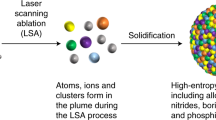
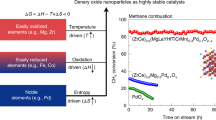
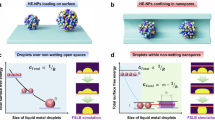
Abstract
‘High entropy’ has become a key concept in materials science over the past two decades, with this concept more recently extended to nanomaterials. High-entropy materials, characterized by the incorporation of five or more principal elements in nearly equal proportions, leverage entropy to promote the formation of compositionally complex single-phase materials rather than phase-segregated alternatives. The extensive compositional space of high-entropy nanomaterials, as well as their distinct structural and catalytic properties, has garnered considerable interest. The synthesis of high-quality single-phase high-entropy nanoparticles is important to fully realizing their potential to drive innovation, and numerous synthetic routes exist. Top-down methods begin with bulk high-entropy materials and break them down into nanosized structures, whereas bottom-up strategies start from atoms and build nanomaterials through nucleation and growth. In this Review, we categorize and compare the synthetic methods for high-entropy alloy and high-entropy intermetallic nanoparticles. Our discussion reveals that colloidal synthesis offers excellent control over the composition, size and shape of high-entropy nanoparticles while also providing pathways to metastable states that are not always accessible by other methods.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
George, E. P., Raabe, D. & Ritchie, R. O. High-entropy alloys. Nat. Rev. Mater. 4, 515–534 (2019).
Pedersen, J. K., Batchelor, T. A. A., Bagger, A. & Rossmeisl, J. High-entropy alloys as catalysts for the CO2 and CO reduction reactions. ACS Catal. 10, 2169–2176 (2020).
Banko, L. et al. Unravelling composition–activity–stability trends in high entropy alloy electrocatalysts by using a data-guided combinatorial synthesis strategy and computational modeling. Adv. Energy Mater. 12, 2103312 (2022).
Li, M., Henein, H., Zhou, C. & Liu, J. Towards high-entropy alloys with high-temperature corrosion resistance and structural stability. J. Mater. Sci. Technol. 174, 133–144 (2024).
Murty, B. S., Yeh, J.-W., Ranganathan, S. & Bhattacharjee, P. P. High-Entropy Alloys (Elsevier, 2019).
Dixit, S. et al. Refractory high-entropy alloy coatings for high-temperature aerospace and energy applications. J. Therm. Spray Technol. 31, 1021–1031 (2022).
Yang, C. et al. A library of polymetallic alloy nanotubes: from binary to septenary. J. Am. Chem. Soc. 147, 9865–9878 (2025).
Ahmad, A. et al. Unlocking the potential of high entropy alloys in electrochemical water splitting: a review. Small 20, 2311929 (2024).
Hsu, W.-L., Tsai, C.-W., Yeh, A.-C. & Yeh, J.-W. Clarifying the four core effects of high-entropy materials. Nat. Rev. Chem. 8, 471–485 (2024).
Al Zoubi, W., Putri, R. A. K., Abukhadra, M. R. & Ko, Y. G. Recent experimental and theoretical advances in the design and science of high-entropy alloy nanoparticles. Nano Energy 110, 108362 (2023).
Otto, F., Yang, Y., Bei, H. & George, E. P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 61, 2628–2638 (2013).
Li, H. et al. Nano high-entropy materials: synthesis strategies and catalytic applications. Small Struct. 1, 2000033 (2020).
Ren, J.-T., Chen, L., Wang, H.-Y. & Yuan, Z.-Y. High-entropy alloys in electrocatalysis: from fundamentals to applications. Chem. Soc. Rev. 52, 8319–8373 (2023).
Zhang, Z. et al. Recent research progress on high-entropy alloys as electrocatalytic materials. J. Alloys Compd. 918, 165585 (2022).
Qin, Y.-C. et al. Noble metal-based high-entropy alloys as advanced electrocatalysts for energy conversion. Rare Met. 40, 2354–2368 (2021).
Liu, J. et al. Recent progress in intermetallic nanocrystals for electrocatalysis: from binary to ternary to high-entropy intermetallics. SmartMat 4, e1210 (2023).
Li, Y. et al. Nanoscale design for high entropy alloy electrocatalysts. Small 20, 2310006 (2024).
Kusada, K., Wu, D. & Kitagawa, H. New aspects of platinum group metal-based solid-solution alloy nanoparticles: binary to high-entropy alloys. Chem. Eur. J. 26, 5105–5130 (2020).
Xu, X., Shao, Z. & Jiang, S. P. High-entropy materials for water electrolysis. Energy Technol. 10, 2200573 (2022).
Yao, Y. et al. Computationally aided, entropy-driven synthesis of highly efficient and durable multi-elemental alloy catalysts. Sci. Adv. 6, eaaz0510 (2020).
Wang, Z. et al. Research progress on high entropy alloys and high entropy derivatives as OER catalysts. J. Environ. Chem. Eng. 11, 109080 (2023).
Chang, X., Zeng, M., Liu, K. & Fu, L. Phase engineering of high-entropy alloys. Adv. Mater. 32, 1907226 (2020).
Sun, Y. & Dai, S. Synthesis of high-entropy materials. Nat. Synth. 3, 1457–1470 (2024).
Deng, C., Wang, T., Wu, P., Zhu, W. & Dai, S. High entropy materials for catalysis: a critical review of fundamental concepts and applications. Nano Energy 120, 109153 (2024).
Tomboc, G. M., Kwon, T., Joo, J. & Lee, K. High entropy alloy electrocatalysts: a critical assessment of fabrication and performance. J. Mater. Chem. A 8, 14844–14862 (2020).
Li, H., Lai, J., Li, Z. & Wang, L. Multi-sites electrocatalysis in high-entropy alloys. Adv. Funct. Mater. 31, 2106715 (2021).
Yao, Y. et al. High-entropy nanoparticles: synthesis–structure–property relationships and data-driven discovery. Science 376, eabn3103 (2022).
Yu, L. et al. High-entropy alloy catalysts: from bulk to nano toward highly efficient carbon and nitrogen catalysis. Carbon Energy 4, 731–761 (2022).
Xu, H. et al. Designing strategies and enhancing mechanism for multicomponent high-entropy catalysts. Chem. Sci. 14, 771–790 (2023).
Zheng, H., Luo, G., Zhang, A., Lu, X. & He, L. The synthesis and catalytic applications of nanosized high-entropy alloys. ChemCatChem 13, 806–817 (2021).
Kumar Katiyar, N., Biswas, K., Yeh, J.-W., Sharma, S. & Sekhar Tiwary, C. A perspective on the catalysis using the high entropy alloys. Nano Energy 88, 106261 (2021).
Cantor, B., Chang, I. T. H., Knight, P. & Vincent, A. J. B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 375–377, 213–218 (2004).
Yeh, J.-W. et al. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater. 6, 299–303 (2004).
Tong, C.-J. et al. Microstructure characterization of Alx CoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 36, 881–893 (2005).
Wang, Y. P., Li, B. S. & Fu, H. Z. Solid solution or intermetallics in a high-entropy alloy. Adv. Eng. Mater. 11, 641–644 (2009).
Ma, Y. et al. High-entropy energy materials: challenges and new opportunities. Energy Environ. Sci. 14, 2883–2905 (2021).
Zhang, Q. et al. Preparation of high entropy alloys and application to catalytical water electrolysis. APL Mater. 10, 070701 (2022).
Zhao, Y. J. et al. A hexagonal close-packed high-entropy alloy: the effect of entropy. Mater. Des. 96, 10–15 (2016).
Yao, Y. et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 359, 1489–1494 (2018).
Wang, D. et al. Structurally ordered high-entropy intermetallic nanoparticles with enhanced C–C bond cleavage for ethanol oxidation. SmartMat 4, e1117 (2023).
Ding, Z. et al. High entropy intermetallic-oxide core–shell nanostructure as superb oxygen evolution reaction catalyst. Adv. Sustain. Syst. 4, 1900105 (2020).
Firstov, G. et al. Electronic and crystal structure of the high entropy TiZrHfCoNiCu intermetallics undergoing martensitic transformation. MATEC Web Conf. 33, 06006 (2015).
Ferrando, R., Jellinek, J. & Johnston, R. L. Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem. Rev. 108, 845–910 (2008).
Zhang, J., Shen, L., Jiang, Y. & Sun, S. Random alloy and intermetallic nanocatalysts in fuel cell reactions. Nanoscale 12, 19557–19581 (2020).
Zhou, M., Li, C. & Fang, J. Noble-metal based random alloy and intermetallic nanocrystals: syntheses and applications. Chem. Rev. 121, 736–795 (2021).
Cortie, M. B. & McDonagh, A. M. Synthesis and optical properties of hybrid and alloy plasmonic nanoparticles. Chem. Rev. 111, 3713–3735 (2011).
Gamler, J. T. L., Ashberry, H. M., Skrabalak, S. E. & Koczkur, K. M. Random alloyed versus intermetallic nanoparticles: a comparison of electrocatalytic performance. Adv. Mater. 30, 1801563 (2018).
Li, J. & Sun, S. Intermetallic nanoparticles: synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 52, 2015–2025 (2019).
Xiao, W., Lei, W., Gong, M., Xin, H. L. & Wang, D. Recent advances of structurally ordered intermetallic nanoparticles for electrocatalysis. ACS Catal. 8, 3237–3256 (2018).
Zerdoumi, R., Ludwig, A. & Schuhmann, W. High entropy intermetallic compounds: a discovery platform for structure–property correlations and materials design principles in electrocatalysis. Curr. Opin. Electrochem. 48, 101590 (2024).
Hodnik, N. et al. Effect of ordering of PtCu3 nanoparticle structure on the activity and stability for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 16, 13610–13615 (2014).
Yan, Y. et al. Intermetallic nanocrystals: syntheses and catalytic applications. Adv. Mater. 29, 1605997 (2017).
Dippo, O. F. & Vecchio, K. S. A universal configurational entropy metric for high-entropy materials. Scr. Mater. 201, 113974 (2021).
Xing, F., Ma, J., Shimizu, K. & Furukawa, S. High-entropy intermetallics on ceria as efficient catalysts for the oxidative dehydrogenation of propane using CO2. Nat. Commun. 13, 5065 (2022).
Chen, W. et al. High-entropy intermetallic PtRhBiSnSb nanoplates for highly efficient alcohol oxidation electrocatalysis. Adv. Mater. 34, 2206276 (2022).
Lin, F., Li, M., Zeng, L., Luo, M. & Guo, S. Intermetallic nanocrystals for fuel-cells-based electrocatalysis. Chem. Rev. 123, 12507–12593 (2023).
Wang, C., Chen, D. P., Sang, X., Unocic, R. R. & Skrabalak, S. E. Size-dependent disorder–order transformation in the synthesis of monodisperse intermetallic PdCu nanocatalysts. ACS Nano 10, 6345–6353 (2016).
Greeley, J. et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 1, 552–556 (2009).
Pérez-Ramírez, J. & López, N. Strategies to break linear scaling relationships. Nat. Catal. 2, 971–976 (2019).
Chen, Z. W. et al. Unusual Sabatier principle on high entropy alloy catalysts for hydrogen evolution reactions. Nat. Commun. 15, 359 (2024).
Narayanan, R. & El-Sayed, M. A. Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 4, 1343–1348 (2004).
Batchelor, T. A. A. et al. High-entropy alloys as a discovery platform for electrocatalysis. Joule 3, 834–845 (2019).
Löffler, T., Ludwig, A., Rossmeisl, J. & Schuhmann, W. What makes high-entropy alloys exceptional electrocatalysts? Angew. Chem. Int. Ed. 60, 26894–26903 (2021).
Cao, G. et al. Liquid metal for high-entropy alloy nanoparticles synthesis. Nature 619, 73–77 (2023).
Liao, Y. et al. High-entropy-alloy nanoparticles with 21 ultra-mixed elements for efficient photothermal conversion. Natl Sci. Rev. 9, nwac041 (2022).
Skrabalak, S. E. Mashing up metals with carbothermal shock. Science 359, 1467–1467 (2018).
Yao, Y. et al. Extreme mixing in nanoscale transition metal alloys. Matter 4, 2340–2353 (2021).
Kar, N. et al. Retrosynthetic design of core–shell nanoparticles for thermal conversion to monodisperse high-entropy alloy nanoparticles. Nat. Synth. 3, 175–184 (2023).
Dey, G. R., McCormick, C. R., Soliman, S. S., Darling, A. J. & Schaak, R. E. Chemical insights into the formation of colloidal high entropy alloy nanoparticles. ACS Nano 17, 5943–5955 (2023).
Dey, G. R. et al. Colloidal nanoparticles of high entropy materials: capabilities, challenges, and opportunities in synthesis and characterization. ACS Nanosci. Au 4, 3–20 (2024).
Kamaruddin, H., Jianghong, Z., Yu, L., Yuefan, W. & Yizhong, H. A review of noble metal-free high entropy alloys for water splitting applications. J. Mater. Chem. A 12, 9933–9961 (2024).
Löffler, T. et al. Discovery of a multinary noble metal-free oxygen reduction catalyst. Adv. Energy Mater. 8, 1802269 (2018).
Xie, P. et al. Highly efficient decomposition of ammonia using high-entropy alloy catalysts. Nat. Commun. 10, 4011 (2019).
Pedersen, A. et al. Comparative techno-economic and life-cycle analysis of precious versus non-precious metal electrocatalysts: the case of PEM fuel cell cathodes. Green Chem. 25, 10458–10471 (2023).
An, K. & Somorjai, G. A. Size and shape control of metal nanoparticles for reaction selectivity in catalysis. ChemCatChem 4, 1512–1524 (2012).
Zaera, F. Shape-controlled nanostructures in heterogeneous catalysis. ChemSusChem 6, 1797–1820 (2013).
Lee, H. Utilization of shape-controlled nanoparticles as catalysts with enhanced activity and selectivity. RSC Adv. 4, 41017–41027 (2014).
Cobley, C. M., Skrabalak, S. E., Campbell, D. J. & Xia, Y. Shape-controlled synthesis of silver nanoparticles for plasmonic and sensing applications. Plasmonics 4, 171–179 (2009).
Wu, Z., Yang, S. & Wu, W. Shape control of inorganic nanoparticles from solution. Nanoscale 8, 1237–1259 (2016).
Nguyen, T.-D. From formation mechanisms to synthetic methods toward shape-controlled oxide nanoparticles. Nanoscale 5, 9455–9482 (2013).
Galeano, C. et al. Toward highly stable electrocatalysts via nanoparticle pore confinement. J. Am. Chem. Soc. 134, 20457–20465 (2012).
Peng, H. et al. Large-scale and facile synthesis of a porous high-entropy alloy CrMnFeCoNi as an efficient catalyst. J. Mater. Chem. A 8, 18318–18326 (2020).
Cai, Z.-X. et al. Nanoporous ultra-high-entropy alloys containing fourteen elements for water splitting electrocatalysis. Chem. Sci. 12, 11306–11315 (2021).
Chen, Z., Wen, J., Wang, C. & Kang, X. Convex cube-shaped Pt34Fe5Ni20Cu31Mo9Ru high entropy alloy catalysts toward high-performance multifunctional electrocatalysis. Small 18, 2204255 (2022).
Abid, N. et al. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: a review. Adv. Colloid Interface Sci. 300, 102597 (2022).
Sun, X. & Sun, Y. Synthesis of metallic high-entropy alloy nanoparticles. Chem. Soc. Rev. 53, 4400–4433 (2024).
Jamkhande, P. G., Ghule, N. W., Bamer, A. H. & Kalaskar, M. G. Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 53, 101174 (2019).
Dwivedi, A., Koch, C. C. & Rajulapati, K. V. On the single phase fcc solid solution in nanocrystalline Cr-Nb-Ti-V-Zn high-entropy alloy. Mater. Lett. 183, 44–47 (2016).
Wu, H., Huang, S., Zhu, C., Zhu, H. & Xie, Z. Excellent mechanical properties of in-situ TiC/FeCrNiCuV0.1 high entropy alloy matrix composites. Mater. Lett. 257, 126729 (2019).
Sure, J., Vishnu, D. S. M. & Schwandt, C. Direct electrochemical synthesis of high-entropy alloys from metal oxides. Appl. Mater. Today 9, 111–121 (2017).
Soare, V. et al. Electrochemical deposition and microstructural characterization of AlCrFeMnNi and AlCrCuFeMnNi high entropy alloy thin films. Appl. Surf. Sci. 358, 533–539 (2015).
Yu, H.-D., Regulacio, M. D., Ye, E. & Han, M.-Y. Chemical routes to top-down nanofabrication. Chem. Soc. Rev. 42, 6006–6018 (2013).
Benjamin, J. S. Dispersion strengthened superalloys by mechanical alloying. Metall. Trans. 1, 2943–2951 (1970).
Zhang, D. L. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 49, 537–560 (2004).
Murty, B. S. & Ranganathan, S. Novel materials synthesis by mechanical alloying/milling. Int. Mater. Rev. 43, 101–141 (1998).
Zhang, R.-Z., Gucci, F., Zhu, H., Chen, K. & Reece, M. J. Data-driven design of ecofriendly thermoelectric high-entropy sulfides. Inorg. Chem. 57, 13027–13033 (2018).
Wu, S., Pan, Y., Wang, N., Lu, T. & Dai, W. Azo dye degradation behavior of AlFeMnTiM (M = Cr, Co, Ni) high-entropy alloys. Int. J. Miner. Metall. Mater. 26, 124–132 (2019).
Rekha, M. Y., Mallik, N. & Srivastava, C. First report on high entropy alloy nanoparticle decorated graphene. Sci. Rep. 8, 8737 (2018).
Varalakshmi, S., Kamaraj, M. & Murty, B. S. Synthesis and characterization of nanocrystalline AlFeTiCrZnCu high entropy solid solution by mechanical alloying. J. Alloys Compd. 460, 253–257 (2008).
Zeng, Y. et al. Electrochemical dealloying with simultaneous phase separation. Acta Mater. 171, 8–17 (2019).
Yao, R.-Q. et al. Nanoporous surface high-entropy alloys as highly efficient multisite electrocatalysts for nonacidic hydrogen evolution reaction. Adv. Funct. Mater. 31, 2009613 (2021).
Erlebacher, J., Aziz, M. J., Karma, A., Dimitrov, N. & Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 410, 450–453 (2001).
Qiu, H.-J. et al. Nanoporous high-entropy alloys for highly stable and efficient catalysts. J. Mater. Chem. A 7, 6499–6506 (2019).
Jin, Z. et al. Nanoporous Al-Ni-Co-Ir-Mo high-entropy alloy for record-high water splitting activity in acidic environments. Small 15, 1904180 (2019).
Yu, T. et al. Twelve-component free-standing nanoporous high-entropy alloys for multifunctional electrocatalysis. ACS Mater. Lett. 4, 181–189 (2022).
Jia, Z. et al. A self-supported high-entropy metallic glass with a nanosponge architecture for efficient hydrogen evolution under alkaline and acidic conditions. Adv. Funct. Mater. 31, 2101586 (2021).
Lung, J.-K. et al. Preparation of gold nanoparticles by arc discharge in water. J. Alloys Compd. 434–435, 655–658 (2007).
Wu, Q. et al. High entropy alloys: from bulk metallic materials to nanoparticles. Metall. Mater. Trans. A 49, 4986–4990 (2018).
Feng, J. et al. Unconventional alloys confined in nanoparticles: building blocks for new matter. Matter 3, 1646–1663 (2020).
Mao, A. et al. Plasma arc discharge synthesis of multicomponent Co-Cr-Cu-Fe-Ni nanoparticles. J. Alloys Compd. 775, 1177–1183 (2019).
Yan, Z. & Chrisey, D. B. Pulsed laser ablation in liquid for micro-/nanostructure generation. J. Photochem. Photobiol. C Photochem. Rev. 13, 204–223 (2012).
Waag, F. et al. Kinetically-controlled laser-synthesis of colloidal high-entropy alloy nanoparticles. RSC Adv. 9, 18547–18558 (2019).
Fritze, S. et al. Influence of deposition temperature on the phase evolution of HfNbTiVZr high-entropy thin films. Materials 12, 587 (2019).
Zhang, Y., Yan, X.-H., Liao, W.-B. & Zhao, K. Effects of nitrogen content on the structure and mechanical properties of (Al0.5CrFeNiTi0.25)Nx high-entropy films by reactive sputtering. Entropy 20, 624 (2018).
Tsai, C. F., Wu, P.-W., Lin, P., Chao, C. G. & Yeh, K. Y. Sputter deposition of multi-element nanoparticles as electrocatalysts for methanol oxidation. Jpn J. Appl. Phys. 47, 5755–5761 (2008).
Li, S.-Y. et al. Sputter-deposited high entropy alloy thin film electrocatalyst for enhanced oxygen evolution reaction performance. Small 18, 2106127 (2022).
Xin, Y. et al. High-entropy alloys as a platform for catalysis: progress, challenges, and opportunities. ACS Catal. 10, 11280–11306 (2020).
Raabe, D., Li, Z. & Ponge, D. Metastability alloy design. MRS Bull. 44, 266–272 (2019).
Oehring, M., Yan, Z. H., Klassen, T. & Bormann, R. Competition between stable and metastable phases during mechanical alloying and ball milling. Phys. Stat. Sol. A 131, 671–689 (1992).
Lacey, S. D. et al. Stable multimetallic nanoparticles for oxygen electrocatalysis. Nano Lett. 19, 5149–5158 (2019).
Zhang, C., Oliaee, S. N., Hwang, S. Y., Kong, X. & Peng, Z. A generic wet impregnation method for preparing substrate-supported platinum group metal and alloy nanoparticles with controlled particle morphology. Nano Lett. 16, 164–169 (2016).
Cui, M. et al. Multi-principal elemental intermetallic nanoparticles synthesized via a disorder-to-order transition. Sci. Adv. 8, eabm4322 (2022).
Ahn, J. et al. Rapid Joule heating synthesis of oxide-socketed high-entropy alloy nanoparticles as CO2 conversion catalysts. ACS Nano 17, 12188–12199 (2023).
Cui, X. et al. Rapid high-temperature liquid shock synthesis of high-entropy alloys for hydrogen evolution reaction. ACS Nano 18, 2948–2957 (2024).
Cha, J.-H. et al. Flash-thermal shock synthesis of high-entropy alloys toward high-performance water splitting. Adv. Mater. 35, 2305222 (2023).
Liu, Y., Tian, X., Han, Y.-C., Chen, Y. & Hu, W. High-temperature shock synthesis of high-entropy-alloy nanoparticles for catalysis. Chin. J. Catal. 48, 66–89 (2023).
Yang, Y. et al. Aerosol synthesis of high entropy alloy nanoparticles. Langmuir 36, 1985–1992 (2020).
Didenko, Y. T. & Suslick, K. S. Chemical aerosol flow synthesis of semiconductor nanoparticles. J. Am. Chem. Soc. 127, 12196–12197 (2005).
Motl, N. E., Mann, A. K. P. & Skrabalak, S. E. Aerosol-assisted synthesis and assembly of nanoscale building blocks. J. Mater. Chem. A 1, 5193–5202 (2013).
Suslick, K. S. & Price, G. J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 29, 295–326 (1999).
Liu, M. et al. Entropy-maximized synthesis of multimetallic nanoparticle catalysts via a ultrasonication-assisted wet chemistry method under ambient conditions. Adv. Mater. Interfaces 6, 1900015 (2019).
Rybakov, K. I., Olevsky, E. A. & Krikun, E. V. Microwave sintering: fundamentals and modeling. J. Am. Ceram. Soc. 96, 1003–1020 (2013).
Roy, R., Agrawal, D., Cheng, J. & Gedevanishvili, S. Full sintering of powdered-metal bodies in a microwave field. Nature 399, 668–670 (1999).
Qiao, H. et al. Scalable synthesis of high entropy alloy nanoparticles by microwave heating. ACS Nano 15, 14928–14937 (2021).
Kunal, P. et al. Continuous flow synthesis of Rh and RhAg alloy nanoparticle catalysts enables scalable production and improved morphological control. Chem. Mater. 29, 4341–4350 (2017).
Tang, J., Xu, J. L., Ye, Z. G., Li, X. B. & Luo, J. M. Microwave sintered porous CoCrFeNiMo high entropy alloy as an efficient electrocatalyst for alkaline oxygen evolution reaction. J. Mater. Sci. Technol. 79, 171–177 (2021).
Mallik, A. & Ray, B. C. Evolution of principle and practice of electrodeposited thin film: a review on effect of temperature and sonication. Int. J. Electrochem. 2011, e568023 (2011).
Glasscott, M. W. et al. Electrosynthesis of high-entropy metallic glass nanoparticles for designer, multi-functional electrocatalysis. Nat. Commun. 10, 2650 (2019).
Chang, S.-Q., Cheng, C.-C., Cheng, P.-Y., Huang, C.-L. & Lu, S.-Y. Pulse electrodeposited FeCoNiMnW high entropy alloys as efficient and stable bifunctional electrocatalysts for acidic water splitting. Chem. Eng. J. 446, 137452 (2022).
Yao, C.-Z. et al. Electrochemical preparation and magnetic study of Bi–Fe–Co–Ni–Mn high entropy alloy. Electrochim. Acta 53, 8359–8365 (2008).
Zhu, H. et al. A high-entropy atomic environment converts inactive to active sites for electrocatalysis. Energy Environ. Sci. 16, 619–628 (2023).
Yao, Y. et al. High entropy alloy nanoparticles encapsulated in graphitised hollow carbon tubes for oxygen reduction electrocatalysis. Dalton Trans. 52, 4142–4151 (2023).
Wang, D. et al. Tailoring lattice strain in ultra-fine high-entropy alloys for active and stable methanol oxidation. Sci. China Mater. 64, 2454–2466 (2021).
Vasquez, Y., Henkes, A. E., Chris Bauer, J. & Schaak, R. E. Nanocrystal conversion chemistry: a unified and materials-general strategy for the template-based synthesis of nanocrystalline solids. J. Solid State Chem. 181, 1509–1523 (2008).
Wang, Y. et al. Ordering-dependent hydrogen evolution and oxygen reduction electrocatalysis of high-entropy intermetallic Pt4FeCoCuNi. Adv. Mater. 35, 2302067 (2023).
Gao, S. et al. Synthesis of high-entropy alloy nanoparticles on supports by the fast moving bed pyrolysis. Nat. Commun. 11, 2016 (2020).
Wong, A., Liu, Q., Griffin, S., Nicholls, A. & Regalbuto, J. R. Synthesis of ultrasmall, homogeneously alloyed, bimetallic nanoparticles on silica supports. Science 358, 1427–1430 (2017).
Demazeau, G. Solvothermal reactions: an original route for the synthesis of novel materials. J. Mater. Sci. 43, 2104–2114 (2008).
Lai, J., Niu, W., Luque, R. & Xu, G. Solvothermal synthesis of metal nanocrystals and their applications. Nano Today 10, 240–267 (2015).
Huo, Y., Xiu, S., Meng, L.-Y. & Quan, B. Solvothermal synthesis and applications of micro/nano carbons: a review. Chem. Eng. J. 451, 138572 (2023).
Bondesgaard, M., Broge, N. L. N., Mamakhel, A., Bremholm, M. & Iversen, B. B. General solvothermal synthesis method for complete solubility range bimetallic and high-entropy alloy nanocatalysts. Adv. Funct. Mater. 29, 1905933 (2019).
Broge, N. L. N., Bondesgaard, M., Søndergaard-Pedersen, F., Roelsgaard, M. & Iversen, B. B. Autocatalytic formation of high-entropy alloy nanoparticles. Angew. Chem. Int. Ed. 59, 21920–21924 (2020).
Broge, N. L. N., Bertelsen, A. D., Søndergaard-Pedersen, F. & Iversen, B. B. Facile solvothermal synthesis of Pt–Ir–Pd–Rh–Ru–Cu–Ni–Co high-entropy alloy nanoparticles. Chem. Mater. 35, 144–153 (2023).
Mei, Y. et al. High-entropy alloy with Mo-coordination as efficient electrocatalyst for oxygen evolution reaction. ACS Catal. 12, 10808–10817 (2022).
Zhang, Z. et al. Off-equilibrium hydrothermal synthesis of high-entropy alloy nanoparticles. J. Am. Chem. Soc. 147, 9640–9652 (2025).
Zuo, X. et al. A hollow PdCuMoNiCo high-entropy alloy as an efficient bi-functional electrocatalyst for oxygen reduction and formic acid oxidation. J. Mater. Chem. A 10, 14857–14865 (2022).
Bondesgaard, M. et al. Supercritical flow synthesis of Pt1–xRux nanoparticles: comparative phase diagram study of nanostructure versus bulk. Chem. Mater. 29, 3265–3273 (2017).
Kusada, K. et al. Nonequilibrium flow-synthesis of solid-solution alloy nanoparticles: from immiscible binary to high-entropy alloys. J. Phys. Chem. C 125, 458–463 (2021).
Darr, J. A., Zhang, J., Makwana, N. M. & Weng, X. Continuous hydrothermal synthesis of inorganic nanoparticles: applications and future directions. Chem. Rev. 117, 11125–11238 (2017).
Rodrigues, T. S. et al. Synthesis of colloidal metal nanocrystals: a comprehensive review on the reductants. Chem. Eur. J. 24, 16944–16963 (2018).
Pu, Y., Cai, F., Wang, D., Wang, J.-X. & Chen, J.-F. Colloidal synthesis of semiconductor quantum dots toward large-scale production: a review. Ind. Eng. Chem. Res. 57, 1790–1802 (2018).
Landfester, K. Synthesis of colloidal particles in miniemulsions. Annu. Rev. Mater. Res. 36, 231–279 (2006).
Wu, C.-Y. et al. A catalyst family of high-entropy alloy atomic layers with square atomic arrangements comprising iron- and platinum-group metals. Sci. Adv. 10, eadl3693 (2024).
Wang, C. et al. Facet-controlled synthesis of platinum-group-metal quaternary alloys: the case of nanocubes and {100} facets. J. Am. Chem. Soc. 145, 2553–2560 (2023).
Wu, D. et al. Platinum-group-metal high-entropy-alloy nanoparticles. J. Am. Chem. Soc. 142, 13833–13838 (2020).
Liu, Y.-H. et al. Toward controllable and predictable synthesis of high-entropy alloy nanocrystals. Sci. Adv. 9, eadf9931 (2023).
Wang, C., He, J. & Xia, Y. Controlling the composition and elemental distribution of bi- and multi-metallic nanocrystals via dropwise addition. Nat. Synth. 3, 1076–1082 (2024).
Zhan, C. et al. Subnanometer high-entropy alloy nanowires enable remarkable hydrogen oxidation catalysis. Nat. Commun. 12, 6261 (2021).
Mathiesen, J. K. et al. Why colloidal syntheses of bimetallic nanoparticles cannot be generalized. ACS Nano 18, 26937–26947 (2024).
Chen, Y. et al. Synthesis of monodisperse high entropy alloy nanocatalysts from core@shell nanoparticles. Nanoscale Horiz. 6, 231–237 (2021).
Bueno, S. L. A. et al. Quinary, senary, and septenary high entropy alloy nanoparticle catalysts from core@shell nanoparticles and the significance of intraparticle heterogeneity. ACS Nano 16, 18873–18885 (2022).
Wang, Y. et al. Synthesis of high-entropy alloy nanoparticles by step-alloying strategy as superior multifunctional electrocatalyst. Adv. Mater. 35, 2302499 (2023).
Kar, N., Leonardi, A., McCoy, M., Selvaraj, R. & Skrabalak, S. E. A programmable nanoparticle conversion pathway to monodisperse polyelemental high entropy alloy, intermetallic, and multiphase nanoparticles. Angew. Chem. Int. Ed. 64, e202505523 (2025) .
Feng, G. et al. Engineering structurally ordered high-entropy intermetallic nanoparticles with high-activity facets for oxygen reduction in practical fuel cells. J. Am. Chem. Soc. 145, 11140–11150 (2023).
Kar, N. et al. Reaction stoichiometry directs the architecture of trimetallic nanostructures produced via galvanic replacement. Nanoscale 15, 3749–3756 (2023).
Xia, X., Wang, Y., Ruditskiy, A. & Xia, Y. 25th anniversary article: galvanic replacement: a simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 25, 6313–6333 (2013).
Tao, L. et al. A general synthetic method for high-entropy alloy subnanometer ribbons. J. Am. Chem. Soc. 144, 10582–10590 (2022).
Li, M. et al. Programmable synthesis of high-entropy nanoalloys for efficient ethanol oxidation reaction. ACS Nano 17, 13659–13671 (2023).
Zheng, S. et al. Ultrathin template approach to synthesize high-entropy intermetallic nanoparticles for hydrogen evolution reaction. Small Struct. 5, 2300537 (2024).
Garlyyev, B. et al. Revealing the nature of active sites in electrocatalysis. Chem. Sci. 10, 8060–8075 (2019).
Somorjai, G. A., McCrea, K. R. & Zhu, J. Active sites in heterogeneous catalysis: development of molecular concepts and future challenges. Top. Catal. 18, 157–166 (2002).
Somorjai, G. A. in Advances in Catalysis Vol. 26 (eds Eley, D. D., Pines, H. & Weisz, P. B.) 1–68 (Academic Press, 1977).
Hsiao, Y.-C. et al. A library of seed@high-entropy-alloy core–shell nanocrystals with controlled facets for catalysis. Adv. Mater. 37, 2411464 (2025).
Long, M. et al. IrPdCuFeNiCoMo based core–shell icosahedron nanocrystals and nanocages for efficient and robust acidic oxygen evolution. Angew. Chem. Int. Ed. 137, e202419956 (2025).
Ming, T. et al. Growth of tetrahexahedral gold nanocrystals with high-index facets. J. Am. Chem. Soc. 131, 16350–16351 (2009).
Yu, Y., Zhang, Q., Lu, X. & Lee, J. Y. Seed-mediated synthesis of monodisperse concave trisoctahedral gold nanocrystals with controllable sizes. J. Phys. Chem. C 114, 11119–11126 (2010).
Zhan, C. et al. Medium/high-entropy amalgamated core/shell nanoplate achieves efficient formic acid catalysis for direct formic acid fuel cell. Angew. Chem. Int. Ed. 135, e202213783 (2023).
Anderson, B. D. & Tracy, J. B. Nanoparticle conversion chemistry: Kirkendall effect, galvanic exchange, and anion exchange. Nanoscale 6, 12195–12216 (2014).
Li, X. et al. A radical-assisted approach to high-entropy alloy nanoparticle electrocatalysts under ambient conditions. ACS Nano 19, 7851–7863 (2025).
Veglak, J. M., Tsai, A., Soliman, S. S., Dey, G. R. & Schaak, R. E. Disentangling competitive and synergistic chemical reactivities during the seeded growth of high-entropy alloys on high-entropy metal sulfide nanoparticles. J. Am. Chem. Soc. 146, 19521–19536 (2024).
Jin, Z. et al. A fourteen-component high-entropy alloy@oxide bifunctional electrocatalyst with a record-low ΔE of 0.61 V for highly reversible Zn–air batteries. Chem. Sci. 13, 12056–12064 (2022).
Kar, N. & Skrabalak, S. E. High-entropy alloy nanoparticles through retrosynthetic design. Nat. Synth. 3, 156–157 (2024).
Yan, J. et al. Anomalous size effect on yield strength enabled by compositional heterogeneity in high-entropy alloy nanoparticles. Nat. Commun. 13, 2789 (2022).
Moniri, S. et al. Three-dimensional atomic structure and local chemical order of medium- and high-entropy nanoalloys. Nature 624, 564–569 (2023).
Song, B. et al. In situ oxidation studies of high-entropy alloy nanoparticles. ACS Nano 14, 15131–15143 (2020).
Song, B. et al. Revealing high-temperature reduction dynamics of high-entropy alloy nanoparticles via in situ transmission electron microscopy. Nano Lett. 21, 1742–1748 (2021).
Huang, Y. et al. Unraveling reactivity origin of oxygen reduction at high-entropy alloy electrocatalysts with a computational and data-driven approach. J. Phys. Chem. C 128, 11183–11189 (2024).
Lu, Z., Chen, Z. W. & Singh, C. V. Neural network-assisted development of high-entropy alloy catalysts: decoupling ligand and coordination effects. Matter 3, 1318–1333 (2020).
Aykol, M., Herring, P. & Anapolsky, A. Machine learning for continuous innovation in battery technologies. Nat. Rev. Mater. 5, 725–727 (2020).
Attia, P. M. et al. Closed-loop optimization of fast-charging protocols for batteries with machine learning. Nature 578, 397–402 (2020).
Senkov, O. N., Miller, J. D., Miracle, D. B. & Woodward, C. Accelerated exploration of multi-principal element alloys with solid solution phases. Nat. Commun. 6, 6529 (2015).
Acknowledgements
This article is supported by a US National Science Foundation grant NSF CHE 2203349.
Author information
Authors and Affiliations
Contributions
N.K. conducted the literature search and prepared the first draft, with S.E.S. providing feedback and revision support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks Tung-Han Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kar, N., Skrabalak, S.E. Synthetic methods for high-entropy nanomaterials. Nat Rev Mater 10, 638–653 (2025). https://doi.org/10.1038/s41578-025-00829-8
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41578-025-00829-8