, R. M. CSF1R ligands IL-34 and CSF1 are differentially required for microglia development and maintenance in white and gray matter brain regions. Front. Immunol. 10, 2199 (2019).
Casano, A. M. & Peri, F. Microglia: multitasking specialists of the brain. Dev. Cell 32, 469–477 (2015).
Ginhoux, F. & Prinz, M. Origin of microglia: current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 7, 1–15 (2015).
Kennedy, K. M. et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature 613, 639–649 (2023).
Vuong, H. E. et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 586, 281–286 (2020).
Pronovost, G. N. et al. The maternal microbiome promotes placental development in mice. Sci. Adv. 9, 1–12 (2023).
Jessa, S. et al. Stalled developmental programs at the root of pediatric brain tumors. Nat. Genet. 51, 1702–1713 (2019).
Cowan, C. S. M., Dinan, T. G. & Cryan, J. F. Annual research review: critical windows—the microbiota–gut–brain axis in neurocognitive development. J. Child. Psychol. Psychiatry 3, 353–371 (2019).
Perry, V. H. & Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 10, 217–224 (2014).
Perry, V. H. & Teeling, J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 35, 601–612 (2013).
Henry, C. J., Huang, Y., Wynne, A. M. & Godbout, J. P. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain. Behav. Immun. 23, 309–317 (2009).
Tejera, D. et al. Systemic inflammation impairs microglial Aβ clearance through NLRP 3 inflammasome. EMBO J. 38, e101064 (2019).
Ghosh, T. S., Shanahan, F. & O’Toole, P. W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 19, 565–584 (2022).
Boehme, M. et al. Mid-life microbiota crises: middle age is associated with pervasive neuroimmune alterations that are reversed by targeting the gut microbiome. Mol. Psychiatry 25, 2567–2583 (2020).
Mossad, O. et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat. Neurosci. 25, 295–305 (2022).
Boehme, M. et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat. Aging 1, 666–676 (2021).
Bennett, M. L. & Barres, B. A. A genetically distinct microglial subset promotes myelination. EMBO J. 36, 3269–3271 (2017).
Guedes, J. R., Ferreira, P. A., Costa, J. M., Cardoso, A. L. & Peça, J. Microglia-dependent remodeling of neuronal circuits. J. Neurochem. 163, 74–93 (2022).
Hughes, A. N. & Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23, 1055–1066 (2020).
Wake, H., Moorhouse, A. J., Miyamoto, A. & Nabekura, J. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 36, 209–217 (2013).
Szepesi, Z., Manouchehrian, O., Bachiller, S. & Deierborg, T. Bidirectional microglia–neuron communication in health and disease. Front. Cell. Neurosci. 12, 323 (2018).
Lui, H. et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165, 921–935 (2016).
Scott-Hewitt, N. et al. Microglial-derived C1q integrates into neuronal ribonucleoprotein complexes and impacts protein homeostasis in the aging brain. Cell 8, 4193–4212 (2024).
Camacho-Hernández, N. P. & Peña-Ortega, F. Fractalkine/CX3CR1-dependent modulation of synaptic and network plasticity in health and disease. Neural Plast. 2023, 4637073 (2023).
Lehrman, E. K. et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron 100, 120–134.e6 (2018).
Kim, H. J. et al. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 22, 1576–1584 (2017).
Chafee, M. V. & Averbeck, B. B. Unmasking schizophrenia: synaptic pruning in adolescence reveals a latent physiological vulnerability in prefrontal recurrent networks. Biol. Psychiatry 92, 436–439 (2022).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Bruckner, J. J. et al. The microbiota promotes social behavior by modulating microglial remodeling of forebrain neurons. PLoS Biol. 20, e3001838 (2022).
Muller, P. A. et al. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature 583, 441–446 (2020).
Hoyles, L. et al. Regulation of blood–brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome 9, 235 (2021).
Rosito, M. et al. Antibiotics treatment promotes vasculogenesis in the brain of glioma-bearing mice. Cell Death Dis. 15, 210 (2024).
Çalışkan, G. et al. Antibiotic-induced gut dysbiosis leads to activation of microglia and impairment of cholinergic gamma oscillations in the hippocampus. Brain. Behav. Immun. 99, 203–217 (2022).
He, H. et al. Gut microbiota regulate stress resistance by influencing microglia–neuron interactions in the hippocampus. Brain Behav. Immun. Health 36, 100729 (2024).
He, H., He, H., Mo, L., You, Z. & Zhang, J. Priming of microglia with dysfunctional gut microbiota impairs hippocampal neurogenesis and fosters stress vulnerability of mice. Brain. Behav. Immun. 115, 280–294 (2024).
Luck, B. et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci. Rep. 10, 7737 (2020).
Williamson, J. M. & Lyons, D. A. Myelin dynamics throughout life: an ever-changing landscape? Front. Cell. Neurosci. 12, 424 (2018).
Irfan, M., Evonuk, K. S. & DeSilva, T. M. Microglia phagocytose oligodendrocyte progenitor cells and synapses during early postnatal development: implications for white versus gray matter maturation. FEBS J. 289, 2110–2127 (2022).
Nemes-Baran, A. D., White, D. R. & DeSilva, T. M. Fractalkine-dependent microglial pruning of viable oligodendrocyte progenitor cells regulates myelination. Cell Rep. 32, 108047 (2020).
Giera, S. et al. Microglial transglutaminase-2 drives myelination and myelin repair via GPR56/ ADGRG1 in oligodendrocyte precursor cells. eLIFE 7, e33385 (2018).
Djannatian, M. et al. Myelination generates aberrant ultrastructure that is resolved by microglia. J. Cell Biol. 222, e202204010 (2023).
McNamara, N. B. et al. Microglia regulate central nervous system myelin growth and integrity. Nature 613, 120–129 (2023).
Benmamar-Badel, A., Owens, T. & Wlodarczyk, A. Protective microglial subset in development, aging, and disease: lessons from transcriptomic studies. Front. Immunol. 11, 430 (2020).
Hagemeyer, N. et al. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 134, 441–458 (2017).
Shen, X. et al. Definition of a mouse microglial subset that regulates neuronal development and proinflammatory responses in the brain. Proc. Natl Acad. Sci. USA 119, e2116241119 (2022).
Wlodarczyk, A. et al. CSF1R stimulation promotes increased neuroprotection by CD11c+ microglia in EAE. Front. Cell. Neurosci. 12, 1–10 (2019).
Wlodarczyk, A. et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308 (2017).
Hoban, A. E. et al. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 6, e774 (2016).
Ahmed, S. et al. Early influences of microbiota on white matter development in germ-free piglets. Front. Cell. Neurosci. 15, 807170 (2021).
Gacias, M. et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLIFE 6, e13442 (2016).
Keogh, C. E. et al. Myelin as a regulator of development of the microbiota–gut–brain axis. Brain. Behav. Immun. 91, 437–450 (2021).
Lynch, C. M. K. et al. Critical windows of early-life microbiota disruption on behaviour, neuroimmune function, and neurodevelopment. Brain. Behav. Immun. 108, 309–327 (2023).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12 (2016).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
McMurran, C. E. et al. The microbiota regulates murine inflammatory responses to toxin-induced CNS demyelination but has minimal impact on remyelination. Proc. Natl Acad. Sci. USA 50, 25311–25321 (2019).
Rothhammer, V. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018).
Juricek, L. et al. AhR-deficiency as a cause of demyelinating disease and inflammation. Sci. Rep. 7, 9794 (2017).
Juricek, L. & Coumoul, X. The aryl hydrocarbon receptor and the nervous system. Int. J. Mol. Sci. 19, 2504 (2018).
Shackleford, G. et al. Involvement of aryl hydrocarbon receptor in myelination and in human nerve sheath tumorigenesis. Proc. Natl Acad. Sci. USA 115, 1319–1328 (2018).
Rothhammer, V. & Quintana, F. J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 19, 184–197 (2019).
Wang, T. et al. Microbiota–indole 3-propionic acid–brain axis mediates abnormal synaptic pruning of hippocampal microglia and susceptibility to ASD in IUGR offspring. Microbiome 11, 245 (2023).
Wang, Y. et al. Microglial aryl hydrocarbon receptor enhances phagocytic function via SYK and promotes remyelination in the cuprizone mouse model of demyelination. J. Neuroinflamm. 20, 83 (2023).
Harry, G. J. Microglia in neurodegenerative events—an initiator or a significant other? Int. J. Mol. Sci. 22, 5818 (2021).
Zhang, W., Xiao, D., Mao, Q. & Xia, H. Role of neuroinflammation in neurodegeneration development. Signal. Transduct. Target. Ther. 8, 267 (2023).
Krasemann, S. et al. The TREM2–APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9 (2017).
Young, A. M. H. et al. A map of transcriptional heterogeneity and regulatory variation in human microglia. Nat. Genet. 53, 861–868 (2021).
Schneider, E., O’Riordan, K. J., Clarke, G. & Cryan, J. F. Feeding gut microbes to nourish the brain: unravelling the diet–microbiota–gut–brain axis. Nat. Metab. 6, 1454–1478 (2024).
Baufeld, C., Osterloh, A., Prokop, S., Miller, K. R. & Heppner, F. L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 132, 361–375 (2016).
Fernández-Arjona, M. D. M., León-Rodríguez, A., Grondona, J. M. & López-Ávalos, M. D. Long-term priming of hypothalamic microglia is associated with energy balance disturbances under diet-induced obesity. Glia 70, 1734–1761 (2022).
Wang, X. L. & Li, L. Microglia regulate neuronal circuits in homeostatic and high-fat diet-induced inflammatory conditions. Front. Cell. Neurosci. 15, 722028 (2021).
Vijaya, A. K. et al. Prebiotics mitigate the detrimental effects of high-fat diet on memory, anxiety and microglia functionality in ageing mice. Brain. Behav. Immun. 122, 167–184 (2024).
Yin, Z. et al. Low-fat diet with caloric restriction reduces white matter microglia activation during aging. Front. Mol. Neurosci. 11, 65 (2018).
Abdel-Haq, R. et al. A prebiotic diet modulates microglial states and motor deficits in α-synuclein overexpressing mice. eLife 11, e81453 (2022).
Barros-Santos, T. & Clarke, G. Gut-initiated neuroprotection in Parkinson’s disease: when microbes turn the tables in the battle against neuroinflammation. Brain. Behav. Immun. 108, 350–352 (2023).
Sun, M. F. et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain. Behav. Immun. 70, 48–60 (2018).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
Hill, C. et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
Castro-Gomez, S. & Heneka, M. T. Innate immune activation in neurodegenerative diseases. Immunity 57, 790–814 (2024).
Heneka, M. T. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).
Ising, C. et al. NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673 (2019).
Venegas, C. et al. Microglia-derived ASC specks crossseed amyloid-β in Alzheimer’s disease. Nature 552, 355–361 (2017).
Abdelhamid, M. et al. Probiotic Bifidobacterium breve prevents memory impairment through the reduction of both amyloid-β production and microglia activation in APP knock-in mouse. J. Alzheimers Dis. 85, 1555–1571 (2022).
Cox, L. M. et al. The microbiota restrains neurodegenerative microglia in a model of amyotrophic lateral sclerosis. Microbiome 10, 47 (2022).
Medawar, E. et al. Prebiotic diet changes neural correlates of food decision-making in overweight adults: a randomised controlled within-subject cross-over trial. Gut 73, 298–310 (2023).
Tillisch, K. et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144, 1394–1401 (2013).
Bagga, D. et al. Influence of 4-week multi-strain probiotic administration on resting-state functional connectivity in healthy volunteers. Eur. J. Nutr. 58, 1821–1827 (2019).
Janssen, B., Vugts, D. J., Windhorst, A. D. & Mach, R. H. PET imaging of microglial activation—beyond targeting TSPO. Molecules 23, 607 (2018).
Gerhard, A. et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 21, 404–412 (2006).
Pajares, M. et al. Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells 9, 1687 (2020).
Zhang, W. et al. Aggregated α‐synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 19, 533–542 (2005).
Scheiblich, H. et al. Microglia jointly degrade fibrillar α-synuclein cargo by distribution through tunneling nanotubes. Cell 184, 5089–5106.e21 (2021).
Unger, M. M. et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 32, 66–72 (2016).
Tan, A. H. et al. Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat. Disord. 20, 535–540 (2014).
Liu, J. et al. Microbiota-microglia crosstalk between Blautia producta and neuroinflammation of Parkinson’s disease: a bench-to-bedside translational approach. Brain. Behav. Immun. 117, 270–282 (2024).
Rossano, S. M. et al. Microglia measured by TSPO PET are associated with Alzheimer’s disease pathology and mediate key steps in a disease progression model. Alzheimers Dement. J. Alzheimers Assoc. 20, 2397–2407 (2024).
Haney, M. S. et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature 628, 154–161 (2024).
Miao, J. et al. Microglia in Alzheimer’s disease: pathogenesis, mechanisms, and therapeutic potentials. Front. Aging Neurosci. 15, 1201982 (2023).
Chen, C. et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 71, 2233–2252 (2022).
Wasén, C. et al. Bacteroidota inhibit microglia clearance of amyloid-β and promote plaque deposition in Alzheimer’s disease mouse models. Nat. Commun. 15, 3872 (2024).
Colombo, A. V. et al. Microbiota-derived short chain fatty acids modulate microglia and promote aβ plaque deposition. eLife 10, e59826 (2021).
Dodiya, H. B. et al. Gut microbiota-driven brain Aβ amyloidosis in mice requires microglia. J. Exp. Med. 219, e20200895 (2021).
Brettschneider, J. et al. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS One 7, 39216 (2012).
Dols-Icardo, O. et al. Motor cortex transcriptome reveals microglial key events in amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 7, e829 (2020).
Frakes, A. E. et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 81, 1009–1023 (2014).
Guo, K. et al. Gut microbiome correlates with plasma lipids in amyotrophic lateral sclerosis. Brain 147, 665–679 (2024).
Zeng, Q. et al. The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci. Rep. 10, 12998 (2020).
Nicholson, K. et al. The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 22, 186–194 (2021).
Singh, S. B. et al. The utility of PET imaging in depression. Front. Psychiatry 15, 1322118 (2024).
Fan, C. et al. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Mol. Ther. 30, 1300–1314 (2022).
Duan, N. et al. Therapeutic targeting of STING–TBK1–IRF3 signalling ameliorates chronic stress induced depression-like behaviours by modulating neuroinflammation and microglia phagocytosis. Neurobiol. Dis. 169, 105739 (2022).
He, L. et al. Nrf2 regulates the arginase 1+ microglia phenotype through the initiation of TREM2 transcription, ameliorating depression-like behavior in mice. Transl. Psychiatry 12, 459 (2022).
Barandouzi, Z. A., Starkweather, A. R., Henderson, W. A., Gyamfi, A. & Cong, X. S. Altered composition of gut microbiota in depression: a systematic review. Front. Psychiatry 11, 541 (2020).
Marin, I. A. et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 7, 43859 (2017).
Hao, W. et al. Gut dysbiosis induces the development of depression-like behavior through abnormal synapse pruning in microglia-mediated by complement C3. Microbiome 12, 34 (2024).
Li, H. et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflamm. 18, 254 (2021).
Liao, X., Chen, M. & Li, Y. The glial perspective of autism spectrum disorder convergent evidence from postmortem brain and PET studies. Front. Neuroendocrinol. 70, 101064 (2023).
Bose, R. et al. Bi-allelic NRXN1α deletion in microglia derived from iPSC of an autistic patient increases interleukin-6 production and impairs supporting function on neuronal networking. Brain. Behav. Immun. 123, 28–42 (2025).
Zhan, Y. et al. Deficient neuron–microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 (2014).
Martínez-Cerdeño, V. Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Dev. Neurobiol. 77, 393–404 (2017).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
Kong, Q. et al. Bifidobacterium longum CCFM1077 ameliorated neurotransmitter disorder and neuroinflammation closely linked to regulation in the kynurenine pathway of autistic-like rats. Nutrients 14, 1615 (2022).
Bloomfield, P. S. et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C]PBR28 PET brain imaging study. Am. J. Psychiatry 173, 44–52 (2016).
Na, K. S., Jung, H. Y. & Kim, Y. K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 48, 277–286 (2014).
Hartmann, S. M., Heider, J., Wüst, R., Fallgatter, A. J. & Volkmer, H. Microglia–neuron interactions in schizophrenia. Front. Cell. Neurosci. 18, 1345349 (2024).
Sekar, A. et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016).
Zheng, P. et al. The gut microbiome from patients with schizophrenia modulates the glutamate–glutamine–GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 5, eaau8317 (2019).
Samochowiec, J. & Misiak, B. Gut microbiota and microbiome in schizophrenia. Curr. Opin. Psychiatry 34, 503–507 (2021).
Airas, L., Rissanen, E. & Rinne, J. Imaging of microglial activation in MS using PET: research use and potential future clinical application. Mult. Scler. 23, 496–504 (2017).
Heppner, F. L. et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 11, 146–152 (2005).
Absinta, M. et al. A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021).
Beckmann, N. et al. Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol. Commun. 6, 9 (2018).
Cekanaviciute, E. et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl Acad. Sci. USA 40, 10713–20728 (2017).
Akkari, L. et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci. Transl. Med. 12, eaaw7843 (2020).
Yan, D. et al. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene 36, 6049–6058 (2017).
Keane, L. et al. Inhibition of microglial EZH2 leads to anti-tumoral effects in pediatric diffuse midline gliomas. Neuro-Oncol. Adv. 3, 45–60 (2020).
Patrizz, A. et al. Glioma and temozolomide induced alterations in gut microbiome. Sci. Rep. 10, 21002 (2020).
Fan, H. et al. Multi-omics-based investigation of Bifidobacterium’s inhibitory effect on glioma: regulation of tumor and gut microbiota, and MEK/ERK cascade. Front. Microbiol. 15, 1344284 (2024).
Scalise, M., Galluccio, M., Console, L., Pochini, L. & Indiveri, C. The human SLC7A5 (LAT1): the intriguing histidine/large neutral amino acid transporter and its relevance to human health. Front. Chem. 6, 243 (2018).
Koh, A., Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Caetano-Silva, M. E. et al. The emergence of inflammatory microglia during gut inflammation is not affected by FFAR2 expression in intestinal epithelial cells or peripheral myeloid cells. Brain. Behav. Immun. 118, 423–436 (2024).
Halestrap, A. P. & Wilson, M. C. The monocarboxylate transporter family—role and regulation. IUBMB Life 64, 109–119 (2012).
Monsorno, K. et al. Loss of microglial MCT4 leads to defective synaptic pruning and anxiety-like behavior in mice. Nat. Commun. 14, 5749 (2023).
Mishra, S. P., Karunakar, P., Taraphder, S. & Yadav, H. Free fatty acid receptors 2 and 3 as microbial metabolite sensors to shape host health: pharmacophysiological view. Biomedicines 8, 154 (2020).
Bindels, L. B., Dewulf, E. M. & Delzenne, N. M. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 34, 226–232 (2013).
Lee, Y. H. et al. Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia. Glia 63, 1138–1154 (2015).
Baggio, L. L. & Drucker, D. J. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. J. Clin. Invest. 124, 4223–4226 (2014).
Cook, T. M. et al. Vagal neuron expression of the microbiota-derived metabolite receptor, free fatty acid receptor (FFAR3), is necessary for normal feeding behavior. Mol. Metab. 54, 101350 (2021).
Ni, Y., Tong, Q., Xu, M., Gu, J. & Ye, H. Gut microbiota-induced modulation of the central nervous system function in Parkinson’s disease through the gut–brain axis and short-chain fatty acids. Mol. Neurobiol. 62, 2480–2492 (2025).
Dalile, B., Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Acknowledgements
The authors thank their funding sources. L.K. acknowledges support from the ChadTough Defeat DIPG Foundation, and J.F.C. acknowledges the support of Science Foundation Ireland (SFI/12/RC/2273_P2). The authors also thank C. Cuesta for her help in generating the original version of Fig. 2.
Author information
Authors and Affiliations
Contributions
All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Jacob Allen, who co-reviewed with Elisa Caetano-Silva, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keane, L., Clarke, G. & Cryan, J.F. A role for microglia in mediating the microbiota–gut–brain axis. Nat Rev Immunol 25, 847–861 (2025). https://doi.org/10.1038/s41577-025-01188-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-025-01188-9