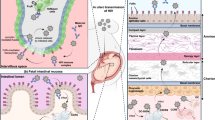
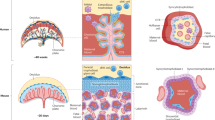
Abstract
Infections are a major threat to human reproductive health, and infections in pregnancy can cause prematurity or stillbirth, or can be vertically transmitted to the fetus leading to congenital infection and severe disease. The acronym ‘TORCH’ (Toxoplasma gondii, other, rubella virus, cytomegalovirus, herpes simplex virus) refers to pathogens directly associated with the development of congenital disease and includes diverse bacteria, viruses and parasites. The placenta restricts vertical transmission during pregnancy and has evolved robust mechanisms of microbial defence. However, microorganisms that cause congenital disease have likely evolved diverse mechanisms to bypass these defences. In this Review, we discuss how TORCH pathogens access the intra-amniotic space and overcome the placental defences that protect against microbial vertical transmission.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
DeSilva, M. et al. Congenital anomalies: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 34, 6015–6026 (2016).
Boyle, B. et al. Estimating global burden of disease due to congenital anomaly: an analysis of European data. Arch. Dis. Child. Fetal Neonatal Ed. 103, F22–F28 (2018).
Christianson, A., Howson, C. & Modell, B. March of Dimes. Global Report on Birth Defect. The Hidden toll of Dying and Disabled Children (March of Dimes Birth Defects Foundation, 2006).
Page, J. M. et al. Stillbirth associated with infection in a diverse U.S. Cohort. Obstet. Gynecol. 134, 1187–1196 (2019).
Lawn, J. E. et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 387, 587–603 (2016).
McClure, E. M. & Goldenberg, R. L. Infection and stillbirth. Semin. Fetal Neonatal Med. 14, 182–189 (2009).
Fouks, Y., Many, A., Shulman, Y., Bak, S. & Shinar, S. The contribution of an infectious workup in understanding stillbirth. Am. J. Perinatol. 38, 377–382 (2021).
Fiumara, N. J. A legacy of syphilis. Arch. Dermatol. 92, 676–678 (1965).
Crane, J. et al. Parvovirus B19 infection in pregnancy. J. Obstet. Gynaecol. Can. 36, 1107–1116 (2014).
Romero, R., Dey, S. K. & Fisher, S. J. Preterm labor: one syndrome, many causes. Science 345, 760–765 (2014).
Venkatesh, K. K. et al. Association of chorioamnionitis and its duration with neonatal morbidity and mortality. J. Perinatol. 39, 673–682 (2019).
Stoll, B. J. et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N. Engl. J. Med. 347, 240–247 (2002).
Oh, J. W., Park, C. W., Moon, K. C., Park, J. S. & Jun, J. K. The relationship among the progression of inflammation in umbilical cord, fetal inflammatory response, early-onset neonatal sepsis, and chorioamnionitis. PLoS ONE 14, e0225328 (2019).
Stoll, B. J. et al. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 174, e200593 (2020).
Nahmias, A. J., Walls, K. W., Stewart, J. A., Herrmann, K. L. & Flynt, W. J. The ToRCH complex-perinatal infections associated with toxoplasma and rubella, cytomegol- and herpes simplex viruses. Pediatr. Res. 5, 405–406 (1971).
Ander, S. E., Diamond, M. S. & Coyne, C. B. Immune responses at the maternal-fetal interface. Sci. Immunol. 4, eaat6114 (2019).
Maltepe, E., Bakardjiev, A. I. & Fisher, S. J. The placenta: transcriptional, epigenetic, and physiological integration during development. J. Clin. Invest. 120, 1016–1025 (2010).
Knöfler, M. et al. Human placenta and trophoblast development: key molecular mechanisms and model systems. Cell. Mol. Life Sci. 76, 405–406 (2019).
Thomas, J. R. et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J. Exp. Med. 218, e20200891 (2020).
Ellery, P. M., Cindrova-Davies, T., Jauniaux, E., Ferguson-Smith, A. C. & Burton, G. J. Evidence for transcriptional activity in the syncytiotrophoblast of the human placenta. Placenta 30, 329–334 (2009).
Guttman, J. A. & Finlay, B. B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 1788, 832–841 (2009).
Zeldovich, V. B. et al. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog. 9, 1–10 (2013).
Ander, S. E. et al. Human placental syncytiotrophoblasts restrict Toxoplasma gondii attachment and replication and respond to infection by producing immunomodulatory chemokines. mBio 9, e01678-17 (2018).
Robbins, J. R., Skrzypczynska, K. M., Zeldovich, V. B., Kapidzic, M. & Bakardjiev, A. I. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 6, e1000732 (2010).
Delorme-Axford, E. et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl Acad. Sci. USA 110, 12048–12053 (2013).
Delorme-Axford, E., Sadovsky, Y. & Coyne, C. B. Lipid raft- and src family kinase-dependent entry of Coxsackievirus B into human placental trophoblasts. J. Virol. 87, 8569–8581 (2013).
Stein, K. R. et al. CD46 facilitates entry and dissemination of human cytomegalovirus. Nat. Commun. 10, 2699 (2019).
Hemmings, D. G., Kilani, R., Nykiforuk, C., Preiksaitis, J. & Guilbert, L. J. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J. Virol. 72, 4970–4979 (1998).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 563, 347–353 (2018).
Pique-Regi, R. et al. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 8, e52004 (2019).
Rinaldi, S. F., Makieva, S., Saunders, P. T., Rossi, A. G. & Norman, J. E. Immune cell and transcriptomic analysis of the human decidua in term and preterm parturition. Mol. Hum. Reprod. 23, 708–724 (2017).
Hamilton, S. et al. Macrophages Infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor1. Biol. Reprod. 86, 39 (2012).
Kwan, M. et al. Dynamic changes in maternal decidual leukocyte populations from first to second trimester gestation. Placenta 35, 1027–1034 (2014).
Smith, S. D., Dunk, C. E., Aplin, J. D., Harris, L. K. & Jones, R. L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol. 174, 1959–1971 (2009).
Harris, L. K., Benagiano, M., D’Elios, M. M., Brosens, I. & Benagiano, G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am. J. Obstet. Gynecol. 221, 457–469 (2019).
Pereira, L. & Maidji, E. Cytomegalovirus infection in the human placenta: maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr. Top. Microbiol. Immunol. 325, 383–395 (2008).
Weisblum, Y. et al. Zika virus infects early- and midgestation human maternal decidual tissues, inducing distinct innate tissue responses in the maternal-fetal interface. J. Virol. 91, e01905–e01916 (2017).
Rizzuto, G., Tagliani, E., Manandhar, P., Erlebacher, A. & Bakardjiev, A. I. Limited colonization undermined by inadequate early immune responses defines the dynamics of decidual listeriosis. Infect. Immun. 85, e00153–17 (2017).
Ning, F., Liu, H. & Lash, G. E. The role of decidual macrophages during normal and pathological pregnancy. Am. J. Reprod. Immunol. 75, 298–309 (2016).
Jiang, X., Du, M. R., Li, M. & Wang, H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell. Mol. Immunol. 15, 1027–1037 (2018).
Crespo, Â. C. et al. Decidual NK cells transfer granulysin to selectively kill bacteria in trophoblasts. Cell 182, 1125–1139.e18 (2020).
Rogers, L. M. et al. Decidual stromal cell-derived PGE2 regulates macrophage responses to microbial threat. Am. J. Reprod. Immunol. 80, e13032 (2018).
Croxatto, D. et al. Stromal cells from human decidua exert a strong inhibitory effect on NK cell function and dendritic cell differentiation. PLoS ONE 9, e89006 (2014).
Castro-Leyva, V. et al. Decidualization mediated by steroid hormones modulates the innate immunity in response to group B streptococcal infection in vitro. Gynecol. Obstet. Invest. 82, 592–600 (2017).
Xu, X. et al. Monocyte chemoattractant protein-1 secreted by decidual stromal cells inhibits NK cells cytotoxicity by up-regulating expression of SOCS3. PLoS ONE 7, e41869 (2012).
Guzeloglu-Kayisli, O. et al. Zika virus–infected decidual cells elicit a gestational age–dependent innate immune response and exaggerate trophoblast zika permissiveness: implication for vertical transmission. J. Immunol. 205, 3083–3094 (2020).
Tabata, T., Petitt, M., Fang-Hoover, J. & Pereira, L. Survey of cellular immune responses to human cytomegalovirus infection in the microenvironment of the uterine–placental interface. Med. Microbiol. Immunol. 208, 475–485 (2019).
Bortolotti, D. et al. Human herpes simplex 1 virus infection of endometrial decidual tissue-derived MSC alters HLA-G expression and immunosuppressive functions. Hum. Immunol. 79, 800–808 (2018).
Deshmukh, H. & Way, S. S. Immunological basis for recurrent fetal loss and pregnancy complications. Annu. Rev. Pathol. Mech. Dis. 14, 185–210 (2019).
Reyes, L. & Golos, T. G. Hofbauer cells: their role in healthy and complicated pregnancy. Front. Immunol. 9, 2628 (2018).
Bayer, A. et al. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19, 705–712 (2016).
Corry, J., Arora, N., Good, C. A., Sadovsky, Y. & Coyne, C. B. Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal-fetal interface. Proc. Natl Acad. Sci. USA 114, 9433–9438 (2017).
Jagger, B. W. et al. Gestational stage and IFN-λ signaling regulate ZIKV infection in utero. Cell Host Microbe 22, 366–376.e3 (2017).
Chen, J. et al. Outcomes of congenital Zika disease depend on timing of infection and maternal-fetal interferon action. Cell Rep. 21, 1588–1599 (2017).
Bayer, A. et al. Chromosome 19 microRNAs exert antiviral activity independent from type III interferon signaling. Placenta 61, 33–38 (2018).
Bayer, A. et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am. J. Obstet. Gynecol. 212, 71.e1–71.e8 (2015).
Dumont, T. M. F. et al. The expression level of C19MC miRNAs in early pregnancy and in response to viral infection. Placenta 53, 23–29 (2017).
Yockey, L. J. et al. Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol. 3, eaao1680 (2018).
Buchrieser, J. et al. IFITM proteins inhibit placental syncytiotrophoblast formation and promote fetal demise. Science 365, 176–180 (2019).
Zani, A. et al. Interferon-induced transmembrane proteins inhibit cell fusion mediated by trophoblast syncytins. J. Biol. Chem. 294, 19844–19851 (2019).
Robbins, J. R., Zeldovich, V. B., Poukchanski, A., Boothroyd, J. C. & Bakardjiev, A. I. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect. Immun. 80, 418–428 (2012).
Megli, C., Morosky, S., Rajasundaram, D. & Coyne, C. B. Inflammasome signaling in human placental trophoblasts regulates immune defense against Listeria monocytogenes infection. J. Exp. Med. 218, e20200649 (2021).
Reis, A. S. et al. Inflammasome activation and IL-1 signaling during placental malaria induce poor pregnancy outcomes. Sci. Adv. 6, eaax6346 (2020).
Thomas, J. et al. Outbreak of listeriosis in South Africa associated with processed meat. N. Engl. J. Med. 382, 632–643 (2020).
Chan, B. T., Hohmann, E., Barshak, M. B. & Pukkila-Worley, R. Treatment of listeriosis in first trimester of pregnancy. Emerg. Infect. Dis. 19, 839–841 (2013).
Chan, L. M., Lin, H. H. & Hsiao, S. M. Successful treatment of maternal Listeria monocytogenes bacteremia in the first trimester of pregnancy: a case report and literature review. Taiwan. J. Obstet. Gynecol. 57, 462–463 (2018).
Al-Tawfiq, J. A. Listeria monocytogenes bacteremia in a twin pregnancy with differential outcome: fetus papyraceus and a full-term delivery. J. Microbiol. Immunol. Infect. 41, 433–436 (2008).
Mylonakis, E., Paliou, M., Hohmann, E. L., Calderwood, S. B. & Wing, E. J. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine 81, 260–269 (2002).
Phelps, C. C. et al. Relative roles of Listeriolysin O, InlA, and InlB in Listeria monocytogenes uptake by host cells. Infect. Immun. 86, e00555-18 (2018).
Lecuit, M. et al. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin. Proc. Natl Acad. Sci. USA 101, 6152–6157 (2004).
Holch, A., Ingmer, H., Licht, T. R. & Gram, L. Listeria monocytogenes strains encoding premature stop codons in inlA invade mice and guinea pig fetuses in orally dosed dams. J. Med. Microbiol. 62, 1799–1806 (2013).
Disson, O. et al. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature 455, 1114–1118 (2008).
Morrison, H. A., Lowe, D., Robbins, J. R. & Bakardjiev, A. I. In vivo virulence characterization of pregnancy-associated Listeria monocytogenes infections. Infect. Immun. 86, e00397-18 (2018).
Bakardjiev, A. I., Theriot, J. A. & Portnoy, D. A. Listeria monocytogenes traffics from maternal organs to the placenta and back. PLoS Pathog. 2, 623–631 (2006).
Le Monnier, A. et al. ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes. Infect. Immun. 75, 950–957 (2007).
Faralla, C. et al. Listeria monocytogenes InlP interacts with afadin and facilitates basement membrane crossing. PLoS Pathog. 14, e1007094 (2018).
Parkash, V. et al. Immunohistochemical detection of Listeria antigens in the placenta in perinatal listeriosis. Int. J. Gynecol. Pathol. 17, 343–350 (1998).
Wolfe, B. et al. Sequelae of fetal infection in a non-human primate model of listeriosis. Front. Microbiol. 10, 2021 (2019).
Radolf, J. D. & Kumar, S. The Treponema pallidum outer membrane. Curr. Topics Microbiol. Immunol. 415, 1–38 (2018).
Gomez, G. B. et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull. World Health Organ. 91, 217–226 (2013).
Korenromp, E. L. et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes — estimates for 2016 and progress since 2012. PLoS ONE 14, e0211720 (2019).
Newman, L. et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med. 10, e1001396 (2013).
Peeling, R. W. & Hook, E. W. The pathogenesis of syphilis: the Great Mimicker, revisited. J. Pathol. 208, 224–232 (2006).
Wicher, V. & Wicher, K. Pathogenesis of maternal-fetal syphilis revisited. Clin. Infect. Dis. 33, 354–363 (2001).
Rac, M. W. F. et al. Progression of ultrasound findings of fetal syphilis after maternal treatment. Am. J. Obstet. Gynecol. 211, 426.e1–426.e6 (2014).
Kimball, A. et al. Missed opportunities for prevention of congenital syphilis — United States, 2018. MMWR 69, 661–665 (2020).
Balaji, G. & Kalaivani, S. Observance of Kassowitz law — late congenital syphilis: palatal perforation and saddle nose deformity as presenting features. Indian J. Sex. Transm. Dis. 34, 35–37 (2013).
Kassowitz, M. Die Vererbung der Syphilis (Kessinger Publishing, 1876).
Dhanaselvi, H. & Kalaivani, S. Untreated late latent syphilis of both spouses with observation of Kassowitz law: adverse pregnancy outcomes in the postpenicillin era. Indian. J. Dermatol. 62, 221–222 (2017).
Wicher, V., Baughn, R. E. & Wicher, K. Congenital and neonatal syphilis in guinea-pigs show a different pattern of immune response. Immunology 82, 404–409 (1994).
Wicher, K., Abbruscato, F., Wicher, V., Baughn, R. & Noordhoek, G. T. Target organs of infection in guinea pigs with acquired or congenital syphilis. Infect. Immun. 64, 3174–3179 (1996).
Sheffield, J. S. et al. Placental histopathology of congenital syphilis. Obstet. Gynecol. 100, 126–133 (2002).
Genest, D. R. et al. Diagnosis of congenital syphilis from placental examination: comparison of histopathology, steiner stain, and polymerase chain reaction for Treponema pallidum DNA. Hum. Pathol. 27, 366–372 (1996).
Myles, T. D., Elam, G., Park-Hwang, E. & Nguyen, T. The Jarisch–Herxheimer reaction and fetal monitoring changes in pregnant women treated for syphilis. Obstet. Gynecol. 92, 859–864 (1998).
Romero, R. et al. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 25, 21–39 (2007).
Nagy, I., Pap, K., Dicső, F. & Arany, I. Chorioamnionitis is still the main cause of preterm birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 206, e84 (2016).
Mendz, G. L., Kaakoush, N. O. & Quinlivan, J. A. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front. Cell. Infect. Microbiol. 3, 58 (2013).
Romero, R. et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J. Matern. Fetal. Neonatal Med. 28, 1394–1409 (2015).
Schrag, S. J. et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N. Engl. J. Med. 347, 233–239 (2002).
Ayala, O. D. et al. Raman microspectroscopy differentiates perinatal pathogens on ex vivo infected human fetal membrane tissues. J. Biophotonics 12, e201800449 (2019).
Verani, J. R., McGee, L., Schrag, S. J. & Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease — revised guidelines from CDC, 2010. MMWR Recomm. Rep. 59, 1–36 (2010).
Doster, R. S., Sutton, J. A., Rogers, L. M., Aronoff, D. M. & Gaddy, J. A. Streptococcus agalactiae induces placental macrophages to release extracellular traps loaded with tissue remodeling enzymes via an oxidative burst-dependent mechanism. mBio 9, e02084-18 (2018).
Kothary, V. et al. Group B Streptococcus induces neutrophil recruitment to gestational tissues and elaboration of extracellular traps and nutritional immunity. Front. Cell. Infect. Microbiol. 7, 19 (2017).
Armistead, B., Oler, E., Adams Waldorf, K. & Rajagopal, L. The double life of group B Streptococcus: asymptomatic colonizer and potent pathogen. J. Mol. Biol. 431, 2914–2931 (2019).
Vornhagen, J., Adams Waldorf, K. M. & Rajagopal, L. Perinatal group B streptococcal infections: virulence factors, immunity, and prevention strategies. Trends Microbiol. 25, 919–931 (2017).
Galask, R. P., Varner, M. W., Rosemarie Petzold, C. & Wilbur, S. L. Bacterial attachment to the chorioamniotic membranes. Am. J. Obstet. Gynecol. 148, 915–928 (1984).
Wilkie, G. L. et al. Microbiology and antibiotic resistance in peripartum bacteremia. Obstet. Gynecol. 133, 269–275 (2019).
Sáez-López, E. et al. Vaginal versus obstetric infection Escherichia coli isolates among pregnant women: antimicrobial resistance and genetic virulence profile. PLoS ONE 11, e0146531 (2016).
Pathirana, J. et al. Prevalence of congenital cytomegalovirus infection and associated risk of in-utero HIV acquisition in a high HIV prevalence setting, South Africa. Clin. Infect. Dis. 69, 1789–1796 (2019).
Fowler, K. B. et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 326, 663–667 (1992).
Boppana, S. B., Rivera, L. B., Fowler, K. B., Mach, M. & Britt, W. J. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 344, 1366–1371 (2001).
Enders, G., Daiminger, A., Bäder, U., Exler, S. & Enders, M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J. Clin. Virol. 52, 244–246 (2011).
Uenaka, M. et al. Histopathological analysis of placentas with congenital cytomegalovirus infection. Placenta 75, 62–67 (2019).
Satosar, A., Ramirez, N. C., Bartholomew, D., Davis, J. & Nuovo, G. J. Histologic correlates of viral and bacterial infection of the placenta associated with severe morbidity and mortality in the newborn. Hum. Pathol. 35, 536–545 (2004).
Njue, A. et al. The role of congenital cytomegalovirus infection in adverse birth outcomes: a review of the potential mechanisms. Viruses 13, 20 (2020).
Schleiss, M. R. Congenital cytomegalovirus infection: molecular mechanisms mediating viral pathogenesis. Infect. Disord. Drug Targets 11, 449–465 (2011).
Britt, W. J. Human cytomegalovirus infection in women with preexisting immunity: sources of infection and mechanisms of infection in the presence of antiviral immunity. J. Infect. Dis. 221, S1–S8 (2020).
Schleiss, M. R. & McVoy, M. A. Guinea pig cytomegalovirus: a model for the prevention and treatment of maternal–fetal cytomegalovirus transmission. Future Virol. 5, 207–217 (2010).
Weisblum, Y. et al. Modeling of human cytomegalovirus maternal–fetal transmission in a novel decidual organ culture. J. Virol. 85, 13204–13213 (2011).
Aronoff, D. M., Correa, H., Rogers, L. M., Arav-Boger, R. & Alcendor, D. J. Placental pericytes and cytomegalovirus infectivity: implications for HCMV placental pathology and congenital disease. Am. J. Reprod. Immunol. https://doi.org/10.1111/aji.12728 (2017).
Burgos, J. S. et al. Hematogenous vertical transmission of herpes simplex virus type 1 in mice. J. Virol. 80, 2823–2831 (2006).
Koi, H. et al. Syncytiotrophoblast is a barrier to maternal–fetal transmission of herpes simplex virus. Biol. Reprod. 67, 1572–1579 (2002).
Finger-Jardim, F. et al. Herpes simplex virus: prevalence in placental tissue and incidence in neonatal cord blood samples. J. Med. Virol. 86, 519–524 (2014).
Finger-Jardim, F. et al. Prevalence of herpes simplex virus types 1 and 2 at maternal and fetal sides of the placenta in asymptomatic pregnant women. Am. J. Reprod. Immunol. 78, e12689 (2017).
Gay, N. J. et al. Age specific antibody prevalence to parvovirus B19: how many women are infected in pregnancy? Commun. Dis. Rep. CDR Rev. 4, R104–7 (1994).
Li, J. J., Henwood, T., Van Hal, S. & Charlton, A. Parvovirus infection: an immunohistochemical study using fetal and placental tissue. Pediatr. Dev. Pathol. 18, 30–39 (2015).
Jordan, J. A. & Deloia, J. A. Globoside expression within the human placenta. Placenta 20, 103–108 (1999).
Wegner, C. C. & Jordan, J. A. Human parvovirus B19 VP2 empty capsids bind to human villous trophoblast cells in vitro via the globoside receptor. Infect. Dis. Obstet. Gynecol. 12, 69–78 (2004).
Moffatt, S., Yaegashi, N., Tada, K., Tanaka, N. & Sugamura, K. Human parvovirus B19 nonstructural (NS1) protein induces apoptosis in erythroid lineage cells. J. Virol. 72, 3018–3028 (1998).
Jordan, J. A. & Butchko, A. R. Apoptotic activity in villous trophoblast cells during B19 infection correlates with clinical outcome: assessment by the caspase-related M30 cytodeath antibody. Placenta 23, 547–553 (2002).
Dickinson, J. E., Keil, A. D. & Charles, A. K. Discordant fetal infection for parvovirus B19 in a dichorionic twin pregnancy. Twin Res. Hum. Genet. 9, 456–459 (2006).
Schiesser, M., Sergi, C., Enders, M., Maul, H. & Schnitzler, P. Discordant outcomes in a case of parvovirus B19 transmission into both dichorionic twins. Twin Res. Hum. Genet. 12, 175–179 (2009).
Bernstein, H. B. & Wegman, A. D. HIV infection: antepartum treatment and management. Clin. Obstet. Gynecol. 61, 122–136 (2018).
Maury, W., Potts, B. J. & Rabson, A. B. HIV-1 infection of first-trimester and term human placental tissue: a possible mode of maternal-fetal transmission. J. Infect. Dis. 160, 583–588 (1989).
Amirhessami-Aghili, N. & Spector, S. A. Human immunodeficiency virus type 1 infection of human placenta: potential route for fetal infection. Dis. Markers 9, 348 (1991).
Mattern, C. F. T. et al. Localization of human immunodeficiency virus core antigen in term human placentas. Pediatrics 89, 207–209 (1992).
Peuchmaur, M. et al. HIV proteins absent from placentas of 75 HIV-1-positive women studied by immunohistochemistry. AIDS 5, 741–745 (1991).
Backe, E. et al. Demonstration of HIV-1 infected cells in human placenta by in situ hybridisation and immunostaining. J. Clin. Pathol. 45, 871–874 (1992).
Townsend, C. L. et al. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS 22, 973–981 (2008).
Koay, W. L. A. et al. Prevention of perinatal HIV transmission in an area of high HIV prevalence in the United States. J. Pediatr. 228, 101–109 (2021).
Johnson, E. L. & Chakraborty, R. HIV-1 at the placenta: immune correlates of protection and infection. Curr. Opin. Infect. Dis. 29, 248–255 (2016).
Mlakar, J. et al. Zika virus associated with microcephaly. N. Engl. J. Med. 374, 951–958 (2016).
Brady, O. J. et al. The association between Zika virus infection and microcephaly in Brazil 2015–2017: an observational analysis of over 4 million births. PLoS Med. 16, e1002755 (2019).
Hoen, B. et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N. Engl. J. Med. 378, 985–994 (2018).
van der Linden, V. et al. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth — Brazil. MMWR Morb. Mortal. Wkly Rep. 65, 1343–1348 (2016).
Sobhani, N. C. et al. Discordant Zika virus findings in twin pregnancies complicated by antenatal Zika virus exposure: a prospective cohort. J. Infect. Dis. 221, 1838–1845 (2020).
Amaral, M. S. et al. Differential gene expression elicited by ZIKV infection in trophoblasts from congenital Zika syndrome discordant twins. PLoS Negl. Trop. Dis. 14, e0008424 (2020).
Hastings, A. K. et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep. 19, 558–568 (2017).
Hermanns, K. et al. Zika virus infection in human placental tissue explants is enhanced in the presence of dengue virus antibodies in-vitro. Emerg. Microbes Infect. 7, 198 (2018).
Zimmerman, M. G. et al. Cross-reactive dengue virus antibodies augment Zika virus infection of human placental macrophages. Cell Host Microbe 24, 731–742.e6 (2018).
Rodriguez-Barraquer, I. et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 363, 607–610 (2019).
Pedroso, C. et al. Cross-protection of dengue virus infection against congenital Zika syndrome, northeastern Brazil. Emerg. Infect. Dis. 25, 1485–1493 (2019).
Pantoja, P. et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat. Commun. 8, 15674 (2017).
Breitbach, M. E. et al. Primary infection with dengue or Zika virus does not affect the severity of heterologous secondary infection in macaques. PLoS Pathog. 15, e1007766 (2019).
Katzelnick, L. C. et al. Zika virus infection enhances future risk of severe dengue disease. Science 369, 1123–1128 (2020).
Dudley, D. M. et al. Using macaques to address critical questions in Zika virus research. Annu. Rev. Virol. 6, 481–500 (2019).
Koenig, M. R. et al. Quantitative definition of neurobehavior, vision, hearing and brain volumes in macaques congenitally exposed to Zika virus. PLoS ONE 15, e0235877 (2020).
Bebell, L. M., Oduyebo, T. & Riley, L. E. Ebola virus disease and pregnancy: a review of the current knowledge of Ebola virus pathogenesis, maternal, and neonatal outcomes. Birth Defects Res. 109, 353–362 (2017).
Muehlenbachs, A. et al. Ebola virus disease in pregnancy: clinical, histopathologic and immunohistochemical findings. J. Infect. Dis. 215, 64–69 (2015).
Oduyebo, T. et al. A pregnant patient with ebola virus disease. Obstet. Gynecol. 126, 1273–1275 (2015).
Baudin, M. et al. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: a cross-sectional study. Lancet Glob. Heal. 4, e864–e871 (2016).
Arishi, H. M., Aqeel, A. Y. & Al Hazmi, M. M. Vertical transmission of fatal Rift Valley fever in a newborn. Ann. Trop. Paediatr. 26, 251–253 (2006).
Oymans, J., Wichgers Schreur, P. J., van Keulen, L., Kant, J. & Kortekaas, J. Rift valley fever virus targets the maternal–foetal interface in ovine and human placentas. PLoS Negl. Trop. Dis. 14, 1–18 (2020).
McMillen, C. M. et al. Rift Valley fever virus induces fetal demise in Sprague–Dawley rats through direct placental infection. Sci. Adv. 4, eaau9812 (2018).
O’Leary, D. R. et al. Birth outcomes following west nile virus infection of pregnant women in the United States: 2003–2004. Pediatrics 117, e537–e545 (2006).
Platt, D. J. et al. Zika virus-related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci. Transl. Med. 10, eaao7090 (2018).
Julander, J. G. et al. West Nile virus infection of the placenta. Virology 347, 175–182 (2006).
Chen, H. et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 395, 809–815 (2020).
Pique-Regi, R. et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 9, e58716 (2020).
Li, Y. et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg. Infect. Dis. 26, 1335–1336 (2020).
Egloff, C., Vauloup-Fellous, C., Picone, O., Mandelbrot, L. & Roques, P. Evidence and possible mechanisms of rare maternal-fetal transmission of SARS-CoV-2. J. Clin. Virol. 128, 104447 (2020).
WAPM working group on COVID-19. . Maternal and perinatal outcomes of pregnant women with SARS-COV-2 infection. Ultrasound Obstet. Gynecol. 57, 232–241 (2021).
Flaherman, V. J. et al. Infant outcomes following maternal infection with SARS-CoV-2: first report from the PRIORITY study. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa1411 (2020).
Torgerson, P. R. & Mastroiacovo, P. La charge mondiale de la toxoplasmose: une étude systématique. Bull. World Health Organ. 91, 501–508 (2013).
Galanakis, E. et al. Outcome of toxoplasmosis acquired during pregnancy following treatment in both pregnancy and early infancy. Fetal Diagn. Ther. 22, 444–448 (2007).
Berrébi, A. et al. Long-term outcome of children with congenital toxoplasmosis. Am. J. Obstet. Gynecol. 203, 552.e1–552.e6 (2010).
Dunn, D. et al. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 353, 1829–1833 (1999).
Mendez, O. A. & Koshy, A. A. Toxoplasma gondii: entry, association, and physiological influence on the central nervous system. PLoS Pathog. 13, e1006351 (2017).
Bisio, H. & Soldati-Favre, D. Signaling cascades governing entry into and exit from host cells by Toxoplasma gondii. Annu. Rev. Microbiol. 73, 579–599 (2019).
English, E. D., Adomako-Ankomah, Y. & Boyle, J. P. Secreted effectors in Toxoplasma gondii and related species: determinants of host range and pathogenesis? Parasite Immunol. 37, 127–140 (2015).
Hunter, C. A. & Sibley, L. D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10, 766–778 (2012).
Rudzki, E. N. et al. Toxoplasma gondii GRA28 is required for specific induction of the regulatory chemokine CCL22 in human and mouse cells. Preprint at bioRxiv https://doi.org/10.1101/2020.10.14.335802 (2020).
Luxemburger, C. et al. Effects of malaria during pregnancy on infant mortality in an area of low malaria transmission. Am. J. Epidemiol. 154, 459–465 (1993).
Luxemburger, C. et al. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans. R. Soc. Trop. Med. Hyg. 91, 256–262 (1997).
Guyatt, H. L. & Snow, R. W. Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans. R. Soc. Trop. Med. Hyg. 95, 569–576 (2001).
Desai, M. et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7, 93–104 (2007).
Tobian, A. A. et al. Frequent umbilical cord-blood and maternal-blood infections with Plasmodium falciparum, P. malariae, and P. ovale in Kenya. J. Infect. Dis. 182, 558–563 (2000).
Ouédraogo, A. et al. Transplacental transmission of plasmodium falciparum in a highly malaria endemic area of Burkina Faso. J. Trop. Med. 2012, 109705 (2012).
Beeson, J. G., Amin, N., Kanjala, M. & Rogerson, S. J. Selective accumulation of mature asexual stages of Plasmodium falciparum-infected erythrocytes in the placenta. Infect. Immun. 70, 5412–5415 (2002).
Muehlenbachs, A. et al. A novel histological grading scheme for placental malaria applied in areas of high and low malaria transmission. J. Infect. Dis. 202, 1608–1616 (2010).
Okoko, B. J. et al. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural west African population. J. Infect. Dis. 184, 627–632 (2001).
Owens, S. et al. Placental malaria and immunity to infant measles. Arch. Dis. Child. 91, 507–508 (2006).
Frech, C. & Chen, N. Variant surface antigens of malaria parasites: functional and evolutionary insights from comparative gene family classification and analysis. BMC Genomics 14, 427 (2013).
Beeson, J. G. & Duffy, P. E. The immunology and pathogenesis of malaria during pregnancy. Curr. Top. Microbiol. Immunol. 297, 187–227 (2005).
Salanti, A. et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49, 179–191 (2003).
Tuikue Ndam, N. G. et al. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J. Infect. Dis. 192, 331–335 (2005).
Magistrado, P. et al. VAR2CSA expression on the surface of placenta-derived Plasmodium falciparum-infected erythrocytes. J. Infect. Dis. 198, 1071–1074 (2008).
Chotivanich, K. et al. Plasmodium vivax adherence to placental glycosaminoglycans. PLoS ONE 7, e34509 (2012).
Fried, M. & Duffy, P. E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272, 1502–1504 (1996).
Fried, M., Domingo, G. J., Gowda, C. D., Mutabingwa, T. K. & Duffy, P. E. Plasmodium falciparum: chondroitin sulfate A is the major receptor for adhesion of parasitized erythrocytes in the placenta. Exp. Parasitol. 113, 36–42 (2006).
O’Neil-Dunne, I. et al. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect. Immun. 69, 7487–7492 (2001).
Ricke, C. H. et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165, 3309–3316 (2000).
Oleinikov, A. V. et al. Effects of sex, parity, and sequence variation on seroreactivity to candidate pregnancy malaria vaccine antigens. J. Infect. Dis. 196, 155–164 (2007).
Duffy, P. E. & Fried, M. Antibodies that inhibit plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 71, 6620–6623 (2003).
Fried, M. & Duffy, P. E. Malaria during pregnancy. Cold Spring Harb. Perspect. Med. 7, a025551 (2017).
Staalsoe, T. et al. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363, 283–289 (2004).
Rogerson, S. J. et al. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 68, 115–119 (2003).
Moormann, A. M. et al. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J. Infect. Dis. 180, 1987–1993 (1999).
Lima, F. A. et al. Plasmodium falciparum infection dysregulates placental autophagy. PLoS ONE 14, e0226117 (2019).
Dimasuay, K. G. et al. Impaired placental autophagy in placental malaria. PLoS ONE 12, e0187291 (2017).
Rios, L., Campos, E. E., Menon, R., Zago, M. P. & Garg, N. J. Epidemiology and pathogenesis of maternal–fetal transmission of Trypanosoma cruzi and a case for vaccine development against congenital Chagas disease. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165591 (2020).
Torrico, F. et al. Maternal trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in bolivia. Am. J. Trop. Med. Hyg. 70, 201–209 (2004).
Torrico, F. et al. Are maternal re-infections with Trypanosoma cruzi associated with higher morbidity and mortality of congenital Chagas disease? Trop. Med. Int. Heal. 11, 628–635 (2006).
Siriano, L. D. R., Luquetti, A. O., Avelar, J. B., Marra, N. L. & De Castro, A. M. Chagas disease: increased parasitemia during pregnancy detected by hemoculture. Am. J. Trop. Med. Hyg. 84, 569–574 (2011).
Brutus, L. et al. Short report: Detectable Trypanosoma cruzi parasitemia during pregnancy and delivery as a risk factor for congenital chagas disease. Am. J. Trop. Med. Hyg. 83, 1044–1047 (2010).
Duaso, J. et al. Trypanosoma cruzi induces apoptosis in ex vivo infected human chorionic villi. Placenta 32, 356–361 (2011).
Duaso, J. et al. Trypanosoma cruzi induces tissue disorganization and destruction of chorionic villi in an ex vivo infection model of human placenta. Placenta 31, 705–711 (2010).
Castillo, C. et al. Trypanosoma cruzi exosomes increases susceptibility to parasite infection in human placental chorionic villi explants. Placenta 51, 123–124 (2017).
Roberts, R. M., Green, J. A. & Schulz, L. C. The evolution of the placenta. Reproduction 152, R179–R189 (2016).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 564, 263–281 (2018).
Turco, M. Y. et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 19, 568–577 (2017).
Acknowledgements
The authors’ work on the placenta is supported by NIH AI145828 (C.B.C.) and a Magee Women’s Research Institute Clinical Trainee Research Award 4032 (C.J.M.). Additionally, C.J.M. is supported by K12 HD000849 awarded to the Reproductive Scientist Development Program. The authors apologize to any authors whose work was not included owing to space limitations.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Microbiology thanks S. Gaw, who co-reviewed with L. Li; N. Gomez-Lopez; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Granulysins
-
Antimicrobial peptides that can cause pores in bacterial cell walls, leading to cell death.
- Osteochondritis
-
Inflammation of the cartilage.
- Hydrops
-
The presence of excess fluid in two or more fetal body compartments, associated with severe perinatal morbidity and death.
- Pyelonephritis
-
Inflammation and infection of the kidney.
- Chorioamnionitis
-
Inflammation of the fetal membranes, classically associated with bacterial infection.
- Intrapartum prophylaxis
-
The process of giving antibiotics, typically penicillins, to individuals in labour for treatment of group B Streptococcus colonization.
- Pericytes
-
Specialized connective cells of mesenchymal origin that classically surround endothelial cells in blood vessels.
- Aplasia cutis
-
A condition in which parts of the skin are missing.
- Ventriculomegaly
-
A condition in which the brain ventricles are abnormally large.
- Microcephaly
-
An abnormally small circumference.
- Chorioretinitis
-
Inflammation of the choroid surrounding the retina of the eye.
- Atrophy
-
Degenerative and small.
- Hydrocephalus
-
Central nervous system abnormality from pathological ventriculomegaly with excess fluid in the brain causing compression of brain parenchyma and requiring treatment.
- Lissencephaly
-
A brain malformation associated with severe developmental delay in which gyration of the brain is absent.
- Apgar score
-
A score assigned to neonates associated with neonatal health after birth. Infants are scored 0–2 points on muscle tone, skin colour, respiratory effort, pulse and reflex irritability at 1, 5 and 10 min after birth.
Rights and permissions
About this article
Cite this article
Megli, C.J., Coyne, C.B. Infections at the maternal–fetal interface: an overview of pathogenesis and defence. Nat Rev Microbiol 20, 67–82 (2022). https://doi.org/10.1038/s41579-021-00610-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41579-021-00610-y
This article is cited by
-
Chronobiology of neurotropic viruses: rhythmic viral entry and arrhythmic host clocks
Cell Discovery (2026)
-
COVID-19-related inflammation of the placenta impedes fetal development in pregnant hamsters
Nature Communications (2026)
-
Modeling the Impact of Tuberculosis and Diabetes in Pregnant Women Using Machine Learning-Based Physics-Informed Neural Networks with Real-World Data Analysis
Brazilian Journal of Physics (2026)
-
Neonatal neurocritical care considerations for prenatally identified neurological disorders
Pediatric Research (2026)
-
Microglia determine an immune-challenged environment and facilitate ibuprofen action in human retinal organoids
Journal of Neuroinflammation (2025)