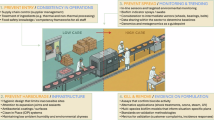
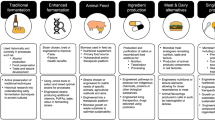
Abstract
Foodborne illnesses pose a substantial health and economic burden, presenting challenges in prevention due to the diverse microbial hazards that can enter and spread within food systems. Various factors, including natural, political and commercial drivers, influence food production and distribution. The risks of foodborne illness will continue to evolve in step with these drivers and with changes to food systems. For example, climate impacts on water availability for agriculture, changes in food sustainability targets and evolving customer preferences can all have an impact on the ecology of foodborne pathogens and the agrifood niches that can carry microorganisms. Whole-genome and metagenome sequencing, combined with microbial surveillance schemes and insights from the food system, can provide authorities and businesses with transformative information to address risks and implement new food safety interventions across the food chain. In this Review, we describe how genome-based approaches have advanced our understanding of the evolution and spread of enduring bacterial foodborne hazards as well as their role in identifying emerging foodborne hazards. Furthermore, foodborne hazards exist in complex microbial communities across the entire food chain, and consideration of these co-existing organisms is essential to understanding the entire ecology supporting pathogen persistence and transmission in an evolving food system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zurek, M. et al. Assessing sustainable food and nutrition security of the EU food system-an integrated approach. Sustainability 10, 4271 (2018).
Parsons, K., Hawkes, C. & Wells, R. Brief 2. Understanding the Food System: Why it Matters for Food Policy (Centre for Food Policy, 2019).
Hasnain, S., Ingram, J. & Zurek, M. Mapping the UK Food System — A Report for the UKRI Transforming UK Food Systems Programme (Environmental Change Institute, 2020).
Food and Agriculture Organization. If It Isn’t Safe, It Isn’t Food https://www.fao.org/newsroom/story/If-it-isn-t-safe-it-isn-t-food/ (2019).
Food and Agriculture Organization. FAO Strategic Priorities for Food Safety within the FAO Strategic Framework 2022-2031 (FAO, 2023).
Climate Change Committee. Progress in Adapting to Climate Change; 2023 Report to Parliament https://www.theccc.org.uk/publication/progress-in-adapting-to-climate-change-2023-report-to-parliament/ (2023).
European Food Safety Authority. Maggiore, A., Afonso, A., Barrucci, F. & De Sanctis, G. Climate Change as a Driver of Emerging Risks for Food and Feed Safety, Plant, Animal Health and Nutritional Quality (EFSA, 2020).
Morgado, M. E. et al. Climate change, extreme events, and increased risk of salmonellosis: foodborne diseases active surveillance network (FoodNet), 2004-2014. Env. Health 20, 105 (2021).
Food and Agriculture Organization. Final Meeting Report: Technical Meeting on the Impact of Whole Genome Sequencing (WGS) on Food Safety Management: Within a One Health Approach (FAO, 2016).
Morse, S. S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1, 7–15 (1995).
Grad, Y. H. et al. Genomic epidemiology of the Escherichia coli O104:H4 outbreaks in Europe, 2011. Proc. Natl Acad. Sci. USA 109, 3065–3070 (2012).
Kingsley, R. A. et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 19, 2279–2287 (2009).
Seribelli, A. A. et al. Phylogenetic analysis revealed that Salmonella Typhimurium ST313 isolated from humans and food in Brazil presented a high genomic similarity. Braz. J. Microbiol. 51, 53–64 (2020).
Almeida, F. et al. Multilocus sequence typing of Salmonella Typhimurium reveals the presence of the highly invasive ST313 in Brazil. Infect. Genet. Evol. 51, 41–44 (2017).
Bian, X. et al. Campylobacter abundance in breastfed infants and identification of a new species in the Global Enterics Multicenter Study. mSphere 5, e00735-19 (2020).
Ramees, T. P. et al. Arcobacter: an emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control — a comprehensive review. Vet. Q. 37, 136–161 (2017).
Centers for Disease Control and Prevention. Reoccurring, Emerging, and Persisting Enteric Bacterial Strains https://www.cdc.gov/ncezid/dfwed/outbreak-response/rep-strains.html (2023).
World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007-2015 (WHO, 2015).
Devleesschauwer, B., Haagsma, J. A., Mangen, M.-J. J., Lake, R. J. & Havelaar, A. H. In: Food Safety Economics (ed. Roberts, T.) (Springer, 2018).
Abebe, E., Gugsa, G. & Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 4674235 (2020).
Bintsis, T. Foodborne pathogens. AIMS Microbiol. 3, 529–563 (2017).
Foley, S. L., Lynne, A. M. & Nayak, R. Molecular typing methodologies for microbial source tracking and epidemiological investigations of gram-negative bacterial foodborne pathogens. Infect. Genet. Evol. 9, 430–440 (2009).
Deng, X., den Bakker, H. C. & Hendriksen, R. S. Genomic epidemiology: whole-genome-sequencing-powered surveillance and outbreak investigation of foodborne bacterial pathogens. Annu. Rev. Food Sci. Technol. 7, 353–374 (2016).
Taboada, E. N., Graham, M. R., Carrico, J. A. & Van Domselaar, G. Food safety in the age of next generation sequencing, bioinformatics, and open data access. Front. Microbiol. 8, 909 (2017).
Threlfall, E. J. Epidemic Salmonella Typhimurium DT104 — a truly international multiresistant clone. J. Antimicrob. Chemother. 46, 7–10 (2000).
Petrovska, L. et al. Microevolution of monophasic Salmonella Typhimurium during epidemic, United Kingdom, 2005-2010. Emerg. Infect. Dis. 22, 617–624 (2016).
Alikhan, N. F. et al. Dynamics of Salmonella enterica and antimicrobial resistance in the Brazilian poultry industry and global impacts on public health. PLoS Genet. 18, e1010174 (2022).
Langridge, G. C. et al. Patterns of genome evolution that have accompanied host adaptation in Salmonella. Proc. Natl Acad. Sci. USA 112, 863–868 (2015).
Moura, A. et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2, 16185 (2016).
Ma, L.-C., Zhao, H.-Q., Wu, L. B., Cheng, Z.-L. & Liu, C. Impact of the microbiome on human, animal and environmental health from a One Health perspective. Sci. One Health 2, 100037 (2023).
Srikumar, S. et al. RNA sequencing-based transcriptional overview of xerotolerance in Cronobacter sakazakii SP291. Appl. Environ. Microbiol. 85, e01993-18 (2019).
Luo, N. et al. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc. Natl Acad. Sci. USA 102, 541–546 (2005).
Marmion, M., Macori, G., Ferone, M., Whyte, P. & Scannell, A. G. M. Survive and thrive: control mechanisms that facilitate bacterial adaptation to survive manufacturing-related stress. Int. J. Food Microbiol. 368, 109612 (2022).
Gauvry, E. et al. Knowledge of the physiology of spore-forming bacteria can explain the origin of spores in the food environment. Res. Microbiol. 168, 369–378 (2017).
NicAogain, K. & O’Byrne, C. P. The role of stress and stress adaptations in determining the fate of the bacterial pathogen Listeria monocytogenes in the food chain. Front. Microbiol. 7, 1865 (2016).
Teh, A. H. T., Lee, S. M. & Dykes, G. A. Association of some Campylobacter jejuni with Pseudomonas aeruginosa biofilms increases attachment under conditions mimicking those in the environment. PLoS One 14, e0215275 (2019).
Whelan, M. V. X. et al. Acquisition of fluoroquinolone resistance leads to increased biofilm formation and pathogenicity in Campylobacter jejuni. Sci. Rep. 9, 18216 (2019).
Ong, K. J. et al. Food safety considerations and research priorities for the cultured meat and seafood industry. Compr. Rev. Food Sci. Food Saf. 20, 5421–5448 (2021).
Food and Agriculture Organization of the United Nations & World Health Organization. Food Safety Aspects of Cell-based Food (FAO & WHO, 2023).
Bartula, K., Begley, M., Latour, N. & Callanan, M. Growth of food-borne pathogens Listeria and Salmonella and spore-forming Paenibacillus and Bacillus in commercial plant-based milk alternatives. Food Microbiol. 109, 104143 (2023).
Li, M. et al. Global disease burden of pathogens in animal source foods, 2010. PLoS One 14, e0216545 (2019).
Jaffee, S., Henson, S., Unnevehr, L., Grace, D. & Cassou, E. The Safe Food Imperative: Accelerating Progress in Low- and Middle-income Countries (World Bank, 2019).
Hellberg, R. S. & Chu, E. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: a review. Crit. Rev. Microbiol. 42, 548–572 (2016).
Jones, B. A. et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl Acad. Sci. USA 110, 8399–8404 (2013).
Mora, C. et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Change 12, 869–875 (2022).
Archer, E. J. et al. Climate warming and increasing Vibrio vulnificus infections in North America. Sci. Rep. 13, 3893 (2023).
Vezzulli, L. et al. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl Acad. Sci. USA 113, E5062–E5071 (2016).
Baker-Austin, C. et al. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Change 3, 73–77 (2013).
Food Standards Agency. The Burden of Foodborne Disease in the UK 2018 (FSA, 2020).
Kristensen, J. M., Nierychlo, M., Albertsen, M. & Nielsen, P. H. Bacteria from the genus Arcobacter are abundant in effluent from wastewater treatment plants. Appl. Environ. Microbiol. 86, e03044-19 (2020).
Billington, C., Kingsbury, J. M. & Rivas, L. Metagenomics approaches for improving food safety: a review. J. Food Prot. 85, 448–464 (2022).
Bloomfield, S. J. et al. Determination and quantification of microbial communities and antimicrobial resistance on food through host DNA-depleted metagenomics. Food Microbiol. 110, 104162 (2023).
Carleton, H. A. et al. Metagenomic approaches for public health surveillance of foodborne infections: opportunities and challenges. Foodborne Pathog. Dis. 16, 474–479 (2019).
Ray, L. C. et al. Changing diagnostic testing practices for foodborne pathogens, Foodborne Diseases Active Surveillance Network, 2012-2019. Open Forum Infect. Dis. 9, ofac344 (2022).
Anyansi, C., Straub, T. J., Manson, A. L., Earl, A. M. & Abeel, T. Computational methods for strain-level microbial detection in colony and metagenome sequencing data. Front. Microbiol. 11, 1925 (2020).
Kang, X., Luo, X. & Schonhuth, A. StrainXpress: strain aware metagenome assembly from short reads. Nucleic Acids Res. 50, e101 (2022).
Rolon, M. L., Voloshchuk, O., Bartlett, K. V., LaBorde, L. F. & Kovac, J. Multi-species biofilms of environmental microbiota isolated from fruit packing facilities promoted tolerance of Listeria monocytogenes to benzalkonium chloride. Biofilm 7, 100177 (2024).
Lupolova, N., Dallman, T. J., Matthews, L., Bono, J. L. & Gally, D. L. Support vector machine applied to predict the zoonotic potential of E. coli O157 cattle isolates. Proc. Natl Acad. Sci. USA 113, 11312–11317 (2016).
Im, H., Hwang, S. H., Kim, B. S. & Choi, S. H. Pathogenic potential assessment of the Shiga toxin-producing Escherichia coli by a source attribution-considered machine learning model. Proc. Natl Acad. Sci. USA 118, e2018877118 (2021).
Burgaya, J. et al. The bacterial genetic determinants of Escherichia coli capacity to cause bloodstream infections in humans. PLoS Genet. 19, e1010842 (2023).
Sadilek, A. et al. Machine-learned epidemiology: real-time detection of foodborne illness at scale. NPJ Digit. Med. 1, 36 (2018).
Franz, E. et al. Phylogeographic analysis reveals multiple international transmission events have driven the global emergence of Escherichia coli O157:H7. Clin. Infect. Dis. 69, 428–437 (2019).
Moura, A. et al. Emergence and global spread of Listeria monocytogenes main clinical clonal complex. Sci. Adv. 7, eabj9805 (2021).
Bayliss, S. C. et al. Rapid geographical source attribution of Salmonella enterica serovar enteritidis genomes using hierarchical machine learning. eLife 12, e84167 (2023).
Brown, B., Allard, M., Bazaco, M. C., Blankenship, J. & Minor, T. An economic evaluation of the whole genome sequencing source tracking program in the U.S. PLoS One 16, e0258262 (2021).
Thomas, J. et al. Outbreak of listeriosis in South Africa associated with processed meat. N. Engl. J. Med. 382, 632–643 (2020).
Kovanen, S. M. et al. Multilocus sequence typing (MLST) and whole-genome MLST of Campylobacter jejuni isolates from human infections in three districts during a seasonal peak in Finland. J. Clin. Microbiol. 52, 4147–4154 (2014).
Yang, C. et al. Outbreak dynamics of foodborne pathogen Vibrio parahaemolyticus over a seventeen year period implies hidden reservoirs. Nat. Microbiol. 7, 1221–1229 (2022).
Pightling, A. W. et al. Interpreting whole-genome sequence analyses of foodborne bacteria for regulatory applications and outbreak investigations. Front. Microbiol. 9, 1482 (2018).
Duval, A., Opatowski, L. & Brisse, S. Defining genomic epidemiology thresholds for common-source bacterial outbreaks: a modelling study. Lancet Microbe 4, e349–e357 (2023).
Hoffmann, M. et al. Tracing origins of the Salmonella Bareilly strain causing a food-borne outbreak in the United States. J. Infect. Dis. 213, 502–508 (2016).
Dooley, D. M. et al. FoodOn: a harmonized food ontology to increase global food traceability, quality control and data integration. NPJ Sci. Food 2, 23 (2018).
National Center for Biotechnology Information. NCBI Datasets https://www.ncbi.nlm.nih.gov/datasets/ (2024).
Timme, R. E. et al. GenomeTrakr proficiency testing for foodborne pathogen surveillance: an exercise from 2015. Microb. Genom. 4, e000185 (2018).
Moran-Gilad, J. et al. Proficiency testing for bacterial whole genome sequencing: an end-user survey of current capabilities, requirements and priorities. BMC Infect. Dis. 15, 174 (2015).
Jolley, K. A., Bray, J. E. & Maiden, M. C. J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 3, 124 (2018).
Baker, K. S. et al. Genomics for public health and international surveillance of antimicrobial resistance. Lancet Microbe 4, e1047–e1055 (2023).
Kovac, J., den Bakker, H., Carroll, L. M. & Wiedmann, M. Precision food safety: a systems approach to food safety facilitated by genomics tools. TrAC Trends Anal. Chem. 96, 52–61 (2017).
World Health Organization. Whole Genome Sequencing as a Tool to Strengthen Foodborne Disease Surveillance and Response: Module 1: Introductory Module (WHO, 2023).
Jackson, B. R. et al. Implementation of nationwide real-time whole-genome sequencing to enhance listeriosis outbreak detection and investigation. Clin. Infect. Dis. 63, 380–386 (2016).
Dallman, T. J. et al. Whole-genome sequencing for national surveillance of Shiga toxin-producing Escherichia coli O157. Clin. Infect. Dis. 61, 305–312 (2015).
Grace, D. Food safety in low and middle income countries. Int. J. Env. Res. Public Health 12, 10490–10507 (2015).
Apruzzese, I. et al. Investing in food safety for developing countries: opportunities and challenges in applying whole-genome sequencing for food safety management. Foodborne Pathog. Dis. 16, 463–473 (2019).
Hadfield, J. et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 34, 4121–4123 (2018).
Argimon, S. et al. Microreact: visualizing and sharing data for genomic epidemiology and phylogeography. Microb. Genom. 2, e000093 (2016).
Argimon, S. et al. A global resource for genomic predictions of antimicrobial resistance and surveillance of Salmonella Typhi at pathogenwatch. Nat. Commun. 12, 2879 (2021).
Gangavarapu, K. et al. Outbreak.info genomic reports: scalable and dynamic surveillance of SARS-CoV-2 variants and mutations. Nat. Methods 20, 512–522 (2023).
Reuter, S. et al. Directional gene flow and ecological separation in Yersinia enterocolitica. Microb. Genom. 1, e000030 (2015).
Rodrigues, J. A. et al. Pangenomic analyses of antibiotic-resistant Campylobacter jejuni reveal unique lineage distributions and epidemiological associations. Microb. Genom. 9, mgen001073 (2023).
Neves, A. et al. The Swiss Pathogen Surveillance Platform — towards a nation-wide One Health data exchange platform for bacterial, viral and fungal genomics and associated metadata. Micro. Genom. 9, mgen001073 (2023).
Sears, A. et al. Marked campylobacteriosis decline after interventions aimed at poultry, New Zealand. Emerg. Infect. Dis. 17, 1007–1015 (2011).
Gardner, T. J. et al. Outbreak of campylobacteriosis associated with consumption of raw peas. Clin. Infect. Dis. 53, 26–32 (2011).
Cody, A. J., Maiden, M. C., Strachan, N. J. & McCarthy, N. D. A systematic review of source attribution of human campylobacteriosis using multilocus sequence typing. Eur. Surveill. 24, 1800696 (2019).
Pires, S. M., Vieira, A. R., Hald, T. & Cole, D. Source attribution of human salmonellosis: an overview of methods and estimates. Foodborne Pathog. Dis. 11, 667–676 (2014).
Mullner, P. et al. Assigning the source of human campylobacteriosis in New Zealand: a comparative genetic and epidemiological approach. Infect. Genet. Evol. 9, 1311–1319 (2009).
Sheppard, S. K. et al. Campylobacter genotyping to determine the source of human infection. Clin. Infect. Dis. 48, 1072–1078 (2009).
Mughini-Gras, L. et al. Tracing the sources of human salmonellosis: a multi-model comparison of phenotyping and genotyping methods. Infect. Genet. Evol. 28, 251–260 (2014).
Wilson, D. J. et al. Tracing the source of campylobacteriosis. PLoS Genet. 4, e1000203 (2008).
Liao, S. J., Marshall, J., Hazelton, M. L. & French, N. P. Extending statistical models for source attribution of zoonotic diseases: a study of campylobacteriosis. J. R. Soc. Interface 16, 20180534 (2019).
Arning, N., Sheppard, S. K., Bayliss, S., Clifton, D. A. & Wilson, D. J. Machine learning to predict the source of campylobacteriosis using whole genome data. PLoS Genet. 17, e1009436 (2021).
Munck, N., Njage, P. M. K., Leekitcharoenphon, P., Litrup, E. & Hald, T. Application of whole-genome sequences and machine learning in source attribution of Salmonella Typhimurium. Risk Anal. 40, 1693–1705 (2020).
Wainaina, L. et al. Source attribution of human campylobacteriosis using whole-genome sequencing data and network analysis. Pathogens 11, 645 (2022).
Jehanne, Q. et al. Genome-wide identification of host-segregating single-nucleotide polymorphisms for source attribution of clinical Campylobacter coli isolates. Appl. Environ. Microbiol. 86, e01787-20 (2020).
Duarte, A. S. R. et al. Metagenomics-based approach to source-attribution of antimicrobial resistance determinants — identification of reservoir resistome signatures. Front. Microbiol. 11, 601407 (2020).
Pasquali, F., Remondini, D., Snary, E. L., Hald, T. & Guillier, L. Editorial: integrating whole genome sequencing into source attribution and risk assessment of foodborne bacterial pathogens. Front. Microbiol. 12, 795098 (2021).
Tanui, C. K., Benefo, E. O., Karanth, S. & Pradhan, A. K. A machine learning model for food source attribution of Listeria monocytogenes. Pathogens 11, 691 (2022).
Liao, J. et al. Comparative genomics unveils extensive genomic variation between populations of Listeria species in natural and food-associated environments. ISME Commun. 3, 85 (2023).
Beck, K. L. et al. Monitoring the microbiome for food safety and quality using deep shotgun sequencing. NPJ Sci. Food 5, 3 (2021).
Mughini-Gras, L. et al. Risk factors for human salmonellosis originating from pigs, cattle, broiler chickens and egg laying hens: a combined case-control and source attribution analysis. PLoS One 9, e87933 (2014).
Lake, R. J. et al. Source attributed case-control study of campylobacteriosis in New Zealand. Int. J. Infect. Dis. 103, 268–277 (2021).
Amini, S. How next-generation sequencing will impact your food safety program. FoodNavigator Europe https://www.foodnavigator.com/Article/2018/12/10/How-next-generation-sequencing-will-impact-your-food-safety-program (William Reed, 2018).
Gerner-Smidt, P. Whole-Genome Sequencing for Food Safety. Food Safety Magazine https://www.food-safety.com/articles/7205-whole-genome-sequencing-for-food-safety (BNP Media, 2021).
Global Food Safety Initiative. A Culture of Food Safety: A Position Paper from the Global Food Safety Initiative (GFSI) (GFSI, 2018).
Espinoza, M. S. A., Flink, C., Boisen, N., Scheutz, F. & Käsbohrer, A. Microbiological sampling and analyses in the food business operators’ HACCP-based self-control programmes. Front. Food Sci. Technol. https://doi.org/10.3389/frfst.2023.1110359 (2023).
Chilled Food Association. Principles of an Environmental Monitoring Program for the Management of Listeria monocytogenes (CFA, 2023).
Jagadeesan, B. et al. The use of next generation sequencing for improving food safety: translation into practice. Food Microbiol. 79, 96–115 (2019).
Tran, M. et al. Economic evaluations of whole-genome sequencing for pathogen identification in public health surveillance and health-care-associated infections: a systematic review. Lancet Microbe 4, e953–e962 (2023).
Jain, S., Mukhopadhyay, K. & Thomassin, P. J. An economic analysis of Salmonella detection in fresh produce, poultry, and eggs using whole genome sequencing technology in Canada. Food Res. Int. 116, 802–809 (2019).
World Health Organization. Food Safety https://www.who.int/news-room/fact-sheets/detail/food-safety (2022).
Ogden, N. H., AbdelMalik, P. & Pulliam, J. Emerging infectious diseases: prediction and detection. Can. Commun. Dis. Rep. 43, 206–211 (2017).
Plowright, R. K. et al. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 15, 502–510 (2017).
Falloon, P. et al. What do changing weather and climate shocks and stresses mean for the UK food system? Env. Res. Lett. 17, 051001 (2022).
Elliott, C. In: New Food (Russell Publishing Ltd., 2022). https://www.newfoodmagazine.com/article/168316/five-food-safety-challenges/.
McLauchlin, J. et al. An outbreak of human listeriosis associated with frozen sweet corn consumption: investigations in the UK. Int. J. Food Microbiol. 338, 108994 (2021).
Kindle, P., Nuesch-Inderbinen, M., Cernela, N. & Stephan, R. Detection, isolation, and characterization of Shiga toxin-producing Escherichia coli in flour. J. Food Prot. 82, 164–167 (2019).
Bloomfield, S. J. et al. Genomic analysis of Salmonella enterica serovar Typhimurium DT160 associated with a 14-year outbreak, New Zealand, 1998-2012. Emerg. Infect. Dis. 23, 906–913 (2017).
Pijnacker, R. et al. An international outbreak of Salmonella enterica serotype enteritidis linked to eggs from Poland: a microbiological and epidemiological study. Lancet Infect. Dis. 19, 778–786 (2019).
Centers for Disease Control and Prevention. A-Z Index for Foodborne Illness https://www.cdc.gov/foodsafety/diseases/index.html (2021).
European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2021 zoonoses report. EFSA J. 20, e07666 (2022).
Wagenaar, J. A., French, N. P. & Havelaar, A. H. Preventing Campylobacter at the source: why is it so difficult? Clin. Infect. Dis. 57, 1600–1606 (2013).
Centers for Disease Control and Prevention. Detecting Outbreaks with Whole Genome Sequencing https://www.cdc.gov/amd/how-it-works/detecting-outbreaks-wgs.html (2019).
Acknowledgements
A.E.M. and M.W.G. are supported by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Microbes and Food Safety BB/X011011/1 and its constituent project BBS/E/F/000PR13634 (Theme 1, Microbial threats from foods in established and evolving food systems). This work was also supported in part by BBSRC grants BB/V018221/1 and BB/X002985/1 (UK Food Safety Research Network). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
International Patent Application No. PCT/GB2023/050906 entitled “Determination and quantification of the microbial communities and antimicrobial resistance genes on food” in the name of Quadram Institute Bioscience has been filed (priority date 05/04/2022) and is currently in the international phase; A.E.M. is an inventor. This relates to the aspect of the Review where it is mentioned that the potential detection of pathogens through metagenomics can be affected by the amount of contaminating host DNA and sequencing depth. N.P.F. is a member of the International Commission on Microbiological Specifications for Foods (ICMSF) and an Emeritus Director of the New Zealand Food Safety Science and Research Centre. Both organizations receive support from the food industry and government agencies. Both roles are unpaid and advisory, and related to food safety research, and neither organization influenced the content contributed by N.P.F. to the Review. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Steven Djordjevic, who co-reviewed with Veronica Jarocki; Jasna Kovac; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mather, A.E., Gilmour, M.W., Reid, S.W.J. et al. Foodborne bacterial pathogens: genome-based approaches for enduring and emerging threats in a complex and changing world. Nat Rev Microbiol 22, 543–555 (2024). https://doi.org/10.1038/s41579-024-01051-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41579-024-01051-z
This article is cited by
-
Food safety culture and the control of microbial communities in food production environments
npj Antimicrobials and Resistance (2026)
-
Spatiotemporal drivers of foodborne disease seasonality pattern in a coastal city of China
BMC Public Health (2025)
-
Advancing microbial risk assessment: perspectives from the evolution of detection technologies
npj Science of Food (2025)
-
Soil drivers of food-borne pathogen persistence
Nature Food (2025)
-
Molecular methods for rapid detection and identification of foodborne pathogenic bacteria
World Journal of Microbiology and Biotechnology (2025)