Abstract
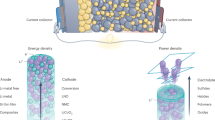
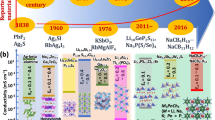
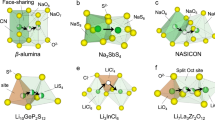
The quest for next-generation energy-storage technologies has pivoted towards all-solid-state batteries, primarily owing to their potential for enhanced safety and energy density. At the centre of this promising technology lie inorganic lithium superionic conductors, which facilitate rapid ion transport comparable to that in their liquid counterparts. Despite their promise, the limited availability of materials that both achieve superionic conductivity and fulfil all practical requirements necessitates the discovery of novel conductors. This Review comprehensively explores the diverse structural and chemical factors that improve ionic conductivity and the atomistic mechanism by which each factor affects it. We emphasize the importance of a dual approach: using structural factors to enable high-conducting prototypes, and chemical factors to further optimize the ionic conductivity. From these insights, we distil over 40 years of conductor development history to the key concepts that paved the way for today’s leading superionic conductors. In detailing the trajectory of ionic conduction advancements, this Review not only charts the progress in the field but also proposes a strategic approach for researchers to efficiently innovate with the ultimate goal of realizing the promise of all-solid-state batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Wang, C. & Sun, X. The promise of solid-state batteries for safe and reliable energy storage. Engineering 21, 32–35 (2023).
Wang, R., Cui, W., Chu, F. & Wu, F. Lithium metal anodes: present and future. J. Energy Chem. 48, 145–159 (2020).
Cheng, D. et al. Manufacturing scale-up of anodeless solid-state lithium thin-film batteries for high volumetric energy density applications. ACS Energy Lett. https://doi.org/10.1021/acsenergylett.3c01839 (2023).
Zhao, Q., Stalin, S., Zhao, C.-Z. & Archer, L. A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 5, 229–252 (2020).
Salamon, M. B. Physics of Superionic Conductors 175–199 (Springer, 2011).
Dieterich, W., Fulde, P. & Peschel, I. Theoretical models for superionic conductors. Adv. Phys. 29, 527–605 (1980).
Diederichsen, K. M., McShane, E. J. & McCloskey, B. D. Promising routes to a high Li+ transference number electrolyte for lithium ion batteries. ACS Energy Lett. 2, 2563–2575 (2017).
Choo, Y., Halat, D. M., Villaluenga, I., Timachova, K. & Balsara, N. P. Diffusion and migration in polymer electrolytes. Prog. Polym. Sci. 103, 101220 (2020).
Aziz, S. B., Woo, T. J., Kadir, M. F. Z. & Ahmed, H. M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 3, 1–17 (2018).
Zhou, L. et al. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 7, 83–93 (2022). In this paper, a chlorospinel superionic conductor (Li2InxSc0.666−xCl4) was used to demonstrate that chloride materials can have excellent stability against uncoated high-voltage cathode materials, with over 3,000 cycles at room temperature.
Kwak, H. et al. Emerging halide superionic conductors for all-solid-state batteries: design, synthesis, and practical applications. ACS Energy Lett. 7, 1776–1805 (2022).
Nikodimos, Y., Su, W. & Hwang, B. J. Halide solid‐state electrolytes: stability and application for high voltage all‐solid‐state Li batteries. Adv. Energy Mater. https://doi.org/10.1002/aenm.202202854 (2023).
Al-Salih, H., Houache, M. S. E., Baranova, E. A. & Abu-Lebdeh, Y. Composite cathodes for solid‐state lithium batteries: ‘catholytes’ the underrated giants. Adv. Energy Sustain. Res. https://doi.org/10.1002/aesr.202200032 (2022).
Kim, M. et al. Carbon-free high-performance cathode for solid-state Li–O2 battery. Sci. Adv. 8, eabm8584 (2022).
Ma, S. B. et al. Mixed ionic–electronic conductor of perovskite LixLayMO3−δ toward carbon‐free cathode for reversible lithium–air batteries. Adv. Energy Mater. 10, 2001767 (2020).
Nuernberg, R. B. Numerical comparison of usual Arrhenius-type equations for modelling ionic transport in solids. Ionics 26, 2405–2412 (2020).
Qi, J. et al. Bridging the gap between simulated and experimental ionic conductivities in lithium superionic conductors. Mater. Today Phys. 21, 100463 (2021).
Arbi, K., Tabellout, M., Lazarraga, M. G., Rojo, J. M. & Sanz, J. Non-Arrhenius conductivity in the fast lithium conductor Li1.2Ti1.8Al0.2(PO4)3: a 7Li NMR and electric impedance study. Phys. Rev. B 72, 094302 (2005).
Winter, G. & Gómez-Bombarelli, R. Simulations with machine learning potentials identify the ion conduction mechanism mediating non-Arrhenius behavior in LGPS. J. Phys. Energy 5, 024004 (2023).
Hargreaves, C. J. et al. A database of experimentally measured lithium solid electrolyte conductivities evaluated with machine learning. npj Comput. Mater. 9, 9 (2023).
Fu, Z., Chen, X. & Zhang, Q. Review on the lithium transport mechanism in solid‐state battery materials.Wiley Interdiscip. Rev. Comput. Mol. Sci. https://doi.org/10.1002/wcms.1621 (2022).
Gao, Y. et al. Classical and emerging characterization techniques for investigation of ion transport mechanisms in crystalline fast ionic conductors. Chem. Rev. 120, 5954–6008 (2020).
Hodge, I. M., Ingram, M. D. & West, A. R. Impedance and modulus spectroscopy of polycrystalline solid electrolytes. J. Electroanal. Chem. Interfacial Electrochem. 74, 125–143 (1976).
Lai, W. & Haile, S. M. Impedance spectroscopy as a tool for chemical and electrochemical analysis of mixed conductors: a case study of ceria. J. Am. Ceram. Soc. 88, 2979–2997 (2005).
Kuhn, A., Duppel, V. & Lotsch, B. V. Tetragonal Li10GeP2S12 and Li7GePS8 — exploring the Li ion dynamics in LGPS Li electrolytes. Energy Environ. Sci. 6, 3548 (2013).
Kuhn, A. et al. NMR relaxometry as a versatile tool to study Li ion dynamics in potential battery materials. Solid State Nucl. Magn. Reson. 42, 2–8 (2012).
Klenk, M. J. et al. Lithium self-diffusion in a model lithium garnet oxide Li5La3Ta2O12: a combined quasi-elastic neutron scattering and molecular dynamics study. Solid State Ion. 312, 1–7 (2017).
Sugiyama, J. et al. Li-ion diffusion in Li4Ti5O12 and LiTi2O4 battery materials detected by muon spin spectroscopy. Phys. Rev. B 92, 014417 (2015).
Poletayev, A. D. et al. The persistence of memory in ionic conduction probed by nonlinear optics. Nature 625, 691–696 (2024).
Poletayev, A. D., Dawson, J. A., Islam, M. S. & Lindenberg, A. M. Defect-driven anomalous transport in fast-ion conducting solid electrolytes. Nat. Mater. 21, 1066–1073 (2022).
Frenkel, D. & Smit, B. in Understanding Molecular Simulation 2nd edn (eds Frenkel, D. & Smit, B.) 63–107 (Academic, 2002).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Henkelman, G., Jóhannesson, G. & Jónsson, H. in Theoretical Methods in Condensed Phase Chemistry (ed. Schwartz, S. D.) 269–302 (Kluwer Academic, 2002).
Brown, I. D. Recent developments in the methods and applications of the bond valence model. Chem. Rev. 109, 6858–6919 (2009).
Adams, S. & Rao, R. P. Transport pathways for mobile ions in disordered solids from the analysis of energy-scaled bond-valence mismatch landscapes. Phys. Chem. Chem. Phys. 11, 3210–3216 (2009).
Chen, D. et al. High throughput identification of Li ion diffusion pathways in typical solid state electrolytes and electrode materials by BV-Ewald method. J. Mater. Chem. A 7, 1300–1306 (2018).
Onsager, L. Reciprocal relations in irreversible processes. I. Phys. Rev. 37, 405–426 (1930).
Onsager, L. Reciprocal relations in irreversible processes. II. Phys. Rev. 38, 2265–2279 (1931).
Prigogine, I. Introduction to Thermodynamics of Irreversible Processes 3rd edn (Wiley-Interscience, 1967).
Murch, G. E. The haven ratio in fast ionic conductors. Solid State Ion. 7, 177–198 (1982).
Fong, K. D., Self, J., McCloskey, B. D. & Persson, K. A. Ion correlations and their impact on transport in polymer-based electrolytes. Macromolecules 54, 2575–2591 (2021).
Wills, I. in Thomas Edison: Success and Innovation through Failure 203–222 (Springer, 2020).
Kasper, H. M. Series of rare earth garnets Ln3+3M2Li+3O12 (M = Te, W). Inorg. Chem. 8, 1000–1002 (1969).
Thangadurai, V., Kaack, H. & Weppner, W. J. F. Novel fast lithium ion conduction in garnet‐type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 86, 437–440 (2003).
Li, Y., Han, J.-T., Wang, C.-A., Xie, H. & Goodenough, J. B. Optimizing Li+ conductivity in a garnet framework. J. Mater. Chem. 22, 15357–15361 (2012).
Aono, H., Sugimoto, E., Sadaoka, Y., Imanaka, N. & Adachi, G. Ionic conductivity and sinterability of lithium titanium phosphate system. Solid State Ion. 40, 38–42 (1990).
Murugan, R., Thangadurai, V. & Weppner, W. Fast lithium ion conduction in garnet‐type Li7La3Zr2O12. Angew. Chem. Int Ed. 46, 7778–7781 (2007). This paper reported that garnet-type oxides can be superionic conductors; they are one of the most widely studied solid electrolyte materials today.
Shon, Y.-J. & Min, K. Extracting chemical information from scientific literature using text mining: building an ionic conductivity database for solid-state electrolytes. ACS Omega https://doi.org/10.1021/acsomega.3c01424 (2023).
Wang, Y. et al. Design principles for solid-state lithium superionic conductors. Nat. Mater. 14, 1026–1031 (2015). In this paper, the authors established how different anion frameworks affect Li-ion conductivities, rationalizing the high ionic conductivities in many of the body-centred-cubic sulfide conductors.
Jun, K. et al. Lithium superionic conductors with corner-sharing frameworks. Nat. Mater. 21, 924–931 (2022). This paper rationalized that distorted Li-ion coordination environments as well as weak interaction between Li and non-Li cations lead to fast Li-ion diffusion in corner-sharing frameworks and presented 10 new oxide-based superionic conductors that share a similar structural feature.
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Ong, S. P. et al. Phase stability, electrochemical stability and ionic conductivity of the Li10±1MP2X12 (M = Ge, Si, Sn, Al or P, and X = O, S or Se) family of superionic conductors. Energy Environ. Sci. 6, 148–156 (2012).
YAMANE, H. et al. Crystal structure of a superionic conductor, Li7P3S11. Solid State Ion. 178, 1163–1167 (2007).
Seino, Y., Ota, T., Takada, K., Hayashi, A. & Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 7, 627–631 (2014).
Kaup, K., Zhou, L., Huq, A. & Nazar, L. F. Impact of the Li substructure on the diffusion pathways in alpha and beta Li3PS4: an in situ high temperature neutron diffraction study. J. Mater. Chem. A 8, 12446–12456 (2020).
Kimura, T. et al. Stabilizing high-temperature α-Li3PS4 by rapidly heating the glass. J. Am. Chem. Soc. 145, 14466–14474 (2023).
Kaup, K. et al. Correlation of structure and fast ion conductivity in the solid solution series Li1+2xZn1–xPS4. Chem. Mater. 30, 592–596 (2018).
Brant, J. A. et al. Fast lithium ion conduction in Li2SnS3: synthesis, physicochemical characterization, and electronic structure. Chem. Mater. 27, 189–196 (2015).
Lin, Z., Liu, Z., Dudney, N. J. & Liang, C. Lithium superionic sulfide cathode for all-solid lithium–sulfur batteries. ACS Nano 7, 2829–2833 (2013).
Roh, J., Lyoo, J. & Hong, S.-T. Enhanced Li-ion conductivity and air stability of Sb-substituted Li4GeS4 toward all-solid-state Li-ion batteries. ACS Appl. Energy Mater. 6, 5446–5455 (2023).
Lyoo, J., Kim, H. J., Hyoung, J., Chae, M. S. & Hong, S.-T. Zn substituted Li4P2S6 as a solid lithium-ion electrolyte for all-solid-state lithium batteries. J. Solid State Chem. 320, 123861 (2023).
Liu, Z. et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 135, 975–978 (2013).
Roh, J., Do, N., Manjón-Sanz, A. & Hong, S.-T. Li2GeS3: lithium ionic conductor with an unprecedented structural type. Inorg. Chem. 62, 15856–15863 (2023).
Gamon, J. et al. Computationally guided discovery of the sulfide Li3AlS3 in the Li–Al–S phase field: structure and lithium conductivity. Chem. Mater. 31, 9699–9714 (2019).
Murayama, M., Sonoyama, N., Yamada, A. & Kanno, R. Material design of new lithium ionic conductor, thio-LISICON, in the Li2S–P2S5 system. Solid State Ion. 170, 173–180 (2004).
Kimura, T. et al. Preparation and characterization of lithium ion conductive Li3SbS4 glass and glass-ceramic electrolytes. Solid State Ion. 333, 45–49 (2019).
Kanno, R., Hata, T., Kawamoto, Y. & Irie, M. Synthesis of a new lithium ionic conductor, thio-LISICON–lithium germanium sulfide system. Solid State Ion. 130, 97–104 (2000).
Kwak, H. et al. Li+ conduction in air-stable Sb-substituted Li4SnS4 for all-solid-state Li-Ion batteries. J. Power Sources 446, 227338 (2020).
Lim, H., Kim, S.-C., Kim, J., Kim, Y.-I. & Kim, S.-J. Structure of Li5AlS4 and comparison with other lithium-containing metal sulfides. J. Solid State Chem. 257, 19–25 (2018).
Huber, S., Preitschaft, C., Weihrich, R. & Pfitzner, A. Preparation, crystal structure, electronic structure, impedance spectroscopy, and Raman spectroscopy of Li3SbS3 and Li3AsS3. Z. Anorg. Allg. Chem. 638, 2542–2548 (2012).
Zhou, L., Minafra, N., Zeier, W. G. & Nazar, L. F. Innovative approaches to Li-argyrodite solid electrolytes for all-solid-state lithium batteries. Acc. Chem. Res. 54, 2717–2728 (2021).
Richards, W. D., Wang, Y., Miara, L. J., Kim, J. C. & Ceder, G. Design of Li1+2xZn1−xPS4, a new lithium ion conductor. Energy Environ. Sci. 9, 3272–3278 (2016).
Suzuki, N. et al. Synthesis and electrochemical properties of I¯4 type Li1+2xZn1−xPS4 solid electrolyte. Chem. Mater. https://doi.org/10.1021/acs.chemmater.7b03833 (2018).
Wang, C., Liang, J., Kim, J. T. & Sun, X. Prospects of halide-based all-solid-state batteries: from material design to practical application. Sci. Adv. 8, eadc9516 (2022).
Wang, S. et al. Lithium chlorides and bromides as promising solid‐state chemistries for fast ion conductors with good electrochemical stability. Angew. Chem. Int. Ed. 58, 8039–8043 (2019).
Park, D. et al. Theoretical design of lithium chloride superionic conductors for all-solid-state high-voltage lithium-ion batteries. ACS Appl. Mater. Interfaces 12, 34806–34814 (2020).
Kanno, R. & Murayama, M. Lithium ionic conductor thio-LISICON: the Li2S GeS2 P2S5 system. J. Electrochem. Soc. 148, A742–A746 (2001).
Hong, H. Y.-P. Crystal structure and ionic conductivity of Li14Zn(GeO4)4 and other new Li+ superionic conductors. Mater. Res Bull. 13, 117–124 (1978).
Aono, H. Ionic conductivity of solid electrolytes based on lithium titanium phosphate. J. Electrochem. Soc. 137, 1023 (1990).
Kim, J., Kim, J., Avdeev, M., Yun, H. & Kim, S.-J. LiTa2PO8: a fast lithium-ion conductor with new framework structure. J. Mater. Chem. A 6, 22478–22482 (2018).
Wang, Q. et al. A new lithium‐ion conductor LiTaSiO5: theoretical prediction, materials synthesis, and ionic conductivity. Adv. Funct. Mater. 29, 1904232 (2019).
Xiong, S. et al. Computation‐guided design of LiTaSiO5, a new lithium ionic conductor with sphene structure. Adv. Energy Mater. 9, 1803821 (2019).
Kang, K., Meng, Y. S., Bréger, J., Grey, C. P. & Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 311, 977–980 (2006).
der Ven, A. V. & Ceder, G. Lithium diffusion mechanisms in layered intercalation compounds. J. Power Sources 97, 529–531 (2001).
Yu, S. et al. Design of a trigonal halide superionic conductor by regulating cation order–disorder. Science 382, 573–579 (2023). This paper reported that in the Li3MCl6 trigonal chloride superionic conductor, two factors, in-plane Li percolation paths and stacking interlayer distances, govern Li-ion conductivity, and they are inversely correlated with each other.
Peng, J. et al. Fast lithium ion conductivity in layered (Li-Ag)CrS2. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.0c08448 (2020).
Delmas, C. et al. On the behavior of the LixNiO2 system: an electrochemical and structural overview. J. Power Sources 68, 120–125 (1997).
Samsonov, G. V. The Oxide Handbook (Springer, 1973).
Jacobs, P. W. M. & Vernon, M. L. Defect energies for magnesium oxide and lithium oxide. J. Chem. Soc. Faraday Trans. 86, 1233–1238 (1990).
Stefano, D. D. et al. Superionic diffusion through frustrated energy landscape. Chem 5, 2450–2460 (2019). This paper reported that in the novel superionic conductor LiTi2(PS4)3 the highly distorted Li-ion sites provide a smooth and frustrated energy landscape.
Waroquiers, D. et al. Statistical analysis of coordination environments in oxides. Chem. Mater. https://doi.org/10.1021/acs.chemmater.7b02766 (2017).
Inaguma, Y., Katsumata, T., Itoh, M. & Morii, Y. Crystal structure of a lithium ion-conducting perovskite La2/3−xLi3xTiO3 (x = 0.05). J. Solid State Chem. 166, 67–72 (2002).
Inaguma, Y. et al. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 86, 689–693 (1993).
He, X. et al. Crystal structural framework of lithium super‐ionic conductors. Adv. Energy Mater. 9, 1902078 (2019).
Aono, H., Sugimoto, E., Sadaoka, Y., Imanaka, N. & Adachi, G. Ionic conductivity of the lithium titanium phosphate (Li1+XMXTi2−X(PO4)3, M = Al, Sc, Y, and La) systems. J. Electrochem. Soc. 136, 590–591 (1989).
Arbi, K., Mandal, S., Rojo, J. M. & Sanz, J. Dependence of ionic conductivity on composition of fast ionic conductors Li1+xTi2−xAlx(PO4)3, 0 ≤ x ≤ 0.7. A parallel NMR and electric impedance study. Chem. Mater. 14, 1091–1097 (2002).
Rossbach, A., Tietz, F. & Grieshammer, S. Structural and transport properties of lithium-conducting NASICON materials. J. Power Sources 391, 1–9 (2018).
Cussen, E. J. Structure and ionic conductivity in lithium garnets. J. Mater. Chem. 20, 5167–5173 (2010).
Xiao, Y. et al. Lithium oxide superionic conductors inspired by garnet and NASICON structures. Adv. Energy Mater. 11, 2101437 (2021). This paper suggested the concept of the activated diffusion network as the common feature of Li stuffing in garnet and NASICON-type superionic conductors, resulting in a graph-based criterion to search for materials similar to garnets and NASICONs.
Deng, B. et al. CHGNet as a pretrained universal neural network potential for charge-informed atomistic modelling. Nat. Mach. Intell. 5, 1031–1041 (2023).
Kozinsky, B. et al. Effects of sublattice symmetry and frustration on ionic transport in garnet solid electrolytes. Phys. Rev. Lett. 116, 055901 (2016).
Xu, M. et al. Mechanisms of Li+ transport in garnet-type cubic Li3+xLa3M2O12 (M = Te, Nb, Zr). Phys. Rev. B 85, 052301 (2012).
Chen, Y. et al. Unlocking Li superionic conductivity in face-centred cubic oxides via face-sharing configurations. Nat. Mater. 23, 535–542 (2024). A face-centred-cubic anion sublattice is generally not considered favourable for Li-ion diffusion, but this paper showed that by introducing face-sharing Li configurations, superionic conductivity can be achieved in close-packed oxides.
Liu, H. et al. A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 585, 63–67 (2020).
Zhang, W. et al. Kinetic pathways of ionic transport in fast-charging lithium titanate. Science 367, 1030–1034 (2020).
He, X., Zhu, Y. & Mo, Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 8, 15893 (2017). This paper demonstrated that in many prototypical Li superionic conductors, Li-ion hops are correlated with each other, with multiple Li ions hopping simultaneously.
Liu, Y., Wang, S., Nolan, A. M., Ling, C., & Mo, Y. Tailoring the cation lattice for chloride lithium‐ion conductors. Adv. Energy Mater. https://doi.org/10.1002/aenm.202002356 (2020).
Zhou, L. et al. A new halospinel superionic conductor for high-voltage all solid state lithium batteries. Energy Environ. Sci. 13, 2056–2063 (2020).
Park, K.-H. et al. High-voltage superionic halide solid electrolytes for all-solid-state Li-ion batteries. ACS Energy Lett. 5, 533–539 (2020).
Kvist, A. & Bengztelius, A. in Fast Ion Transport in Solids (ed. van Gool, W.) 193–199 (North-Holland, 1972).
Aronsson, R., Knape, H. E. G., Lundén, A., Nilsson, L. & Torell, L. M. Neutron, X-ray and Brillouin scattering studies of rotator phases with fast ion conduction. Solid State Ion. 5, 445–447 (1981).
Dissanayake, M. A. K. L., Careem, M. A., Bandaranayake, P. W. S. K. & Wijayasekera, C. N. Ionic conductivity of solid solutions of α-Li2SO4 with Li2WO4: strong evidence for the paddle wheel mechanism of ion transport. Solid State Ion. 48, 277–281 (1991).
Lundén, A. Enhancement of cation mobility in some sulphate phases due to a paddle-wheel mechanism. Solid State Ion. 28, 163–167 (1988).
Secco, E. A. Electrical conductivity measurements to test for rotating sulfate ions in fast ion conductors. Phys. Status Solidi 88, K75–K77 (1985).
Gundusharma, U. M., MacLean, C. & Secco, E. A. Rotating sulfate ion contribution to electrical conductivity in Li2SO4 and LiNaSO4? Solid State Commun. 57, 479–481 (1986).
Jansen, M. Volume effect or paddle‐wheel mechanism — fast alkali‐metal ionic conduction in solids with rotationally disordered complex anions. Angew. Chem. Int. Ed. Engl. 30, 1547–1558 (1991).
Tsai, P. et al. Double paddle‐wheel enhanced sodium ion conduction in an antiperovskite solid electrolyte. Adv. Energy Mater. 13, 2203284 (2023).
Smith, J. G. & Siegel, D. J. Low-temperature paddlewheel effect in glassy solid electrolytes. Nat. Commun. 11, 1483 (2020).
Zhang, Z., Roy, P.-N., Li, H., Avdeev, M. & Nazar, L. F. Coupled cation–anion dynamics enhances cation mobility in room-temperature superionic solid-state electrolytes. J. Am. Chem. Soc. 141, 19360–19372 (2019).
Zhang, Z., & Nazar, L. F. Exploiting the paddle-wheel mechanism for the design of fast ion conductors. Nat. Rev. Mater. https://doi.org/10.1038/s41578-021-00401-0 (2022).
Zhang, Z. et al. Targeting superionic conductivity by turning on anion rotation at room temperature in fast ion conductors. Matter 2, 1667–1684 (2020).
Verdal, N. et al. Anion reorientations in the superionic conducting phase of Na2B12H12. J. Phys. Chem. C 118, 17483–17489 (2014).
Dimitrievska, M. et al. Carbon incorporation and anion dynamics as synergistic drivers for ultrafast diffusion in superionic LiCB11H12 and NaCB11H12. Adv. Energy Mater. 8, 1703422 (2018).
Sun, Y. et al. Rotational cluster anion enabling superionic conductivity in sodium-rich antiperovskite Na3OBH4. J. Am. Chem. Soc. 141, 5640–5644 (2019).
Fang, H. & Jena, P. Argyrodite-type advanced lithium conductors and transport mechanisms beyond paddle-wheel effect. Nat. Commun. 13, 2078 (2022).
Jun, K., Lee, B., Kam, R. & Ceder, G. The non-existence of a paddlewheel effect in superionic conductors. Proc. Natl Acad. Sci. USA 121, e2316493121 (2024). This paper investigated what types of rotational motion of anion groups exist in fast Li-ion conductors and showed that large-angle rotational motion as implied by the term ‘paddlewheel effect’ is not correlated to Li-ion diffusion.
Udovic, T. J. et al. Exceptional superionic conductivity in disordered sodium decahydro‐closo‐decaborate. Adv. Mater. 26, 7622–7626 (2014).
Wilmer, D., Feldmann, H., Lechner, R. E. & Combet, J. Sodium ion conduction in plastic phases: dynamic coupling of cations and anions in the picosecond range. J. Mater. Res. 20, 1973–1978 (2005).
Kweon, K. E. et al. Structural, chemical, and dynamical frustration: origins of superionic conductivity in closo-borate solid electrolytes. Chem. Mater. 29, 9142–9153 (2017).
Hanghofer, I., Gadermaier, B. & Wilkening, H. M. R. Fast rotational dynamics in argyrodite-type Li6PS5X (X: Cl, Br, I) as seen by 31P nuclear magnetic relaxation — on cation–anion coupled transport in thiophosphates. Chem. Mater. 31, 4591–4597 (2019).
Brinek, M., Hiebl, C., Hogrefe, K., Hanghofer, I. & Wilkening, H. M. R. Structural disorder in Li6PS5I speeds 7Li nuclear spin recovery and slows down 31P relaxation — implications for translational and rotational jumps as seen by nuclear magnetic resonance. J. Phys. Chem. C 124, 22934–22940 (2020).
Huynh, D. Q. Metrics for 3D rotations: comparison and analysis. J. Math. Imaging Vis. 35, 155–164 (2009).
Rettie, A. J. E. et al. A two-dimensional type I superionic conductor. Nat. Mater. 20, 1683–1688 (2021).
Gupta, M. K. et al. Fast Na diffusion and anharmonic phonon dynamics in superionic Na3PS4. Energy Environ. Sci. 14, 6554–6563 (2021).
Gupta, M. K. et al. Strongly anharmonic phonons and their role in superionic diffusion and ultralow thermal conductivity of Cu7PSe6. Adv. Energy Mater. https://doi.org/10.1002/aenm.202200596 (2022).
Xu, Z., Chen, X., Zhu, H. & Li, X. Anharmonic cation–anion coupling dynamics assisted lithium‐ion diffusion in sulfide solid electrolytes. Adv. Mater. 34, 2207411 (2022).
Wang, H. et al. Borohydride substitution effects of Li6PS5Cl solid electrolyte. ACS Appl. Energy Mater. 4, 12079–12083 (2021).
Sun, Y. et al. Enhanced ionic conductivity and lack of paddle-wheel effect in pseudohalogen-substituted Li argyrodites. Matter 5, 4379–4395 (2022).
Han, J.-H. et al. Borohydride and halide dual-substituted lithium argyrodites. Mater. Horiz. 11, 251–261 (2023).
Fang, H. & Jena, P. Li-rich antiperovskite superionic conductors based on cluster ions. Proc. Natl Acad. Sci. USA 114, 11046–11051 (2017).
Jang, Y. et al. Lithium superionic conduction in BH4‐substituted thiophosphate solid electrolytes. Adv. Sci. 10, 2204942 (2023).
Muy, S. et al. High-throughput screening of solid-state Li-ion conductors using lattice-dynamics descriptors. iScience 16, 270–282 (2019).
Muy, S. et al. Tuning mobility and stability of lithium ion conductors based on lattice dynamics. Energy Environ. Sci. 11, 850–859 (2018).
Muy, S., Schlem, R., Shao-Horn, Y. & Zeier, W. G. Phonon–ion interactions: designing ion mobility based on lattice dynamics. Adv. Energy Mater. https://doi.org/10.1002/aenm.202002787 (2020).
Deng, Y. et al. Enhancing the lithium ion conductivity in lithium superionic conductor (LISICON) solid electrolytes through a mixed polyanion effect. ACS Appl. Mater. Inter. 9, 7050–7058 (2017).
Kraft, M. A. et al. Influence of lattice polarizability on the ionic conductivity in the lithium superionic argyrodites Li6PS5X (X = Cl, Br, I). J. Am. Chem. Soc. 139, 10909–10918 (2017). This paper systematically investigated the effect of anion framework polarizability on the ionic conductivity by tuning the fractional occupancy of halide ions in argyrodite-type superionic conductors, highlighting the importance of lattice stiffness on optimizing ionic conductivity.
Manthiram, A. & Goodenough, J. B. Lithium insertion into Fe2(SO4)3 frameworks. J. Power Sources 26, 403–408 (1989).
Etourneau, J., Portier, J. & Ménil, F. The role of the inductive effect in solid state chemistry: how the chemist can use it to modify both the structural and the physical properties of the materials. J. Alloy. Compd. 188, 1–7 (1992).
Krauskopf, T., Culver, S. P. & Zeier, W. G. Bottleneck of diffusion and inductive effects in Li10Ge1–xSnxP2S. Chem. Mater. 30, 1791–1798 (2018).
Culver, S. P. et al. Evidence for a solid-electrolyte inductive effect in the superionic conductor Li10Ge1–xSnxP2S12. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.0c10735 (2020). This paper demonstrated that the change of chemical bonding within the framework of the Li10Ge1−xSnxP2S12 system and its effect on ionic conductivity (inductive) can be quantified, making it a viable strategy to optimize ionic conductivities.
Pareek, T. et al. LiSnZr(PO4)3: NASICON-type solid electrolyte with excellent room temperature Li+ conductivity. J. Alloy Compd. 777, 602–611 (2019).
Zhang, Z. et al. Na11Sn2PS12: a new solid state sodium superionic conductor. Energy Environ. Sci. 11, 87–93 (2017).
Yu, Z. et al. Exceptionally high ionic conductivity in Na3P0.62As0.38S4 with improved moisture stability for solid‐state sodium‐ion batteries. Adv. Mater. https://doi.org/10.1002/adma.201605561 (2017).
Zeng, Y. et al. High-entropy mechanism to boost ionic conductivity. Science 378, 1320–1324 (2022). This paper demonstrated that the high-entropy mechanism can improve ionic conductivities and elucidated how high entropy affects the site energy distributions to enhance lithium-ion conductivity.
Lin, J. et al. A high-entropy multicationic substituted lithium argyrodite superionic solid electrolyte. ACS Mater. Lett. 4, 2187–2194 (2022).
Stramare, S., Thangadurai, V. & Weppner, W. Lithium lanthanum titanates: a review. Chem. Mater. 15, 3974–3990 (2003).
Harada, Y., Ishigaki, T., Kawai, H. & Kuwano, J. Lithium ion conductivity of polycrystalline perovskite La0.67−xLi3xTiO3 with ordered and disordered arrangements of the A-site ions. Solid State Ion. 108, 407–413 (1998).
Harada, Y., Hirakoso, Y., Kawai, H. & Kuwano, J. Order–disorder of the A-site ions and lithium ion conductivity in the perovskite solid solution La0.67−xLi3xTiO3 (x = 0.11). Solid State Ion. 121, 245–251 (1999). This paper was presented at the 11th International Conference on Solid State Ionics, Hawaii, 1997.
Schlem, R. et al. Mechanochemical synthesis: a tool to tune cation site disorder and ionic transport properties of Li3MCl6 (M = Y, Er) superionic conductors. Adv. Energy Mater. 10, 1903719 (2020). This paper reported the use of various synthesis methods to tune the degree of cation disorder in Li3Y/ErCl6 and demonstrated that cation disorder obtained via mechanochemical synthesis results in substantially higher ionic conductivity.
Wang, K. et al. A cost-effective and humidity-tolerant chloride solid electrolyte for lithium batteries. Nat. Commun. 12, 4410 (2021).
Liang, J., Li, X., Adair, K. R. & Sun, X. Metal halide superionic conductors for all-solid-state batteries. Acc. Chem. Res. 54, 1023–1033 (2021).
Tanaka, Y. et al. New oxyhalide solid electrolytes with high lithium ionic conductivity >10 mS cm−1 for all‐solid‐state batteries. Angew. Chem. Int. Ed. 62, e202217581 (2023). This paper reported LiMOCl4 (M = Ta, Nb), with an ionic conductivity of up to 12.4 mS cm−1 at room temperature, the highest among any non-sulfide-based conductors, opening up opportunities in the oxychloride chemistry.
Rayavarapu, P. R., Sharma, N., Peterson, V. K. & Adams, S. Variation in structure and Li+-ion migration in argyrodite-type Li6PS5X (X = Cl, Br, I) solid electrolytes. J. Solid State Electrochem. 16, 1807–1813 (2012).
Gautam, A. et al. Engineering the site‐disorder and lithium distribution in the lithium superionic argyrodite Li6PS5Br. Adv. Energy Mater. https://doi.org/10.1002/aenm.202003369 (2020).
Morgan, B. J. Mechanistic origin of superionic lithium diffusion in anion-disordered Li6PS5X argyrodites. Chem. Mater. 33, 2004–2018 (2021).
Liu, Z. et al. High ionic conductivity achieved in Li3Y(Br3Cl3) mixed halide solid electrolyte via promoted diffusion pathways and enhanced grain boundary. ACS Energy Lett. 6, 298–304 (2021).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Feng, X., Chien, P.-H., Patel, S., Wang, Y. & Hu, Y.-Y. Enhanced ion conduction in Li2.5Zn0.25PS4 via anion doping. Chem. Mater. 32, 3036–3042 (2020).
Samson, A. J., Hofstetter, K., Bag, S. & Thangadurai, V. A bird’s-eye view of Li-stuffed garnet-type Li7La3Zr2O12 ceramic electrolytes for advanced all-solid-state Li batteries. Energy Environ. Sci. 12, 2957–2975 (2019).
Yu, C., Zhao, F., Luo, J., Zhang, L. & Sun, X. Recent development of lithium argyrodite solid-state electrolytes for solid-state batteries: synthesis, structure, stability and dynamics. Nano Energy 83, 105858 (2021).
Hou, M., Liang, F., Chen, K., Dai, Y. & Xue, D. Challenges and perspectives of NASICON-type solid electrolytes for all-solid-state lithium batteries. Nanotechnology 31, 132003 (2020).
Kato, Y., Hori, S., & Kanno, R. Li10GeP2S12‐type superionic conductors: synthesis, structure, and ionic transportation. Adv. Energy Mater. https://doi.org/10.1002/aenm.202002153 (2020).
Zheng, J., Perry, B. & Wu, Y. Antiperovskite superionic conductors: a critical review. ACS Mater. Au 1, 92–106 (2021).
Li, X. et al. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 13, 1429–1461 (2020).
Bruce, P. G. & West, A. R. Ionic conductivity of LISICON solid solutions, Li2+2xZn1−xGeO4. J. Solid State Chem. 44, 354–365 (1982).
Bruce, P. G. & West, A. R. Phase diagram of the LISICON, solid electrolyte system, Li4GeO4–Zn2GeO4. Mater. Res. Bull. 15, 379–385 (1980).
Whiteley, J. M., Woo, J. H., Hu, E., Nam, K.-W. & Lee, S.-H. Empowering the lithium metal battery through a silicon-based superionic conductor. J. Electrochem. Soc. 161, A1812–A1817 (2014).
Bron, P. et al. Li10SnP2S12: an affordable lithium superionic conductor. J. Am. Chem. Soc. 135, 15694–15697 (2013).
Bron, P., Dehnen, S. & Roling, B. Li10Si0.3Sn0.7P2S12 — a low-cost and low-grain-boundary-resistance lithium superionic conductor. J. Power Sources 329, 530–535 (2016).
Yang, K., Dong, J., Zhang, L., Li, Y. & Wang, L. Dual doping: an effective method to enhance the electrochemical properties of Li10GeP2S12‐based solid electrolytes. J. Am. Ceram. Soc. 98, 3831–3835 (2015).
Sun, Y., Suzuki, K., Hori, S., Hirayama, M. & Kanno, R. Superionic conductors: Li10+δ[SnySi1–y]1+δP2−δS12 with a Li10GeP2S12-type structure in the Li3PS4–Li4SnS4–Li4SiS4 quasi-ternary system. Chem. Mater. 29, 5858–5864 (2017).
Li, Y. et al. A lithium superionic conductor for millimeter-thick battery electrode. Science 381, 50–53 (2023). This paper showed that using a high-entropy mechanism, an LGPS-type superionic conductor can reach a conductivity of up to 32 mS cm−1 at room temperature.
Goodenough, J. B., Hong, H. Y.-P. & Kafalas, J. A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 11, 203–220 (1976).
Taylor, B. E., English, A. D. & Berzins, T. New solid ionic conductors. Mater. Res. Bull. 12, 171–181 (1977).
Yamamoto, H., Tabuchi, M., Takeuchi, T., Kageyama, H. & Nakamura, O. Ionic conductivity enhancement in LiGe2(PO4)3 solid electrolyte. J. Power Sources 68, 397–401 (1997).
Arbi, K., Rojo, J. M. & Sanz, J. Lithium mobility in titanium based Nasicon Li1+xTi2−xAlx(PO4)3 and LiTi2−xZrx(PO4)3 materials followed by NMR and impedance spectroscopy. J. Eur. Ceram. Soc. 27, 4215–4218 (2007).
Subramanian, M. A., Subramanian, R. & Clearfield, A. Lithium ion conductors in the system AB(IV)2(PO4)3 (B = Ti, Zr and Hf). Solid State Ion. 18, 562–569 (1986).
Yi, E. et al. Materials that can replace liquid electrolytes in Li batteries: superionic conductivities in Li1.7Al0.3Ti1.7Si0.4P2.6O12. Processing combustion synthesized nanopowders to free standing thin films. J. Power Sources 269, 577–588 (2014).
Liu, M. et al. Facile synthesis and electrochemical properties of high lithium ionic conductivity Li1.7Al0.3Ti1.7Si0.4P2.6O12 ceramic solid electrolyte. J. Alloys Compd. 756, 103–110 (2018).
Thangadurai, V., Narayanan, S. & Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem. Soc. Rev. 43, 4714–4727 (2014).
Geiger, C. A. et al. Crystal chemistry and stability of ‘Li7La3Zr2O12’ garnet: a fast lithium-ion conductor. Inorg. Chem. 50, 1089–1097 (2011).
Vema, S., Berge, A. H., Nagendran, S. & Grey, C. P. Clarifying the dopant local structure and effect on ionic conductivity in garnet solid-state electrolytes for lithium-ion batteries. Chem. Mater. 35, 9632–9646 (2023).
O’Callaghan, M. P. & Cussen, E. J. Lithium dimer formation in the Li-conducting garnets Li5+xBaxLa3−xTa2O12 (0 < x ≤ 1.6). Chem. Commun. 2007, 2048–2050 (2007).
Xie, H., Alonso, J. A., Li, Y., Fernández-Díaz, M. T. & Goodenough, J. B. Lithium distribution in aluminum-free cubic Li7La3Zr2O12. Chem. Mater. 23, 3587–3589 (2011).
Jalem, R. et al. Concerted migration mechanism in the Li ion dynamics of garnet-type Li7La3Zr2O12. Chem. Mater. 25, 425–430 (2013).
Awaka, J., Kijima, N., Hayakawa, H. & Akimoto, J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J. Solid State Chem. 182, 2046–2052 (2009).
Wolfenstine, J., Rangasamy, E., Allen, J. L. & Sakamoto, J. High conductivity of dense tetragonal Li7La3Zr2O12. J. Power Sources 208, 193–196 (2012).
Deiseroth, H.-J. et al. Li6PS5X: a class of crystalline li-rich solids with an unusually high Li+ mobility. Angew. Chem. Int. Ed. 47, 755–758 (2008).
Kam, R. L. et al. Crystal structures and phase stability of the Li2S–P2S5 system from first principles. Chem. Mater. 35, 9111–9126 (2023).
Deiseroth, H. et al. Li7PS6 and Li6PS5X (X: Cl, Br, I): possible three‐dimensional diffusion pathways for lithium ions and temperature dependence of the ionic conductivity by impedance measurements. Z. Anorg. Allg. Chem. 637, 1287–1294 (2011).
Ziolkowska, D. A., Arnold, W., Druffel, T., Sunkara, M. & Wang, H. Rapid and economic synthesis of a Li7PS6 solid electrolyte from a liquid approach. ACS Appl. Mater. Interfaces 11, 6015–6021 (2019).
Patel, S. V. et al. Tunable lithium-ion transport in mixed-halide argyrodites Li6–xPS5–xClBrx: an unusual compositional space. Chem. Mater. 33, 1435–1443 (2021).
Zhou, L., Assoud, A., Zhang, Q., Wu, X. & Nazar, L. F. New family of argyrodite thioantimonate lithium superionic conductors. J. Am. Chem. Soc. 141, 19002–19013 (2019). This paper demonstrated that Sb substitution and modification of Li content can lead to an ionic conductivity of 24 mS cm−1 at room temperature, one of the highest values reported for the argyrodite family.
Baktash, A., Reid, J. C., Roman, T. & Searles, D. J. Diffusion of lithium ions in lithium-argyrodite solid-state electrolytes. npj Comput. Mater. 6, 162 (2020).
Feng, X. et al. Enhanced ion conduction by enforcing structural disorder in Li-deficient argyrodites Li6−xPS5−xCl1+x. Energy Storage Mater. 30, 67–73 (2020).
Yu, C. et al. Enabling ultrafast ionic conductivity in Br-based lithium argyrodite electrolytes for solid-state batteries with different anodes. Energy Storage Mater. 30, 238–249 (2020).
Jung, W. D. et al. Superionic halogen-rich li-argyrodites using in situ nanocrystal nucleation and rapid crystal growth. Nano Lett. 20, 2303–2309 (2020).
Yu, C. et al. Superionic conductivity in lithium argyrodite solid-state electrolyte by controlled Cl-doping. Nano Energy 69, 104396 (2020).
Zhang, Z. et al. Enhancing ionic conductivity of solid electrolyte by lithium substitution in halogenated Li-argyrodite. J. Power Sources 450, 227601 (2020).
Schneider, H. et al. Stabilization of highly conductive lithium argyrodites by means of lithium substitution: the case of Li6Fe0.5PS6. ChemistrySelect 4, 3351–3354 (2019).
Zhang, Z. et al. Design and synthesis of room temperature stable Li-argyrodite superionic conductors via cation doping. J. Mater. Chem. A https://doi.org/10.1039/c8ta10790d (2019).
Minafra, N., Culver, S. P., Krauskopf, T., Senyshyn, A. & Zeier, W. G. Effect of Si substitution on the structural and transport properties of superionic Li-argyrodites. J. Mater. Chem. A 6, 645–651 (2017).
Schneider, H. et al. A novel class of halogen-free, super-conductive lithium argyrodites: synthesis and characterization. J. Power Sources 366, 151–160 (2017).
Kraft, M. A. et al. Inducing high ionic conductivity in the lithium superionic argyrodites Li6+xP1−xGexS5I for all-solid-state batteries. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.8b10282 (2018).
Kanno, R., Takeda, Y., Takada, K. & Yamamoto, O. Phase diagram and ionic conductivity of the lithium chloride–iron(II) chloride system. Solid State Ion. 9, 153–156 (1983).
Steiner, H. ‐J. & Lutz, H. D. Neue schnelle Ionenleiter vom Typ MMIIICl6 (MI = Li, Na, Ag; MIII = In, Y). Z. Anorg. Allg. Chem. 613, 26–30 (1992).
Asano, T. et al. Solid halide electrolytes with high lithium‐ion conductivity for application in 4 V class bulk‐type all‐solid‐state batteries. Adv. Mater. 30, 1803075 (2018). This paper demonstrated that Li3YCl6 and Li3YBr6 are superionic conductors at room temperature, which led to extensive research in close-packed chloride chemistries for Li-ion conductors.
Li, X. et al. Structural regulation of halide superionic conductors for all-solid-state lithium batteries. Nat. Commun. 15, 53 (2024).
Wang, Q. et al. Designing lithium halide solid electrolytes. Nat. Commun. 15, 1050 (2024).
Li, X. et al. Origin of superionic Li3Y1–xInxCl6 halide solid electrolytes with high humidity tolerance. Nano Lett. 20, 4384–4392 (2020).
Chen, S. et al. Enabling ultrafast lithium-ion conductivity of Li2ZrCl6 by indium doping. Chin. Chem. Lett. 33, 4635–4639 (2022).
Kwak, H. et al. New cost‐effective halide solid electrolytes for all‐solid‐state batteries: mechanochemically prepared Fe3+‐substituted Li2ZrCl6. Adv. Energy Mater. 11, 2003190 (2021).
Tomita, Y., Fuji-i, A., Ohki, H., Yamada, K. & Okuda, T. New lithium ion conductor Li3InBr6 studied by 7Li NMR. Chem. Lett. 27, 223–224 (1998).
Li, X. et al. Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries. Energy Environ. Sci. 2019, 2665–2671 (2019).
Liang, J. et al. Site-occupation-tuned superionic LixScCl3+x halide solid electrolytes for all-solid-state batteries. J. Am. Chem. Soc. 142, 7012–7022 (2020).
Lutz, H. D., Schmidt, W. & Haeuseler, H. Chloride spinels: a new group of solid lithium electrolytes. J. Phys. Chem. Solids 42, 287–289 (1981).
Luo, J. et al. Halide superionic conductors with non‐close‐packed anion frameworks. Angew. Chem. Int. Ed. 63, e202400424 (2024).
Kim, R. et al. Computational design and experimental synthesis of air-stable solid-state ionic conductors with high conductivity. Chem. Mater. 33, 6909–6917 (2021).
Adams, S. Origin of fast Li+-ion conductivity in the compressible oxyhalide LiNbOCl4. Energy Storage Mater. 38, 103359 (2024).
Jun, K., Wei, G. & Ceder, G. Thermodynamic stability and diffusion mechanism of LiMXCl4 superionic conductors. Preprint at https://doi.org/10.26434/chemrxiv-2024-nm8ks (2024).
Yin, Y.-C. et al. A LaCl3-based lithium superionic conductor compatible with lithium metal. Nature 616, 77–83 (2023). This paper reported the development of a new type of non-close-packed LaCl3 superionic conductor obtained by stuffing extra Li into the structure and introducing La vacancies, demonstrating that non-close-packed chlorides can exhibit superionic conductivity.
Malik, R., Burch, D., Bazant, M. & Ceder, G. Particle size dependence of the ionic diffusivity. Nano Lett. 10, 4123–4127 (2010).
Zhong, P., Gupta, S., Deng, B., Jun, K. & Ceder, G. Effect of cation disorder on lithium transport in halide superionic conductors. ACS Energy Lett. 9, 2775–2781 (2024).
Batatia, I. et al. A foundation model for atomistic materials chemistry. Preprint at https://arxiv.org/abs/2401.00096 (2023).
Min, J., Gubow, L. M., Hargrave, R. J., Siegel, J. B. & Li, Y. Direct measurements of size-independent lithium diffusion and reaction times in individual polycrystalline battery particles. Energy Environ. Sci. 16, 3847–3859 (2023).
Kahle, L., Marcolongo, A. & Marzari, N. High-throughput computational screening for solid-state Li-ion conductors. Energy Environ. Sci. https://doi.org/10.1039/c9ee02457c (2020).
Zhang, Y. et al. Unsupervised discovery of solid-state lithium ion conductors. Nat. Commun. 10, 5260 (2019).
Sebti, E. et al. Stacking faults assist lithium-ion conduction in a halide-based superionic conductor. J. Am. Chem. Soc. 144, 5795–5811 (2022).
Gautam, A. et al. Rapid crystallization and kinetic freezing of site-disorder in the lithium superionic argyrodite Li6PS5Br. Chem. Mater. 31, 10178–10185 (2019).
Szymanski, N. J. et al. An autonomous laboratory for the accelerated synthesis of novel materials. Nature 624, 86–91 (2023).
Lunt, A. M. et al. Modular, multi-robot integration of laboratories: an autonomous workflow for solid-state chemistry. Chem. Sci. 15, 2456–2463 (2023).
Chen, J. et al. Navigating phase diagram complexity to guide robotic inorganic materials synthesis. Nat. Synth. 3, 606–614 (2023).
Chen, C., Lu, Z. & Ciucci, F. Data mining of molecular dynamics data reveals Li diffusion characteristics in garnet Li7La3Zr2O12. Sci. Rep. 7, 40769 (2017).
Jain, A. et al. Commentary. The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Acknowledgements
This work was funded by the Assistant Secretary of Energy Efficiency and Renewable Energy, Vehicle Technologies Office of the US Department of Energy (DOE), under contract number DE-AC02-05CH11231 under the Advanced Battery Materials (BMR) programme. Earlier work was performed with the support of Samsung Electronics. Earlier computational work utilized the resources of the National Energy Research Scientific Computing Center (NERSC), a US Department of Energy Office of Science user facility, as well as the clusters at the National Renewable Energy Laboratory (NREL) under saepssic and ahlssic allocations. K.J. acknowledges support from Kwanjeong Educational Foundation scholarship. G.W. acknowledges support by the US DOE, Office of Science, Office of Advanced Scientific Computing Research, Department of Energy Computational Science Graduate Fellowship under award number DE-SC0023112. This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation or favouring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Author information
Authors and Affiliations
Contributions
K.J., Y.C., G.W. and X.Y. researched data for the article. K.J. and G.C. led the drafting of the manuscript. All authors contributed substantially to discussion of the content and drafting of the article. K.J. and G.C. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks James Dawson and Yoon Seok Jung for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jun, K., Chen, Y., Wei, G. et al. Diffusion mechanisms of fast lithium-ion conductors. Nat Rev Mater 9, 887–905 (2024). https://doi.org/10.1038/s41578-024-00715-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41578-024-00715-9